The Mechanical Consequences of the Interplay of Mineral Distribution and Organic Matrix Orientation in the Claws of the Sea Slater Ligia pallasii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isopod Collection
2.2. Scanning Electron Microscopy (SEM)
2.3. Imaging and Analytical Scanning Transmission Electron Microscopy (STEM)
2.4. X-ray Micro-Computed Tomography
2.5. Nanoindentation Measurements
3. Results
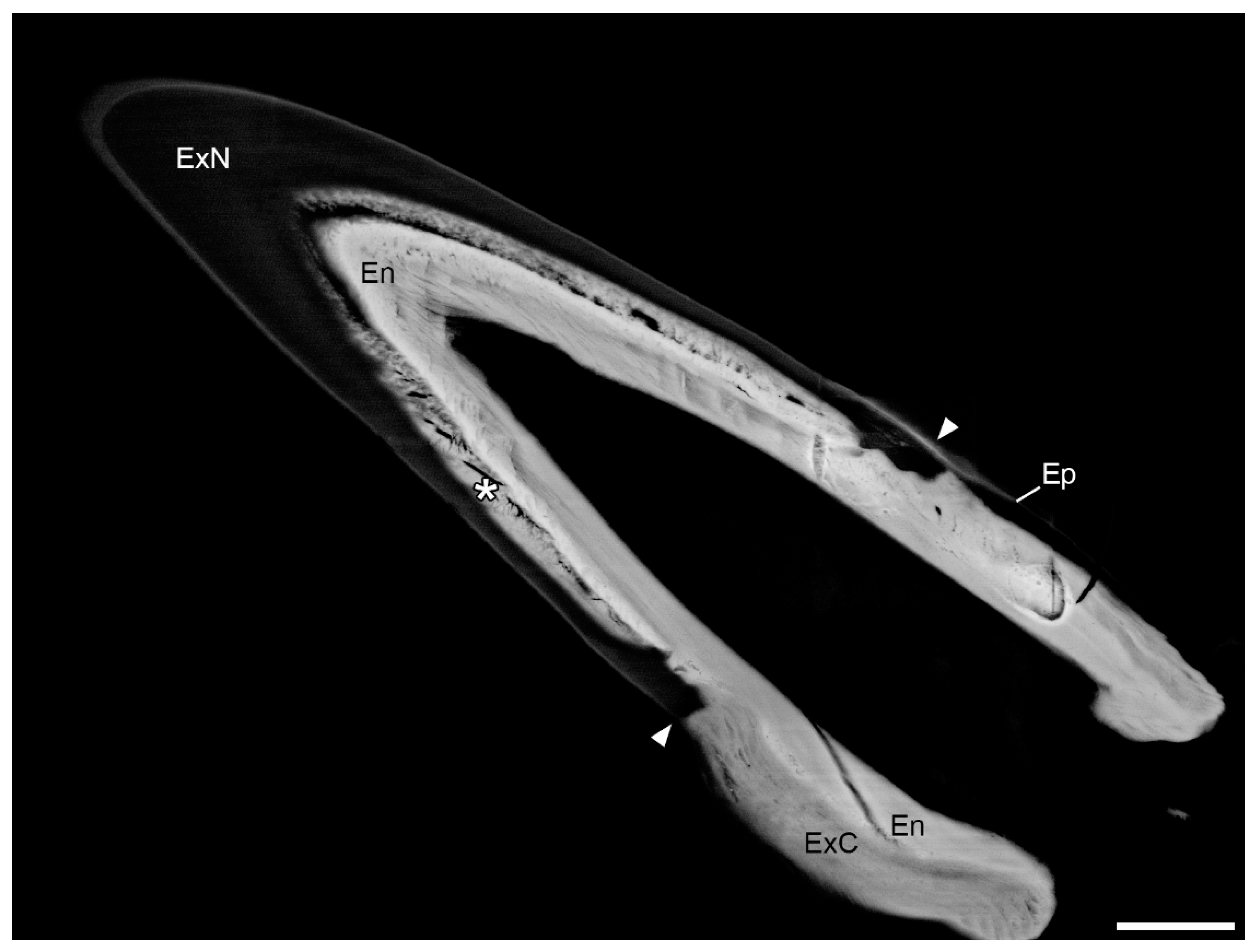
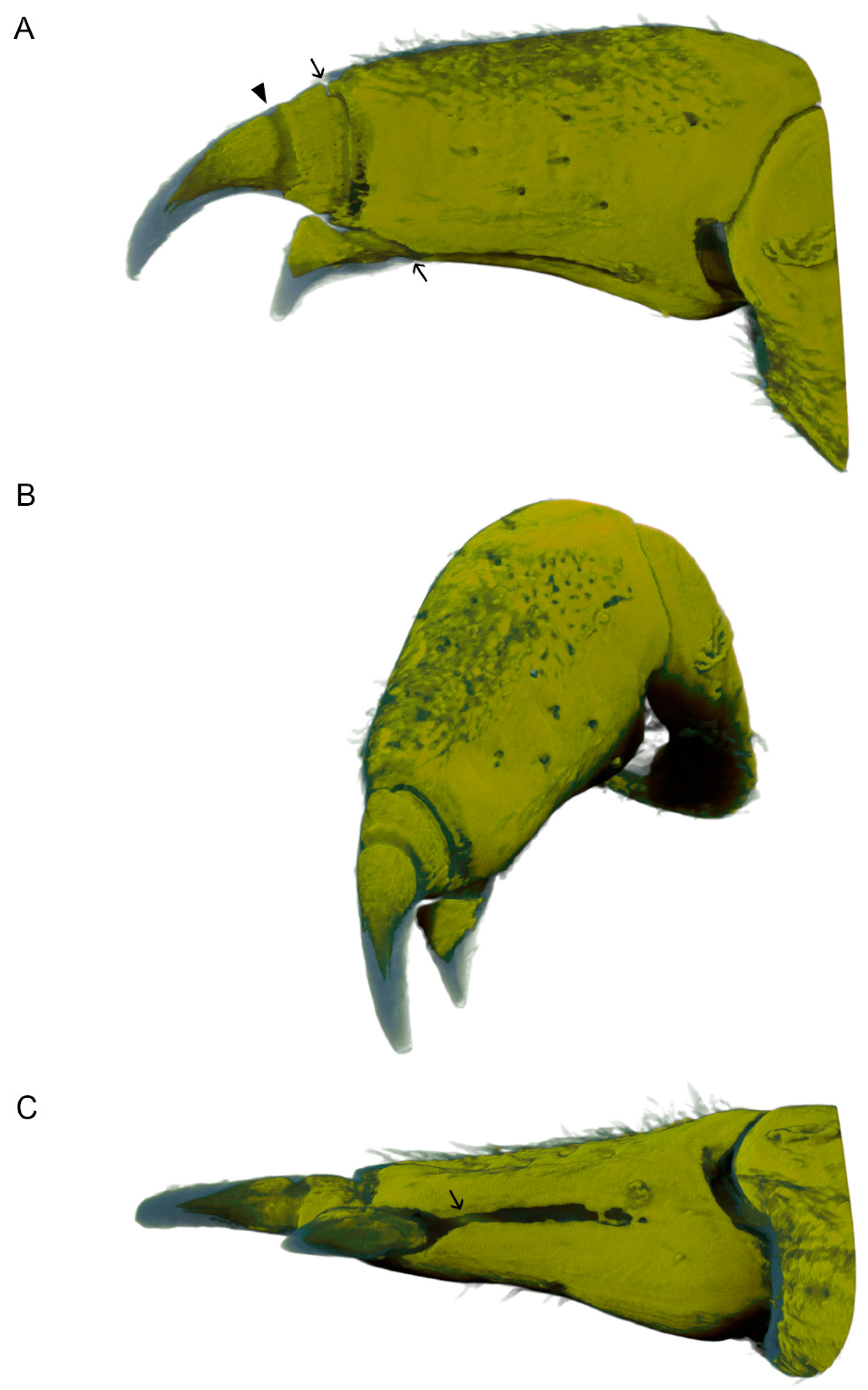
3.1. Morphology of Dactyli in L. pallasii
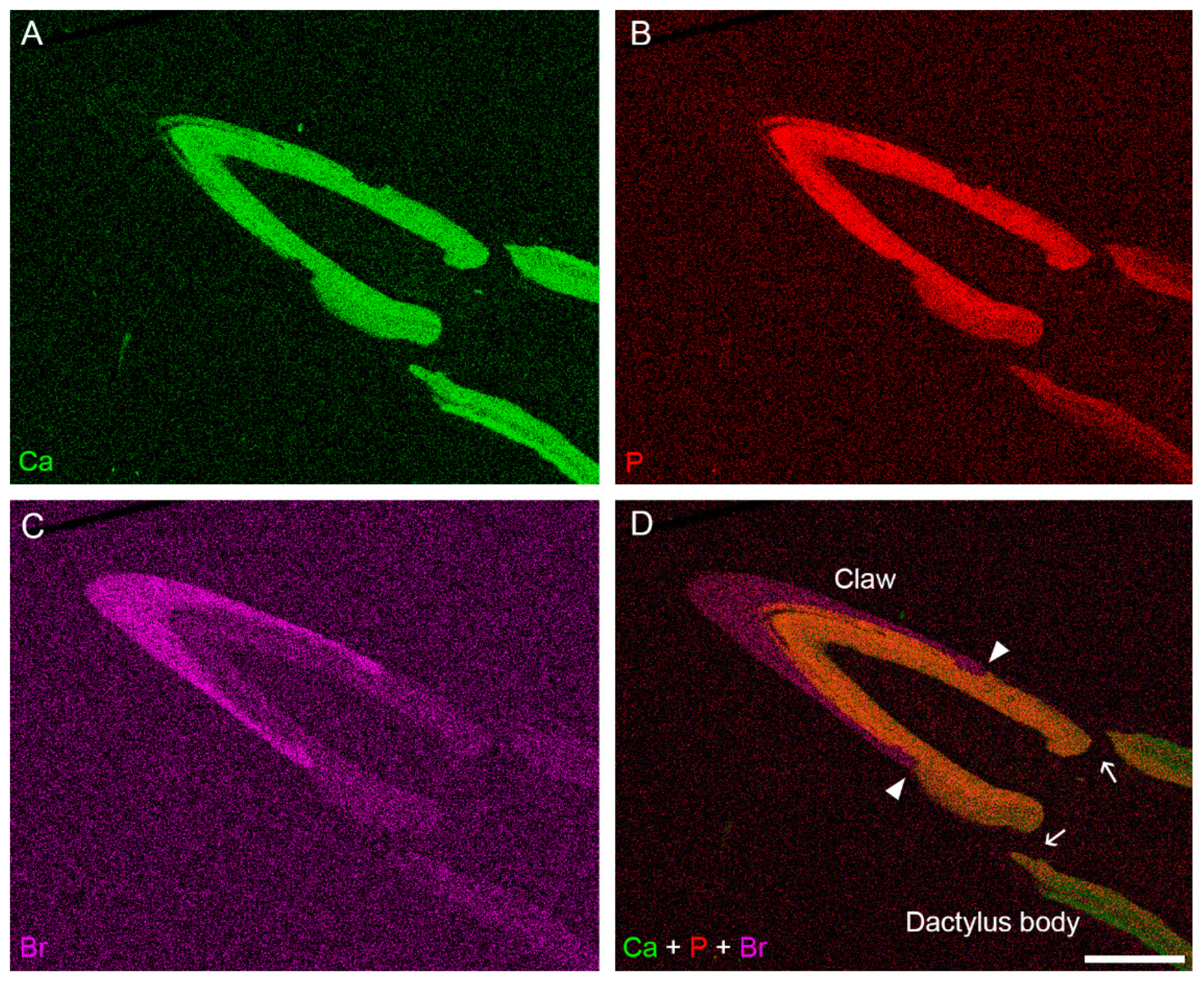
3.2. Distribution of Mineral in the Dactylus Cuticle
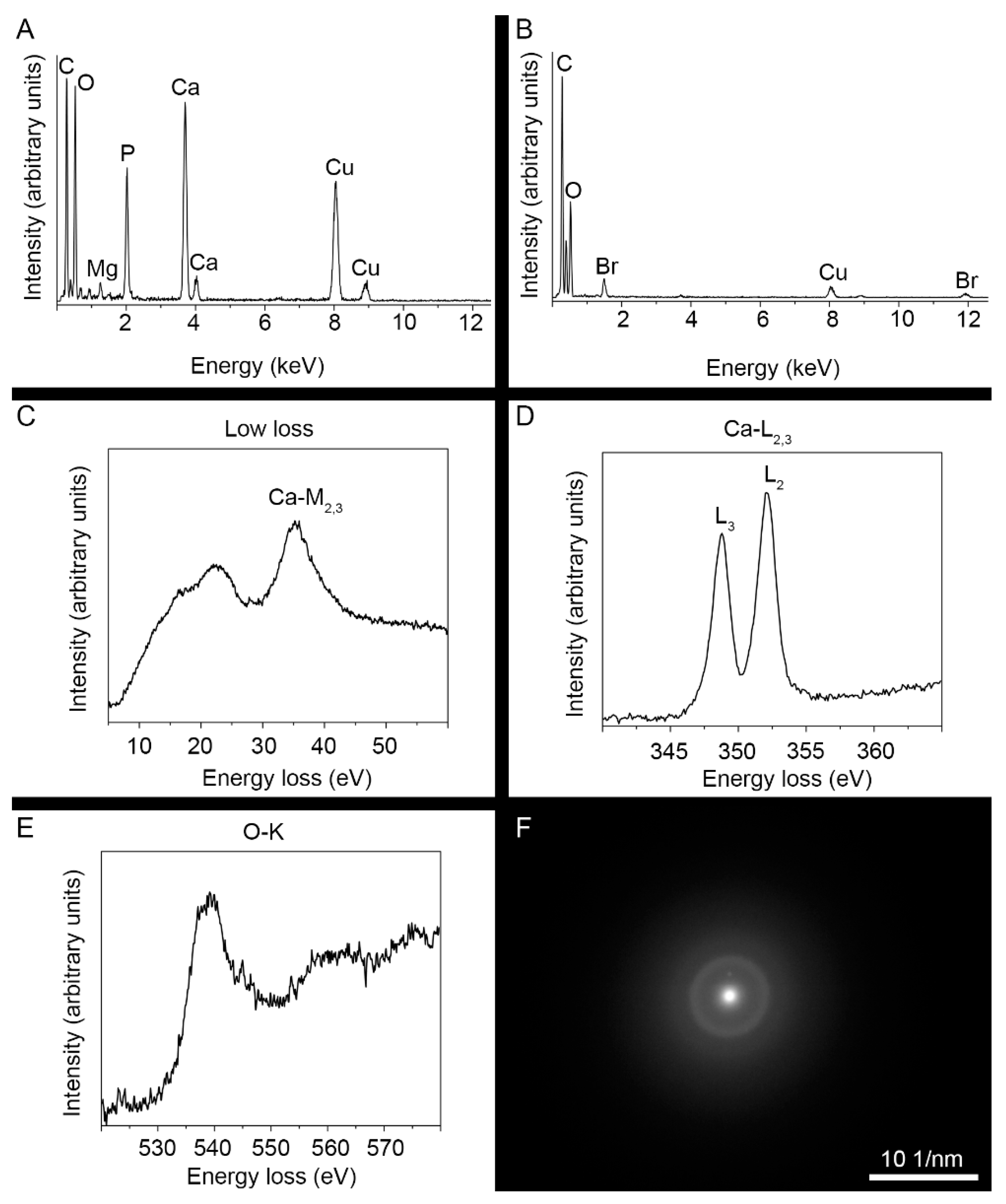
3.3. Ultrastructure and Mineral Composition of the Claw Cuticle
3.4. Mechanical Properties of the Claw Cuticle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, S.; Masic, A.; Bertinetti, L.; Teguh, J.S.; Herrin, J.S.; Zhu, X.; Su, H.; Miserez, A. Textured fluorapatite bonded to calcium sulphate strengthen stomatopod raptorial appendages. Nat. Commun. 2014, 5, 3187. [Google Scholar] [CrossRef] [Green Version]
- Bentov, S.; Zaslansky, P.; Al-Sawalmih, A.; Masic, A.; Fratzl, P.; Sagi, A.; Berman, A.; Aichmayer, B. Enamel-like apatite crown covering amorphous mineral in a crayfish mandible. Nat. Commun. 2012, 3, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, R.M.S.; Bailey, J.; Coon, J.J.; Devaraj, A.; Garrett, R.W.; Goggans, M.S.; Hebner, M.G.; Lee, B.S.; Lee, D.; Lovern, N.; et al. The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Sci. Rep. 2021, 11, 17481. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.B.; Nelson, D.M.; Weiss, C.; Soeldner, A.H. Morphogenesis of opal teeth in calanoid copepods. Mar. Biol. 1990, 106, 91–101. [Google Scholar] [CrossRef]
- Vittori, M.; Srot, V.; Žagar, K.; Bussmann, B.; van Aken, P.A.; Čeh, M.; Štrus, J. Axially aligned organic fibers and amorphous calcium phosphate form the claws of a terrestrial isopod (Crustacea). J. Struct. Biol. 2016, 195, 227–237. [Google Scholar] [CrossRef]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friák, M.; Sachs, C.; Fabritius, H.O.; Raabe, D.; Neugebauer, J. Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef]
- Roer, R.; Abehsera, S.; Sagi, A. Exoskeletons across the Pancrustacea: Comparative morphology, physiology, biochemistry and genetics. Integr. Comp. Biol. 2015, 55, 771–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillaman, R.; Roer, R.; Shafer, T.; Modla, S. The Crustacean Integument: Structure and Function. In The Natural History of the Crustacea. Functional Morphology and Diversity; Watling, L., Thiel, M., Eds.; Oxford University Press: Oxford, UK, 2013; Volume 1, pp. 140–166. [Google Scholar]
- Hild, S.; Marti, O.; Ziegler, A. Spatial distribution of calcite and amorphous calcium carbonate in the cuticle of the terrestrial crustaceans Porcellio scaber and Armadillidium vulgare. J. Struct. Biol. 2008, 163, 100–108. [Google Scholar] [CrossRef]
- Neues, F.; Ziegler, A.; Epple, M. The composition of the mineralized cuticle in marine and terrestrial isopods: A comparative study. CrystEngComm 2007, 9, 1245–1251. [Google Scholar] [CrossRef]
- Currey, J.D.; Nash, A.; Bonfield, W. Calcified cuticle in the stomatopod smashing limb. J. Mater. Sci. 1982, 17, 1939–1944. [Google Scholar] [CrossRef]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The stomatopod dactylus club: A formidable damage-tolerant biological hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Štrus, J.; Tušek-Žnidarič, M.; Repnik, U.; Blejec, A.; Summers, A. Microscopy of crustacean cuticle: Formation of a flexible extracellular matrix in moulting sea slaters Ligia pallasii. J. Mar. Biol. Assoc. UK 2018, 99, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217. [Google Scholar] [CrossRef]
- Hessler, R.R. The structural morphology of walking mechanisms in eumalacostracan crustaceans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 296, 245–298. [Google Scholar]
- Vittori, M. Structure of a hinge joint with textured sliding surfaces in terrestrial isopods (Crustacea: Isopoda: Oniscidea). Zool. Lett. 2021, 7, 7. [Google Scholar] [CrossRef]
- Schmidt, C. Phylogeny of the terrestrial Isopoda (Oniscidea): A review. Arthropod Syst. Phylogeny 2008, 66, 191–226. [Google Scholar]
- Dimitriou, A.C.; Taiti, S.; Sfenthourakis, S. Genetic evidence against monophyly of Oniscidea implies a need to revise scenarios for the origin of terrestrial isopods. Sci. Rep. 2019, 9, 18508. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.S.; Ho, S.Y.; Lo, N. An evolutionary timescale for terrestrial isopods and a lack of molecular support for the monophyly of Oniscidea (Crustacea: Isopoda). Org. Divers. Evol. 2017, 17, 813–820. [Google Scholar] [CrossRef]
- Carefoot, T.H.; Taylor, B.E.; Brett, K. A day in the life of an isopod: Time and energy allocations in the semiterrestrial Ligia pallasii. Isr. J. Ecol. Evol. 1998, 44, 463–471. [Google Scholar]
- Schmalfuss, H. Eco-morphological strategies in terrestrial isopods. Symp. Zool. Soc. Lond. 1984, 53, 49–63. [Google Scholar]
- Alexander, C.G. Observations on receptor mechanisms in Ligia oceanica (Linn.). Comp. Biochem. Physiol. A Physiol. 1971, 40, 339–347. [Google Scholar] [CrossRef]
- Alexander, C.G. Locomotion in the isopod crustacean, Ligia oceanica (Linn.). Comp. Biochem. Physiol. A Physiol. 1972, 42, 1039–1047. [Google Scholar] [CrossRef]
- Carefoot, T.H. Studies on the growth, reproduction, and life cycle of the supralittoral isopod Ligia pallasii. Mar. Biol. 1973, 18, 302–311. [Google Scholar]
- Zidar, P.; Drobne, D.; Štrus, J. Determination of moult stages of Porcellio scaber (Isopoda) for routine use. Crustaceana 1998, 71, 646–654. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Srot, V.; Bussmann, B.; Salzberger, U.; Koch, C.T.; van Aken, P.A. Linking microstructure and nanochemistry in human dental tissues. Microsc. Microanal. 2012, 18, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Srot, V.; Bussmann, B.; Salzberger, U.; Deuschle, J.; Watanabe, M.; Pokorny, B.; Jelenko Turinek, I.; Mark, A.F.; van Aken, P.A. Magnesium-assisted continuous growth of strongly iron-enriched incisors. ACS Nano 2017, 11, 239–248. [Google Scholar] [CrossRef]
- Vittori, M.; Srot, V.; Bussmann, B.; Predel, F.; van Aken, P.A.; Štrus, J. Structural optimization and amorphous calcium phosphate mineralization in sensory setae of a terrestrial crustacean (Isopoda: Oniscidea). Micron 2018, 112, 26–34. [Google Scholar] [CrossRef]
- Huber, J.; Fabritius, H.-O.; Griesshaber, E.; Ziegler, A. Function related adaptations of ultrastructure, mineral phase distribution and mechanical properties in the incisive cuticle of mandibles of Porcellio scaber Latreille, 1804. J. Struct. Biol. 2014, 188, 1–15. [Google Scholar] [CrossRef]
- Gelli, R.; Ridi, F.; Baglioni, P. The importance of being amorphous: Calcium and magnesium phosphates in the human body. Adv. Colloid. Interface. Sci. 2019, 269, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Khalaji-Pirbalouty, V.; Wägele, J.W. Two new species of Ligia Fabricius, 1798 (Crustacea: Isopoda: Ligiidae) from coasts of the Persian and Aden gulfs. Org. Divers. Evol. 2010, 10, 135–145. [Google Scholar] [CrossRef]
- Leela Vallabhan, D. Observations on the moulting phenomenon of an isopod, Sphaeroma walkeri. Proc. Indian Acad. Sci. 1979, 88, 257–264. [Google Scholar] [CrossRef]
- Saber-Samandri, S.; Gross, K.A. Amorphous calcium phosphate offers improved crack resistance: A design feature from nature? Acta Biomater. 2011, 7, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Niedbala, J.C.; Nesson, M.H.; Tao, Y.; Shokes, J.E.; Scott, R.A.; Latimer, M.J. Br-rich tips of calcified crab claws are less hard but more fracture resistant: A comparison of biomineralized and heavy-element biomaterials. J. Struct. Biol. 2009, 166, 272–287. [Google Scholar] [CrossRef]



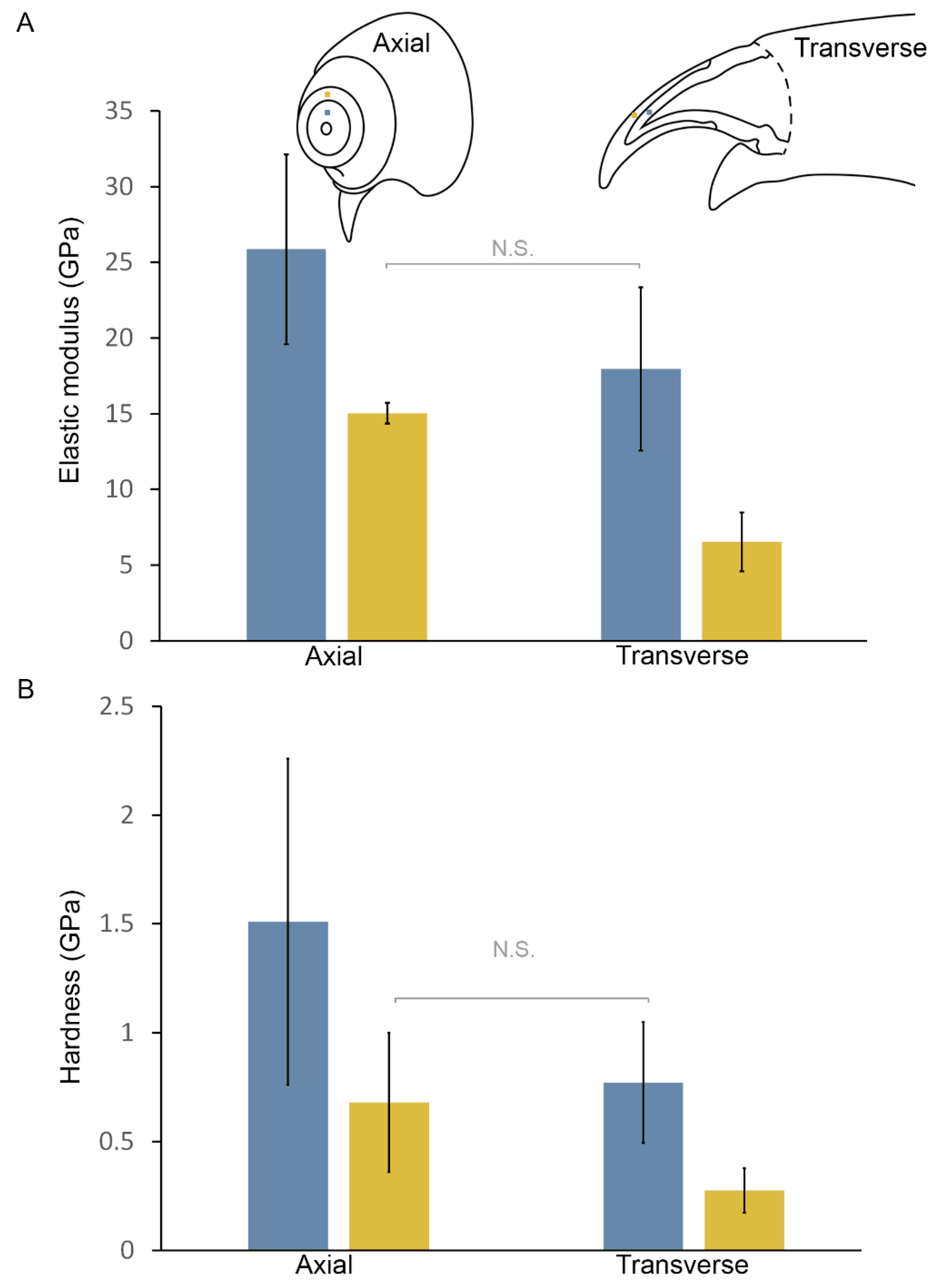
| Mineralized Cuticle, Axial Direction | Mineralized Cuticle, Transverse Direction | Brominated Cuticle, Axial Direction | Brominated Cuticle, Transverse Direction | |
|---|---|---|---|---|
| Elastic modulus (GPa) ± s. d. | 25.9 ± 6.3 | 17.9 ± 5.4 | 15.0 ± 0.7 | 6.5 ± 1.9 |
| Hardness (GPa) ± s. d. | 1.5 ± 0.7 | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.3 ± 0.1 |
| Number of individuals/number of measurements | 1/8 | 2/18 | 1/9 | 2/19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vittori, M.; Srot, V.; Korat, L.; Rejec, M.; Sedmak, P.; Bussmann, B.; Predel, F.; van Aken, P.A.; Štrus, J. The Mechanical Consequences of the Interplay of Mineral Distribution and Organic Matrix Orientation in the Claws of the Sea Slater Ligia pallasii. Minerals 2021, 11, 1373. https://doi.org/10.3390/min11121373
Vittori M, Srot V, Korat L, Rejec M, Sedmak P, Bussmann B, Predel F, van Aken PA, Štrus J. The Mechanical Consequences of the Interplay of Mineral Distribution and Organic Matrix Orientation in the Claws of the Sea Slater Ligia pallasii. Minerals. 2021; 11(12):1373. https://doi.org/10.3390/min11121373
Chicago/Turabian StyleVittori, Miloš, Vesna Srot, Lidija Korat, Matjaž Rejec, Pavel Sedmak, Birgit Bussmann, Felicitas Predel, Peter A. van Aken, and Jasna Štrus. 2021. "The Mechanical Consequences of the Interplay of Mineral Distribution and Organic Matrix Orientation in the Claws of the Sea Slater Ligia pallasii" Minerals 11, no. 12: 1373. https://doi.org/10.3390/min11121373






