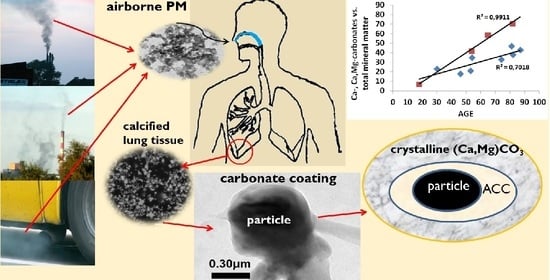
The Impact of Ambient Atmospheric Mineral-Dust Particles on the Calcification of Lungs
Abstract
:1. Introduction
2. Mineral Inventory of Inhaled Dust Particles
3. Site Characterization
4. Materials and Methods
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bendayan, D.; Barziv, Y.; Kramer, M.R. Pulmanory calcification: A review. Respir. Med. 2000, 94, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.D.; Morales, D.V.; Welsh, C.H.; McDermott, M.T.; Shwarz, M.I. Calcium deposition with or without bone formation in the lung. Am. J. Respir. Crit. Care Med. 2002, 165, 1654–1669. [Google Scholar] [CrossRef] [Green Version]
- Farver, C.F. Other Nonneoplastic Focal Lesions, Inclusions, and Depositions. In Pulmonary Pathology, 2nd ed.; Zander, D.S., Farver, C.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 514–533. [Google Scholar]
- Belém, L.C.; Zanetti, G.; Souza, A.S., Jr.; Hochhegger, B.; Giumãres, M.D.; Nobre, L.F.; Rodrigues, R.S.; Marchiori, E. Metastatic pulmonary calcification: State-of-the-art review focused on imaging findings. Respir. Med. 2014, 108, 668–676. [Google Scholar]
- Khan, A.N.; Al-Jahdali, H.H.; Allen, C.M.; Irion, K.L.; Al Ghanem, S.; Koteyar, S.S. The calcified lung nodule: What does it mean? Ann. Thorac Med. 2010, 5, 67–79. [Google Scholar] [CrossRef]
- Ferreira Francisco, F.A.; Pereira e Silva, J.L.; Hochhegger, B.; Zanetti, G.; Marchiori, E. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir Med. 2013, 107, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Gordon, T.; Rom, W.N.; Finkelman, R.B. Interaction of iron and calcium minerals in coals and their role in coal-dust-induced health and environmental problems. Rev. Min. Geochem. 2006, 64, 153–178. [Google Scholar] [CrossRef]
- Pope, C.A., III; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Green, F.H.Y.; Vallyathan, V.; Hahn, F.F. Comparative pathology of environmental lung disease: An overview. Toxicol. Pathol. 2007, 35, 136–147. [Google Scholar] [CrossRef]
- Naccache, J.-M.; Monnet, I.; Nunes, H.; Billon-Galland, M.-A.; Pairon, J.-C.; Guillon, F.; Valeyre, D. Anthracofibrosis attributed to mixed mineral dust exposure: Report of three cases. Thorax 2008, 63, 655–657. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sc. Health C 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Hochgatterer, K.; Moshammer, H.; Haluza, D. Dust is in the air: Effects of occupational exposure to mineral dust on lung function in a 9-year study. Lung 2013, 191, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Camatini, M.; Gaultieri, M.; Sancini, G. Impact of the airborne particulate matter on the human health. In Atmospheric Aerosols: Life Cycles and Effects on Air Quality and Climate; Tomasi, C., Fuzzi, S., Kokhanovsky, A., Eds.; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2017; pp. 597–670. [Google Scholar]
- Riediker, M.; Zink, D.; Kreylink, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Ichihara, G.; Kobayashi, T.; Hisanaga, N.; et al. Particle toxicology and health. Where are we? Part. Fiber Toxicol. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Avila-Casado, C.; Fortoul, T.I.; Vedal, S.; Stevens, B.; Churg, A. Air pollution and retained particles in the lung. Environ. Health Perspect. 2001, 109, 1039–1043. [Google Scholar] [CrossRef]
- Hughes, L.S.; Cass, G.R.; Gone, J.; Ames, M.; Olmez, I. Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles area. Environ. Sci. Technol. 1998, 32, 1153–1161. [Google Scholar] [CrossRef]
- Stettler, L.E.; Platek, S.F.; Riley, R.D.; Mastin, J.P.; Simon, S.D. Lung particulate burden of subjects from the Cincinnati, Ohio urban area. Scan. Microsc. 1991, 5, 85–94. [Google Scholar]
- Gibbs, A.R.; Pooley, F.D. Analysis and interpretation of inorganic mineral particles in “lung” tissues. Thorax 1996, 51, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Sellaro, R.; Sarver, E.; Baxter, D. A standard characterization methodology for respirable coal mine dust using SEM-EDX. Resources 2015, 4, 939–957. [Google Scholar] [CrossRef]
- Churg, A.; Wiggs, B. Types, numbers, sizes, and distribution of mineral particles in the lungs of urban male cigarette smokers. Environ Res. 1987, 42, 121–129. [Google Scholar] [CrossRef]
- Churg, A.; Wright, J.L.; Stevens, B. Exogenous mineral particles in the human bronchial mucosa and lung parenchyma. I. Nonsmokers in the general population. Lung Res. 1990, 16, 159–175. [Google Scholar] [CrossRef]
- Paoletti, L.; Batisti, D.; Caiazza, S.; Petrelli, M.G.; Taggi, F.; De Zorzi, L.; Dinam, A.; Donelli, G. Mineral particles in the lungs of subjects resident in the Rome area and not occupationally exposed to mineral dust. Environ. Res. 1987, 44, 18–28. [Google Scholar]
- Hunt, A.; Abraham, J.L.; Judson, B.; Berry, C.L. Toxicologic and epidemiologic clues from the characterization of the 1952 London smog fine particulate matter in archival autopsy lung tissues. Environ. Health Perspect. 2003, 111, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Domingo-Neumann, R.; Southard, R.J.; Pinkerton, K.E. Mineral dust retained in lung tissue of residents reflects ambient PM10 mineralogy in Fresno, California. In Proceedings of the Geological Society of America Abstracts with Programs 41, 2009, GSA Annual Meeting, Portland, OR, USA, 18–21 October 2009; p. 90. [Google Scholar]
- Lowers, H.A.; Breit, G.N.; Strand, M.; Pillers, R.M.; Meeker, G.P.; Todorov, T.I.; Plumlee, G.S.; Wolf, R.E.; Robinson, M.; Parr, J.; et al. Method to characterize inorganic particulates in lung tissue biopsies using field emission scanning electron microscopy. Toxicol. Mech. Methods 2018, 28, 475–487. [Google Scholar] [CrossRef] [Green Version]
- AAQR: Annual Air Quality Report for the Silesia Voivodship in 2018. Main Inspectorate of Environmental Protection. Regional Department of the Environmental Monitoring in Katowice. April 2019. Available online: http://www.katowice.wios.gov.pl/monitoring/informacje/stan2018/ocena_pow.pdf (accessed on 30 September 2020).
- State of the Environment Report in the Voivodship of Silesia in 2018. Chief Inspectorate of the Environment Protection, Regional Inspectorate of the Environment Protection Katowice 2019. Available online: http://www.katowice.wios.gov.pl/monitoring/raporty/2018/ocena2018.pdf (accessed on 30 May 2020).
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J.; Niewiadomska, E.; Czech, E. The relationship between daily concentration of fine particulate matter in ambient air and exacerbation of respiratory diseases in Silesian Agglomeration, Poland. Int. J. Environ. Res. Public Health 2019, 16, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, M. Short-term effect of changes in fine particulate matter concentrations in ambient air to daily cardio-respiratory mortality in inhabitants of urban-industrial agglomeration (Katowice Agglomeration), Poland. In Air-Quality—New Perspective; Lopez, G., Ed.; Intech Open: London, UK, 2012; pp. 185–198. [Google Scholar] [CrossRef]
- Kapka, L.; Zemła, B.F.; Kozłowska, A.; Olewińska, E.; Pawlas, N. Air quality vs. morbidity to lung cancer in selected provinces and localities of the Silesian region. Przegl. Epidemiol. 2009, 63, 439–444. (In Polish) [Google Scholar]
- Jabłońska, M.; Janeczek, J. Identification of industrial point sources of airborne dust particles in an urban environment by a combined mineralogical and meteorological analyses: A case study from the Upper Silesian conurbation, Poland. Atmos. Pollut. Res. 2019, 10, 980–988. [Google Scholar] [CrossRef]
- Jabłońska, M. Mineral Markers in Lung Tissues of Individuals Exposed to Air Pollution in the Katowice Conurbation; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2013. (In Polish) [Google Scholar]
- Jablonska, M.; Janeczek, J.; Rietmeijer, F.J. Seasonal changes in the mineral compositions of tropospheric dust in the industrial region of Upper Silesia, Poland. Mineral. Mag. 2003, 67, 1231–1241. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey Techniques and Methods; US Geological Survey: Reston, VA, USA, 2013; Book 6, Chapter A43; 497p. Available online: http://pubs.usgs.gov/tm/06/a43 (accessed on 6 July 2020).
- Koczulla, A.-R.; Noeske, S.; Herr, C.; Jörres, R.A.; Römmelt, H.; Vogelmeier, C.; Bals, R. Acute and chronic effects of smoking on inflammation markers in exhaled breath condensate in current smokers. Respiration 2010, 79, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Möller, P.; De Lucia, M. The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines. Geochemistry 2020, 80, 125611. [Google Scholar] [CrossRef]
- Taunton, A.E.; Gunter, M.E.; Druschel, G.K.; Wood, S.A. Geochemistry in the lung: Reaction-path modeling and experimental examination of rock-forming minerals under physiologic conditions. Am. Mineral. 2010, 95, 1624–1635. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.R.C.; Loebeneberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011. [Google Scholar] [CrossRef]
- Utsonomiya, S.; Jensen, K.A.; Keeler, G.J.; Ewing, R.C. Uraninite and fullerene in atmospheric particulates. Environ. Sci. Technol. 2002, 36, 4943–4947. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Zoboli, A.; Vigliaturo, R.; Gieré, R.; Bonasoni, M.P.; Sala, O.; Gualtieri, A.F. Mineral fibres and asbestos bodies in human lung tissue: A case study. Minerals 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Lenders, J.J.M.; Dey, A.; Bomans, P.H.H.; Spielmann, J.; Hendrix, M.M.R.M.; de With, G.; Meldrum, F.C.; Harder, S.; Sommrdijk, N.A.J.M. High-magnesian calcite mesocrystals: A coordination chemistry approach. J. Am. Chem. Soc. 2012, 134, 1367–1373. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Blue, C.R.; Giuffre, A.; Mergelsberg, S.; Han, N.; De Yoreo, J.J.; Dove, P.M. Chemical and physical controls on the transformation of amorphous calcium carbonate into crystalline CaCO3 polymorphs. Geochim. Cosmochim. Acta 2017, 196, 179–196. [Google Scholar] [CrossRef] [Green Version]
- Mayorga, I.C.; Astilleros, J.M.; Fernández-Días, L. Precipitation of CaCO3 polymorphs from aqueous solutions: The role of pH and sulphate groups. Minerals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium carbonate formation and dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef]
- Roggli, V.L.; Mastin, J.P.; Shellbourne, J.D.; Roe, M.; Brody, A.R. Inorganic particles in human lung: Relationship to the inflammatory response. In Inflammatory Cells and Lung Disease; Lynn, W.S., Ed.; Routledge Revivals; CRC Press: Boca Raton, FL, USA, 2019; Chapter 2. [Google Scholar]
- Johnson, B.; Hochholzer, L. Pulmonary blue bodies. Hum. Pathol. 1981, 12, 258–266. [Google Scholar]
- MacNee, W.; Donaldson, K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur. Respir. J. 2003, 21, 47–51. [Google Scholar] [CrossRef]
- Scherbart, A.M.; Langer, J.; Bushmelev, A.; van Berlo, D.; Haberzettl, P.; van Schooten, F.J.; Schmidt, A.M.; Rose, C.R.; Schins, R.P.F.; Albrecht, C. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Part. Fibre Toxicol. 2011, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.B. Regional differences in the lungs. Postgrad. Med. J. 1968, 44, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Klocke, R.A. Catalysis of CO2 reactions by lung carbonic anhydrase. J. Appl. Physiol. 1978, 44, 882–888. [Google Scholar] [CrossRef]
- Arthurs, G.J.; Sudhakar, M. Carbon dioxide transport. Education in Anaesthesia. Crit. Care Pain 2005, 5, 207–210. [Google Scholar]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Gossner, J.; Nau, R. Geriatric Chest Imaging: When and how to image the elderly lung. Age-Related Changes, and Common Pathologies. Radiol. Res. Pract. 2013, 584793. [Google Scholar] [CrossRef] [Green Version]
- Bardelli, F.; Veronesi, G.; Capella, S.; Bellis, D.; Charles, L.; Cedola, A.; Belluso, E. New insights on the biomineralisation process developing in human lungs around inhaled asbestos fibres. Sci. Rep. 2017, 7, 44862. [Google Scholar] [CrossRef] [Green Version]






| Mineral Phase | n | Content (vol. %) | Size (µm) | ||
|---|---|---|---|---|---|
| Mean | Range | Mean * | Range | ||
| Carbonates | 612 | 32 | 5.5–65 | 1.42(06) | 0.22–5.05 |
| Al-silicates1 | 243 | 19 | 12–34 | 2.10(12) | 0.26–5.01 |
| Silica2 | 149 | 10 | 3–17 | 1.90(12) | 0.35–4.65 |
| Fe-oxides3 | 199 | 12 | 5–30 | 1.67(11) | 0.31–4.98 |
| Halides4 | 112 | 7 | 2–9 | 1.69(15) | 0.54–5.04 |
| Iron | 35 | 2 | 1–4 | 1.74(36) | 0.42–4.93 |
| Sulfides5 | 53 | 3 | 1–5 | 1.36(22) | 0.34–4.08 |
| Metals6 | 49 | 3 | 0.4–7 | 1.72(29) | 0.22–5.02 |
| Spinels 7 | 38 | 3 | 1–4 | 1.83(31) | 0.17–5.08 |
| Alloys 8 | 39 | 2 | 1–4 | 1.58(30) | 0.25–4.33 |
| Oxides 9 | 53 | 2 | 0–7 | 1.13(17) | 0.17–4.46 |
| Gypsum | 26 | 2 | 2–5 | 2.11(32) | 0.68–4.76 |
| Barite | 32 | 2 | 1–5 | 1.25(22) | 0.42–3.60 |
| Ca-phosphate | 9 | 1 | 1–3 | 2.70(70) | 0.68–4.76 |
| REE-phosphates | 3 | 0.5 | 0.3–0.9 | 1.47(26) | 0.97–2.08 |
| Females | Males | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 18s | 54ns | 65ns | 82ns | 30s | 46s | 53ns | 54s | 74s | 81sm | 82ns | 87sm |
| CP/TP | 6/83 | 49/117 | 66/112 | 77/108 | 49/211 | 29/158 | 24/113 | 102/289 | 28/85 | 95/201 | 26/68 | 46/107 |
| vol. % | 5.48 | 36.05 | 41.57 | 64.62 | 21.06 | 18.65 | 21.09 | 28.50 | 29.20 | 38.29 | 40.09 | 43.59 |
| Diameter Range | 0.68–2.48 | 0.37–4.46 | 0.23–3.51 | 0.38–5.05 | 0.30–4.45 | 0.22–4.98 | 0.44–3.14 | 0.23–4.43 | 0.39–4.25 | 0.22–3.58 | 0.52–3.97 | 0.56–3.90 |
| Phase | Gamble’s Solution | Blood Plasma | ||||
|---|---|---|---|---|---|---|
| SI | log IAP | Log Ksp | SI | log IAP | log Ksp | |
| Calcite | 1.12 | −7.44 | −8.56 | 0.70 | −7.86 | −8.56 |
| Dolomite | 2.10 | −15.26 | −17.36 | 1.43 | −15.93 | −17.36 |
| HAP | 7.77 | 3.32 | −4.45 | 6.52 | 2.08 | −4.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, M.; Janeczek, J.; Smieja-Król, B. The Impact of Ambient Atmospheric Mineral-Dust Particles on the Calcification of Lungs. Minerals 2021, 11, 125. https://doi.org/10.3390/min11020125
Jabłońska M, Janeczek J, Smieja-Król B. The Impact of Ambient Atmospheric Mineral-Dust Particles on the Calcification of Lungs. Minerals. 2021; 11(2):125. https://doi.org/10.3390/min11020125
Chicago/Turabian StyleJabłońska, Mariola, Janusz Janeczek, and Beata Smieja-Król. 2021. "The Impact of Ambient Atmospheric Mineral-Dust Particles on the Calcification of Lungs" Minerals 11, no. 2: 125. https://doi.org/10.3390/min11020125