Effect of Gypsum Addition on the Mechanical and Microstructural Performance of Sulphide-Rich Cemented Paste Backfill
Abstract
:1. Introduction
2. Materials and Test Methods
2.1. Test Raw Materials
2.1.1. Tailings
2.1.2. Binders and the Mixing Water
2.2. Test Methods
2.2.1. Mix Designs and Sample Preparations
2.2.2. Fluidity Tests
2.2.3. Unconfined Compressive Strength (UCS)
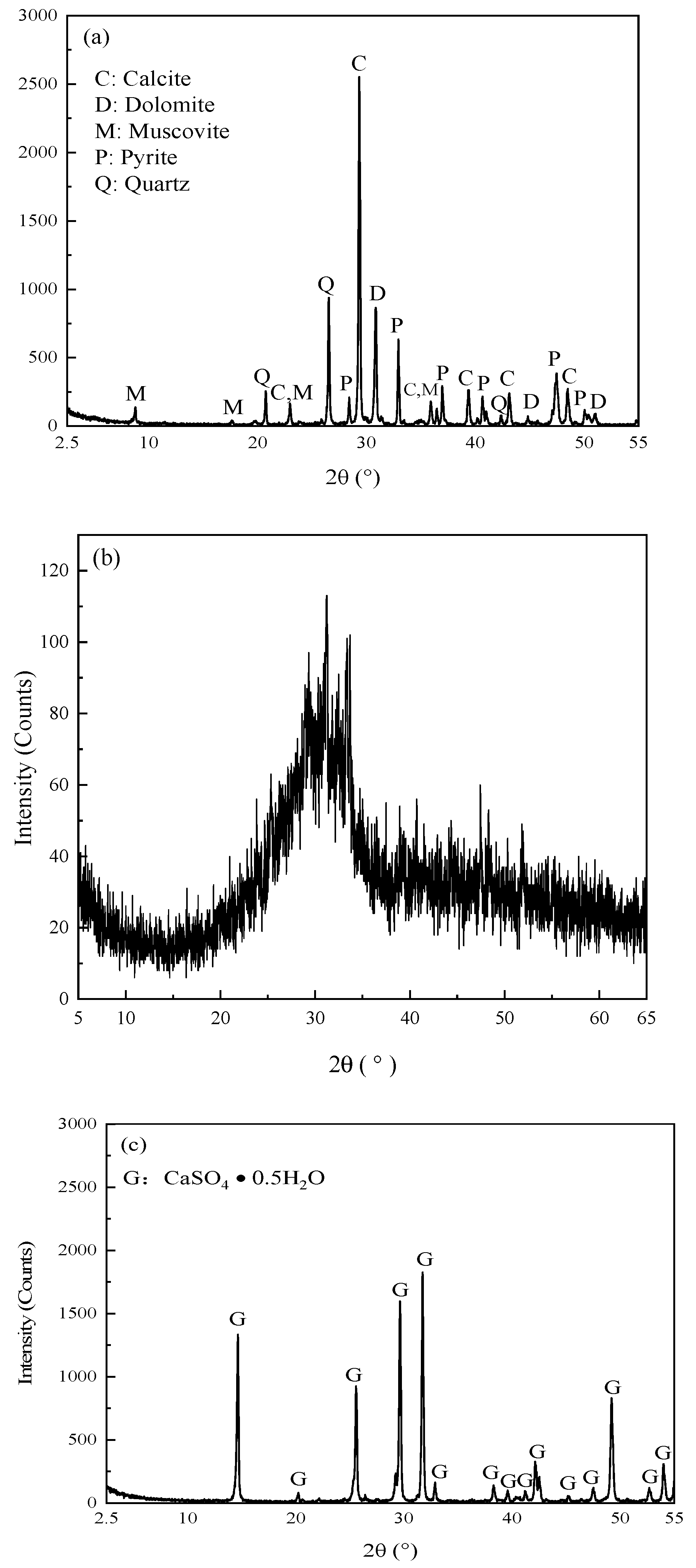
2.2.4. XRD, SEM and MIP Analyses
3. Results and Discussion
3.1. Effects of HG Dosage on the Fluidity of NAS-HG-CPB Pastes
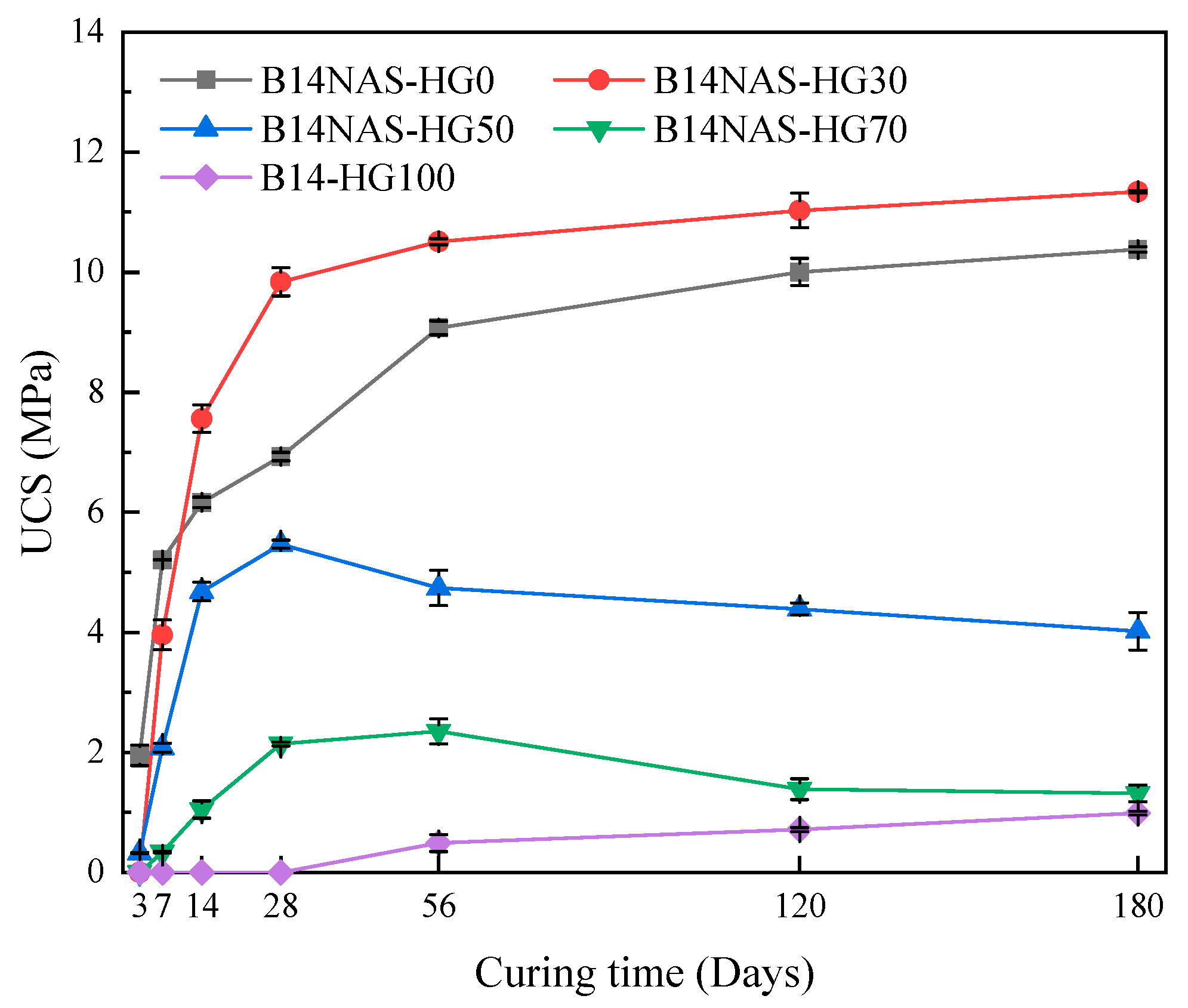
3.2. Effects of HG Dosages on UCS of NAS-HG-CPB
3.3. Mineralogical Composition and Microstructure Analyses
3.3.1. XRD Analyses
3.3.2. SEM Analyses
3.3.3. MIP Analyses
3.4. Hydration Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Chen, B.H.; Zhou, Q. Distribution of heavy metals in water-surface sediments in AMD, Dabao Mountain in North Guangdong Province and influential factors. J. Environ. Sci. 2018, 38, 134–141. (In Chinese) [Google Scholar] [CrossRef]
- Plante, B.; Bussière, B.; Benzaazoua, M. Lab to field scale effects on contaminated neutral drainage prediction from the Tio mine waste rocks. J. Geochem. Explor. 2014, 137, 37–47. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Potvin, R.; Benzaazoua, M.; Zagury, G.J. Dissolution of calcitic marble and dolomitic rock in high iron concentrated acid mine drainage: Application to anoxic limestone drains. Environ. Earth Sci. 2012, 66, 2387–2401. [Google Scholar] [CrossRef]
- Yilmaz, E. Advances in reducing large volumes of environmentally harmful mine waste rocks and tailings. Miner. Resour. Manag. 2011, 27, 89–112. [Google Scholar]
- Qi, C.C.; Fourie, A. Cemented paste backfill for mineral tailings management: Review and future perspectives. Miner. Eng. 2019, 144, 1–21. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H. Practical importance of tailings for cemented paste backfill. In Paste Tailings Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–32. [Google Scholar]
- Mangane, M.B.C.; Argane, R.; Trauchessec, R.; Lecomte, A.; Benzaazoua, M. Influence of superplasticisers on mechanical properties and workability of cemented paste backfill. Miner. Eng. 2018, 116, 3–14. [Google Scholar] [CrossRef]
- Yilmaz, E.; Fall, M. Paste Tailings Management; Springer International Publishing: Cham, Switzerland, 2017; 303p. [Google Scholar]
- Ouellet, S.; Bussière, B.; Aubertin, M.; Benzaazoua, M. Microstructural evolution of cemented paste backfill: Mercury intrusion porosimetry test results. Cem. Concr. Res. 2007, 37, 1654–1665. [Google Scholar] [CrossRef]
- Tariq, A.; Nehdi, M. Developing durable paste backfill from sulphidic tailings. Waste Resour. Manag. 2007, 160, 155–166. [Google Scholar] [CrossRef]
- Yin, S.; Shao, Y.; Wu, A.; Wang, Y.; Chen, X. Expansion and strength properties of cemented backfill using sulphidic mill tailings. Construct. Build. Mater. 2018, 165, 138–148. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Mechanical, hydrological and microstructural assessment of the durability of cemented paste backfill containing alkali-activated slag. Int. J. Min. Reclamat. Environ. 2018, 32, 123–143. [Google Scholar] [CrossRef]
- Atis, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Effect of type, form, and dosage of activators on strength of alkali-activated natural pozzolans. Cem. Concr. Res. 2011, 33, 251–260. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes, 1st ed.; Taylor and Francis: Abingdon, UK, 2006. [Google Scholar]
- Walia, M.K.; Dick, W.A. Selected soil physical properties and aggregate-associated carbon and nitrogen as influenced by gypsum, crop residue, and glucose. Geoderma 2018, 320, 67–73. [Google Scholar] [CrossRef]
- Hua, S.; Wang, K.; Yao, X.; Xu, W.; He, Y. Effects of fibers on mechanical properties and freeze-thaw resistance of phosphogypsum-slag based cementitious materials. Constr. Build. Mater. 2016, 121, 290–299. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, S.; Pan, Y.; Li, Q.; Zhou, J.; Xu, Y.; Qian, G. Characterisation and leaching toxicities of mercury in flue gas desulfurisation gypsum from coal-fired power plants in China. Fuel 2016, 177, 157–163. [Google Scholar] [CrossRef]
- Al Attar, L.; Al-Oudat, M.; Kanakri, S.; Budeir, Y.; Hussam Khalily, H.; Al Hamwi, A. Radiological impacts of phosphogypsum. J. Environ. Manag. 2011, 92, 2151–2158. [Google Scholar] [CrossRef]
- Hammas, I.; Horchani-Naifer, K.; Férid, M. Solubility study and valorization of phosphogypsum salt solution. Int. J. Miner. Process. 2013, 123, 87–93. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Donatella Barca, D. Rare earths concentration from phosphogypsum waste by two-step leaching method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, H.; Shu, Z.; Wang, Y.X.; Yan, C.J. Utilization of waste phosphogypsum to prepare non-fired bricks by a novel Hydration–Recrystallization process. Constr. Build. Mater. 2012, 34, 114–119. [Google Scholar] [CrossRef]
- Ding, W.J.; Chen, Q.J.; Hongjuan Sun, H.J.; Peng, T.J. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, L.; Yu, B.Y. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Peyronnard, O.; Benzaazoua, M. Estimation of the cementitious properties of various industrial by-products for applications requiring low mechanical strength. Resour. Conserv. Recycl. 2011, 56, 22–33. [Google Scholar] [CrossRef]
- Jiang, G.Z.; Wu, A.X.; Wang, Y.M.; Lan, W.T. Low cost and high efficiency utilisation of hemihydrate phosphogypsum: Used as binder to prepare filling material. Constr. Build. Mater. 2018, 167, 263–270. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Rubert, S.; Luz, C.A.; Varela, M.V.F.; Filho, J.I.P.; Hooton, R.D. Hydration mechanisms of supersulfated cement: The role of alkali activator and calcium sulfate content. J. Therm. Anal. Calorim. 2018, 134, 971–980. [Google Scholar] [CrossRef]
- Landriault, D. Backfill in underground mining. In Underground Mining Methods: Engineering Fundamentals and International Case Studies; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2001; pp. 601–614. [Google Scholar]
- Fall, M.; Benzaazoua, M.; Ouellet, S. Effect of tailings properties on paste backfill performance. In Proceedings of the International Symposium on Mining with Backfill, Beijing, China, 19–21 September 2004; pp. 193–202. Available online: https://www.researchgate.net/publication/265273604_Effect_of_tailings_properties_on_paste_backfill_performance (accessed on 28 February 2021).
- Zheng, J.R.; Lv, S.S.; Zhao, Z.B. Method for determination of fluidity of paste slurry of whole tailings. Min. Technol. 2013, 13, 23–25. (In Chinese) [Google Scholar] [CrossRef]
- Kaziliunas, A.; Leskeviciene, V.; Vektaris, B.; Valancius, Z. The study of neutralisation of the dihydrate phosphogypsum impurities. Ceram-Silik. 2006, 50, 178–184. [Google Scholar]
- Wang, S.; Scrivener, K.; Pratt, P. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Komnitsas, K.; Yurramendi, L.; Bartzas, G.; Karmali, V.; Petrakis, E. Factors affecting co-valorization of fayalitic and ferronickel slags for the production of alkali activated materials. Sci. Total Environ. 2020, 721, 137753. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.M.; Wang, Y.R.; Sun, R.; Rui, Y.F. Effects of the n(H2O: Na2Oeq) ratio on the geopolymerization process and microstructures of fly ash-based geopolymers. J. Non-Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Cristelo, N.; Miranda, T.; Palomoa, A. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated binders based on ground granulated blast furnace slag and phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Bartzas, G.; Karmali, V. Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Sci. Total Environ. 2019, 665, 347–357. [Google Scholar] [CrossRef] [PubMed]
- EN12457-3. Characterization of Waste—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 3: Two Stage Batch Test at a Liquid to Solid Ratio of 2 l/kg and 8 l/kg for Materials with High Solid Content and With Particle Size Below 4 mm; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- Van der Sloot, H.A.; Hjelmar, O.; Bjerre Hansen, J.; Woitke, P.; Lepom, P.; Leschber, R.; Bartet, B.; Debrucker, N. Validation of CEN/TC 292 Leaching Tests and Eluate Analysis Methods prEN 12457 1-4, ENV 13370 and ENV 12506. 2001. Available online: https://repository.tno.nl//islandora/object/uuid:d49a977b-011d-46fa-96cd-045623e8a8ad (accessed on 28 February 2021).





| Physical Properties | SSA(a) (m2/kg) | >100 μm (wt. %) | >45 μm (wt. %) | D50 (μm) | <20 μm (wt. %) |
|---|---|---|---|---|---|
| Tailings | 668.7 | 19.0 | 35.8 | 21.3 | 48.8 |
| GGBS | 681.5 | 0 | 3.3 | 9.8 | 76.4 |
| HG | 339.2 | 3.6 | 36.3 | 34.2 | 31.3 |
| Chemical Composition (wt. %) | SiO2 | Al2O3 | Fet (a) | CaO | MgO | Pb | Cu | Zn | S |
|---|---|---|---|---|---|---|---|---|---|
| Tailings | 11.23 | 2.65 | 19.80 | 20.06 | 0.40 | 0.90 | 0.46 | 0.45 | 21.09 |
| GGBS | 31.68 | 12.77 | 2.89 | 40.8 | 4.76 | - | - | - | 0.85 |
| HG | 0.98 | 0.50 | - | 34.68 | 0.26 | - | - | - | 17.1 |
| Pastes | Tailings (Dry Mass) (wt. %) | GGBS (wt. %) | NaOH (wt. %) | HG (a) (wt. %) | Solid Content (wt. %) | NaOH (b) (mol/L) | Fluidity (mm) |
|---|---|---|---|---|---|---|---|
| NAS-HG0 | - | 97 | 3 | - | 71.4 | 2.62 | 180 |
| NAS-HG30 | - | 67.9 | 2.1 | 30 | 72.7 | 1.92 | 180 |
| NAS-HG50 | - | 48.5 | 1.5 | 50 | 72.9 | 1.38 | 180 |
| NAS-HG70 | - | 29.1 | 0.9 | 70 | 71.8 | 0.8 | 180 |
| NAS-HG100 | - | - | - | 100 | 69.3 | - | 180 |
| B14NAS-HG0 | 86 | 13.58 | 0.42 | - | 73.7 | 0.4 | 180 |
| B14NAS-HG30 | 86 | 9.51 | 0.29 | 4.2 | 73.9 | 0.28 | 180 |
| B14NAS-HG50 | 86 | 6.79 | 0.21 | 7.0 | 73.9 | 0.2 | 180 |
| B14NAS-HG70 | 86 | 4.07 | 0.13 | 9.8 | 73.8 | 0.12 | 180 |
| B14-HG100 | 86 | - | - | 14 | 73.4 | - | 180 |
| Pastes | Tailings (Dry Mass) (wt. %) | GGBS (wt. %) | NaOH (wt. %) | HG (a) (wt. %) | Solid Content (wt. %) | Fluidity (mm) | Fluidity (1 h) (mm) |
|---|---|---|---|---|---|---|---|
| B14NAS-HG0 | 86 | 13.58 | 0.42 | - | 73.7 | 180 | 180 |
| B14NAS-HG30 | 86 | 9.51 | 0.29 | 4.2 | 73.9 | 180 | 153 |
| B14NAS-HG50 | 86 | 6.79 | 0.21 | 7.0 | 73.9 | 180 | 139 |
| B14NAS-HG70 | 86 | 4.07 | 0.13 | 9.8 | 73.8 | 180 | 125 |
| B14-HG100 | 86 | - | - | 14 | 73.4 | 180 | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zheng, J.; Guo, L.; Zhao, Y. Effect of Gypsum Addition on the Mechanical and Microstructural Performance of Sulphide-Rich Cemented Paste Backfill. Minerals 2021, 11, 283. https://doi.org/10.3390/min11030283
Tang Y, Zheng J, Guo L, Zhao Y. Effect of Gypsum Addition on the Mechanical and Microstructural Performance of Sulphide-Rich Cemented Paste Backfill. Minerals. 2021; 11(3):283. https://doi.org/10.3390/min11030283
Chicago/Turabian StyleTang, Yu, Juanrong Zheng, Lijie Guo, and Yue Zhao. 2021. "Effect of Gypsum Addition on the Mechanical and Microstructural Performance of Sulphide-Rich Cemented Paste Backfill" Minerals 11, no. 3: 283. https://doi.org/10.3390/min11030283








