Copper Slag of Pyroxene Composition as a Partial Replacement of Natural Aggregate for Concrete Production
Abstract
:1. Introduction
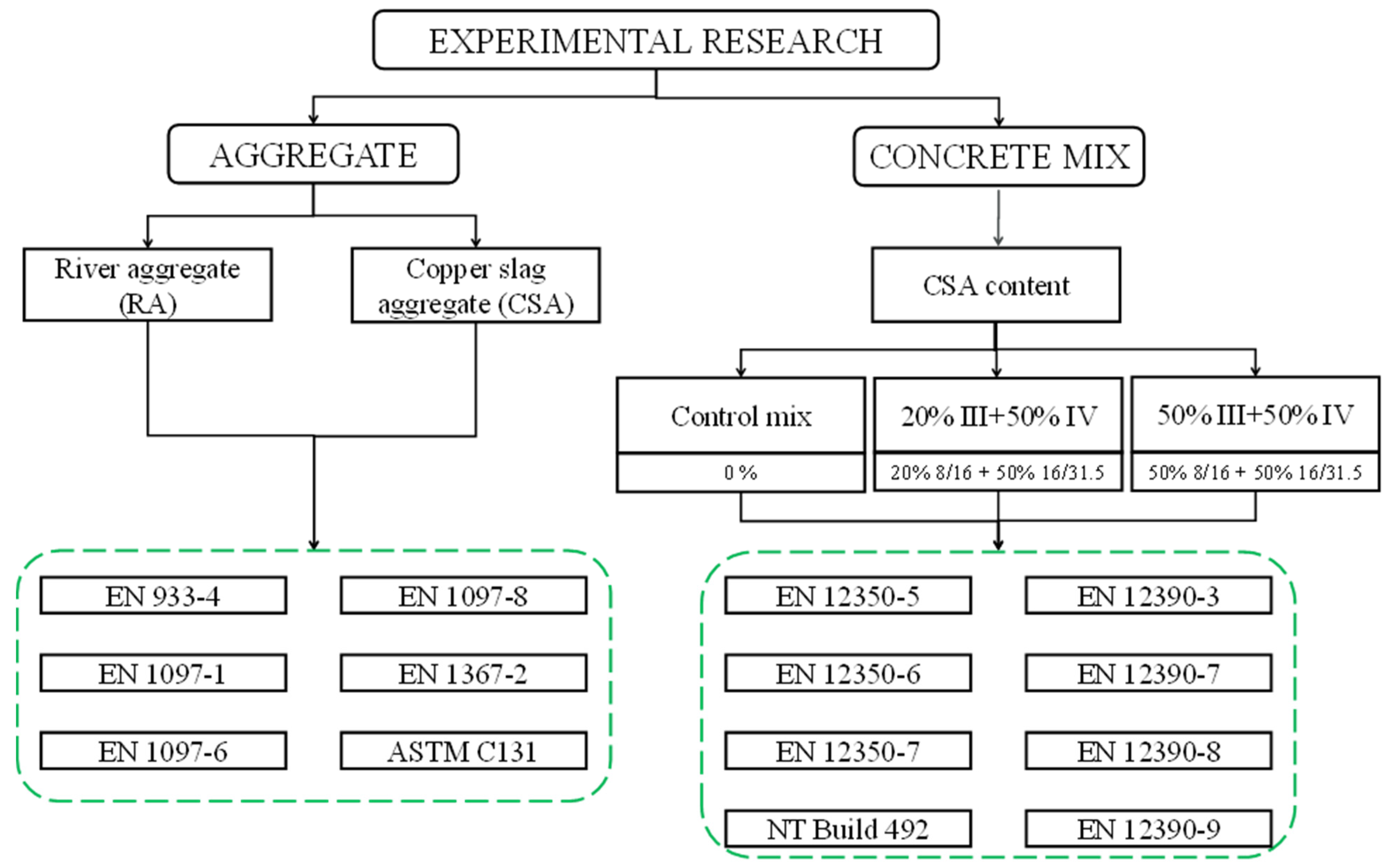
2. Materials and Methods
3. Results
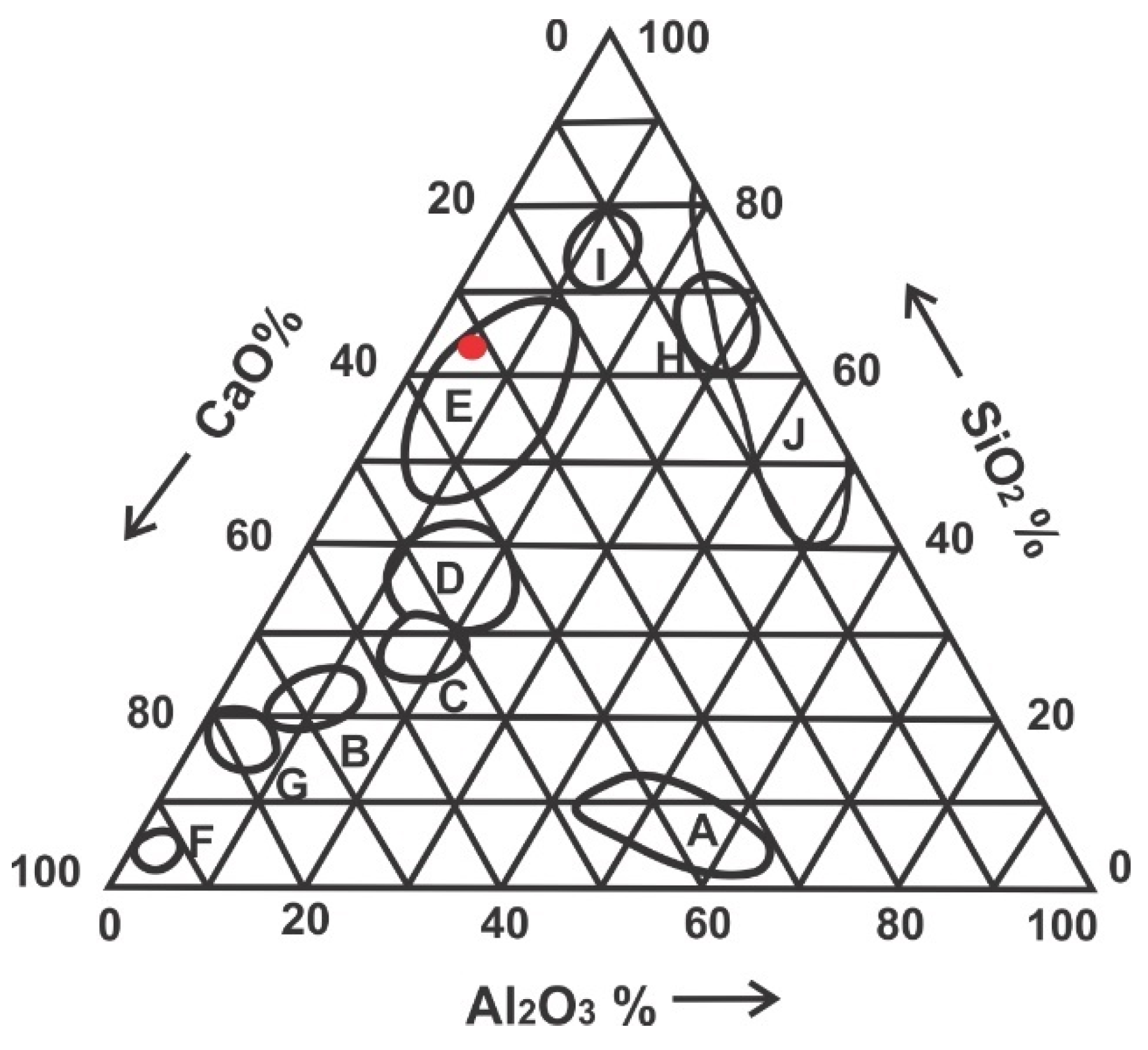
3.1. Chemical Analysis of Copper Slag Aggregate
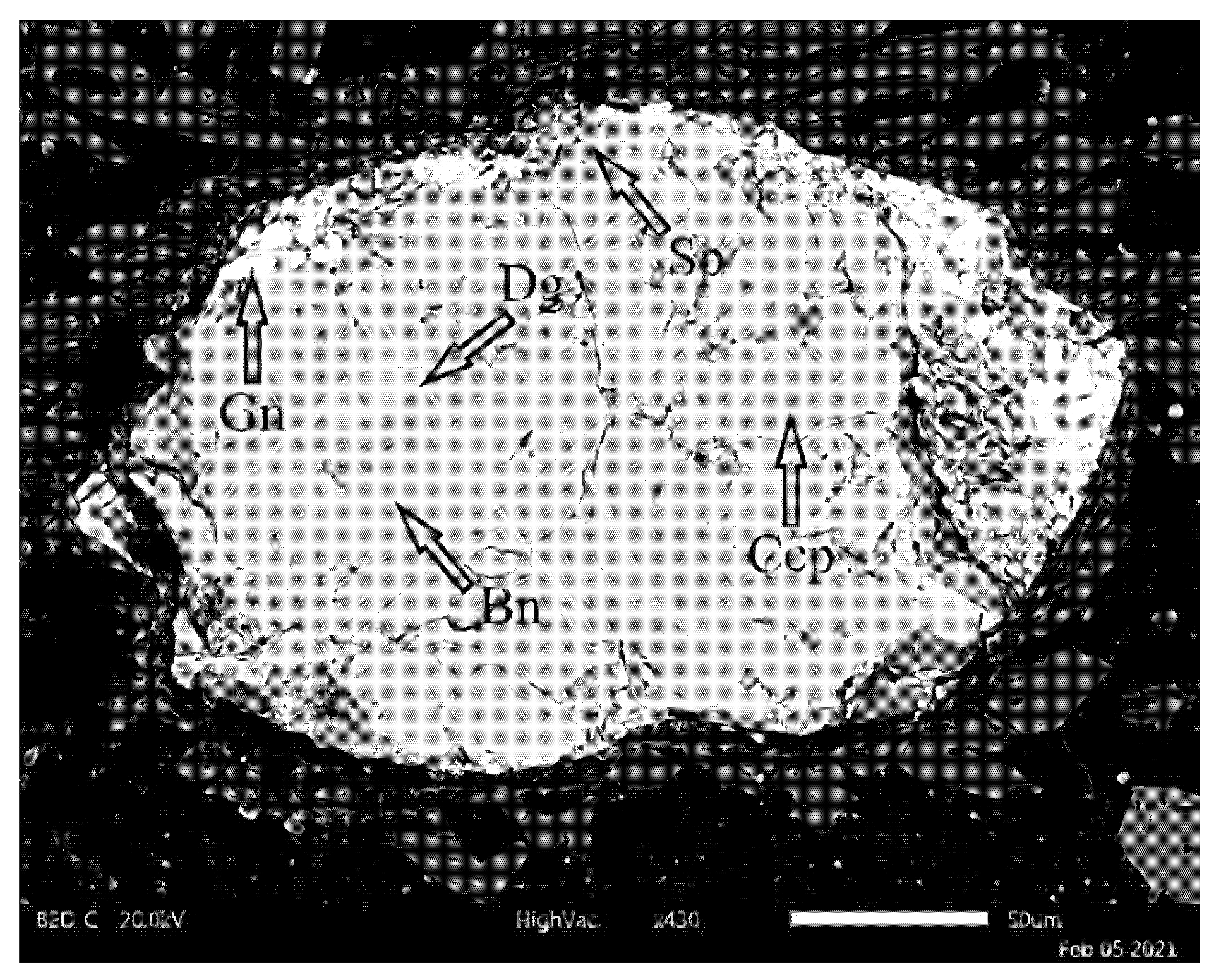
3.2. Mineralogical Analysis of Copper Slag Aggregate
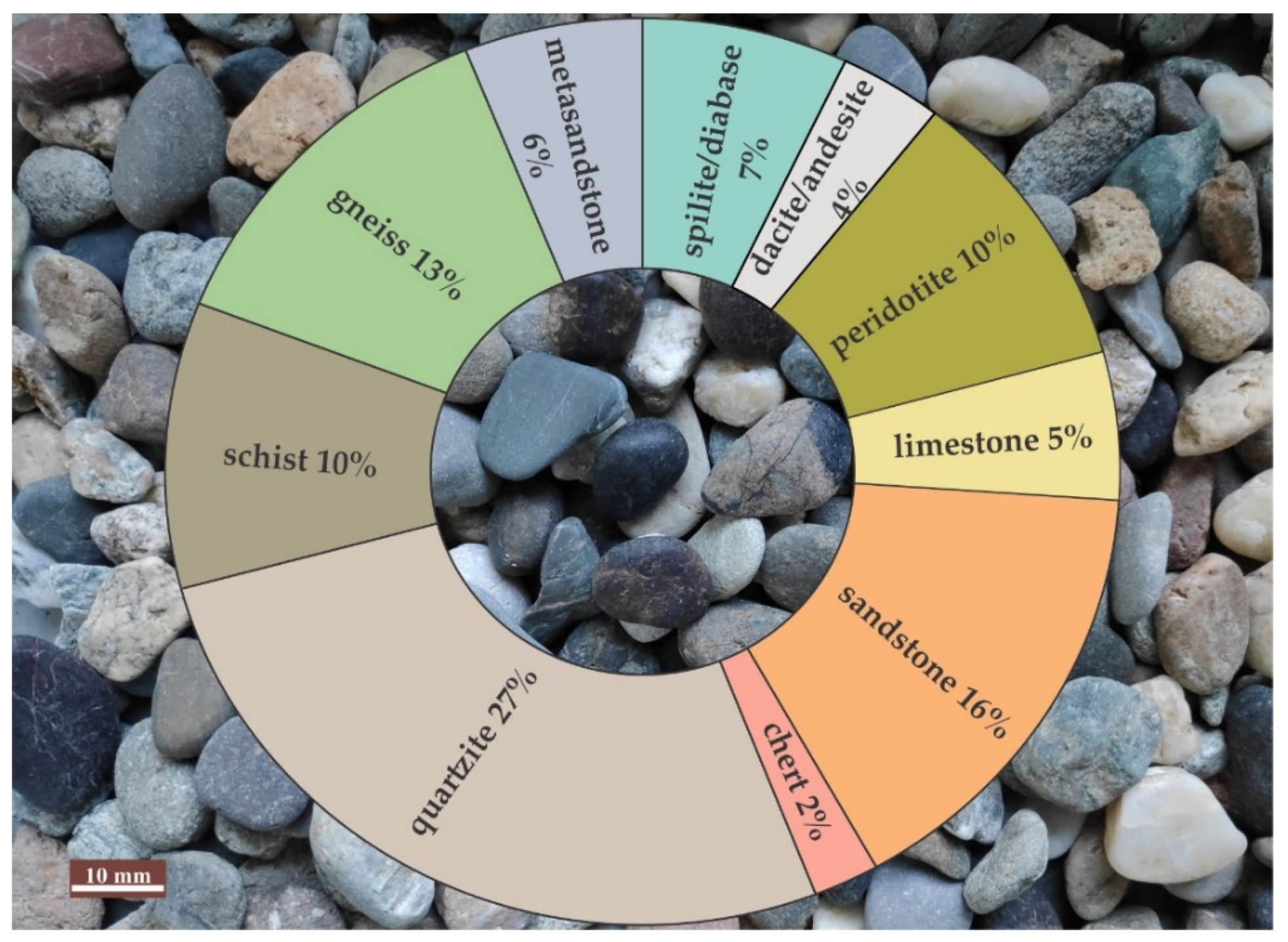
3.3. Petrographic Analysis of River Aggregate
3.4. Geometric, Mechanical and Physical Characteristics of Aggregates
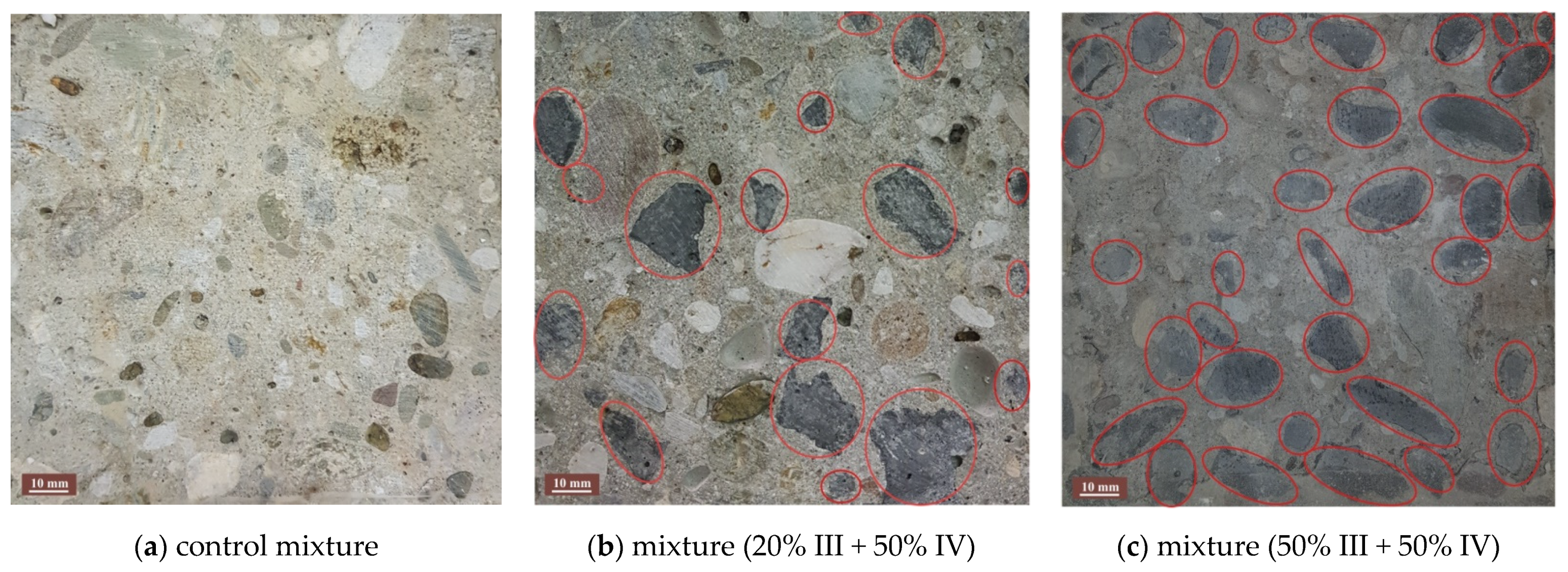
3.5. Concrete Composition and Characteristics of Fresh Concrete
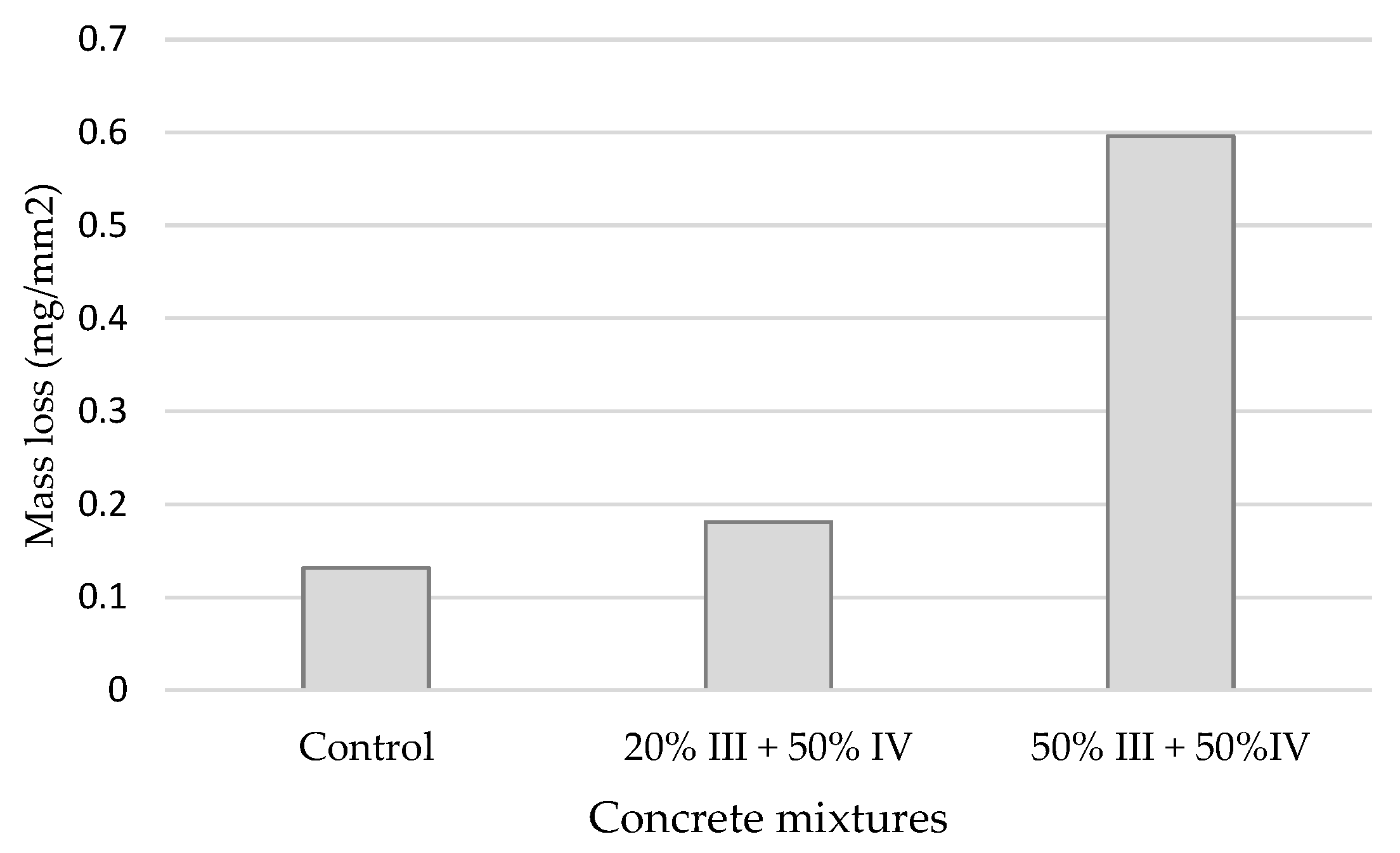
3.6. Physical and Mechanical Characteristics of Hardened Concrete
4. Discussion
5. Conclusions
- Copper slag from RTB Bor is a mineral material with predominant silicon, calcium, iron, and aluminium oxides, while copper content did not exceed 1%;
- The slag consisted of the following minerals: silicate mineral of the clinopyroxene group and devitrified glassy matrix. Two accessory phases were magnetite and sulphide droplets;
- River aggregate consisted mostly of fragments of metamorphic rocks and the same quantity of sedimentary and igneous rocks;
- Compared to RA, CSA had better resistance to fragmentation and wear, higher particle density, and lower water absorption. These characteristics are in accordance with the European technical requirements for aggregates used in concrete;
- The increase of CSA content increased concrete density, both in the fresh and hardened states;
- The replacement of RA fractions 8/16 mm and 16/31.5 mm with CSA aggregate in the amount of 20 + 50% and 50 + 50% by volume led to increases in compressive strength by 12.4% and 10.5%, respectively;
- Lower water absorption of the CSA compared to the RA, with an unchanged amount of cement and water, caused an increase in the porosity of the cement matrix;
- Increased cement-matrix porosity resulted in reduced water penetration resistance and salt-frost resistance in concrete mixtures with CSA;
- The resistance to chloride ion penetration of the CSA mixtures was not significantly changed compared to the control mixture.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tam, C.; Taylor, M.; Gielen, D.; Twigg, C.; Klee, H.; Rocha, P.; Meer, R. Cement Technology Roadmap 2009—Carbon Emissions Reductions up to 2050; World Business Council for Sustainable Development: Geneva, Switzerland, 2009. [Google Scholar]
- Zhao, Q.; Liu, X.; Jiang, J. Effect of curing temperature on creep behavior of fly ash concrete. Constr. Build. Mater. 2015, 96, 326–333. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Hisada, M.; Al-Saidy, A.H.; Al-Oraimi, S.K. Performance of high strength concrete made with copper slag as a fine aggregate. Constr. Build. Mater. 2009, 23, 2132–2140. [Google Scholar] [CrossRef]
- Sankh, A.C.; Biradar, P.M.; Naghathan, S.J.; Ishwargol, M.B. Recent Trends in Replacement of Natural Sand with Different Alternatives Akshay. IOSR J. Mech. Civ. Eng. (IOSR-JMCE) 2002, 12, 180–183. [Google Scholar]
- De Leeuw, J.; Shankman, D.; Wu, G.; de Boer, W.F.; Burnham, J.; He, Q.; Yesou, H.; Xiao, J. Strategic assessment of the magnitude and impacts of sand mining in Poyang Lake, China. Reg. Environ. Chang. 2010, 10, 95–102. [Google Scholar] [CrossRef]
- Kuntikana, G.; Singh, D.N. Contemporary Issues Related to Utilization of Industrial Byproducts. Adv. Civ. Eng. Mater. 2017, 6, 20160050. [Google Scholar] [CrossRef]
- Maharishi, A.; Singh, S.P.; Gupta, L.K. Shehnazdeep Strength and durability studies on slag cement concrete made with copper slag as fine aggregates. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Prasad, P.S.; Ramana, G.V. Feasibility study of copper slag as a structural fill in reinforced soil structures. Geotext. Geomembr. 2016, 44, 623–640. [Google Scholar] [CrossRef]
- Alter, H. The composition and environmental hazard of copper slags in the context of the Basel Convention. Resour. Conserv. Recycl. 2005, 43, 353–360. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, R.A. Sustainable use of copper slag in self compacting concrete containing supplementary cementitious materials. J. Clean. Prod. 2017, 151, 179–192. [Google Scholar] [CrossRef]
- Baghalha, M.; Papangelakis, V.G.; Curlook, W. Factors affecting the leachability of Ni/Co/Cu slags at high temperature. Hydrometallurgy 2007, 85, 42–52. [Google Scholar] [CrossRef]
- Zivkovic, Z.; Mitevska, N.; Mihajlovic, I.; Nikolic, D. The influence of the silicate slag composition on copper losses during smelting of the sulfide concentrates. J. Min. Metall. Sect. B Metall. 2009, 45, 23–34. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Reuse of copper slag as a supplementary cementitious material: Reactivity and safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Dhir, R.K.; de Brito, J.; Mangabhai, R.; Lye, C.Q. Use of Copper Slag in Geotechnical Applications. In Sustainable Construction Materials: Copper Slag; Elsevier: Amsterdam, The Netherlands, 2017; pp. 211–245. [Google Scholar]
- Dimitrijevic, M.; Urosevic, D.; Milic, S.; Sokic, M.; Markovic, R. Dissolution of copper from smelting slag by leaching in chloride media. J. Min. Metall. Sect. B Metall. 2017, 53, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Khan, R.A. Durability assessment of self compacting concrete incorporating copper slag as fine aggregates. Constr. Build. Mater. 2017, 155, 617–629. [Google Scholar] [CrossRef]
- Dey, A.; Dev, D.; Saha, P. Use of copper slag as sustainable aggregate. Icsci 2014 2015, 1, 229–240. [Google Scholar]
- Khanzadi, M.; Behnood, A. Mechanical properties of high-strength concrete incorporating copper slag as coarse aggregate. Constr. Build. Mater. 2009, 23, 2183–2188. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Al-Saidy, A.H.; Taha, R. Effect of copper slag as a fine aggregate on the properties of cement mortars and concrete. Constr. Build. Mater. 2011, 25, 933–938. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Neves, R.; Branco, F.; de Brito, J. Field assessment of the relationship between natural and accelerated concrete carbonation resistance. Cem. Concr. Compos. 2013, 41, 9–15. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K. Premchand Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Shi, C.; Qian, J. High performance cementing materials from industrial slags—A review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Mavroulidou, M. Mechanical Properties and Durability of Concrete with Water Cooled Copper Slag Aggregate. Waste Biomass Valorization 2017, 8, 1841–1854. [Google Scholar] [CrossRef] [Green Version]
- Lori, A.R.; Hassani, A.; Sedghi, R. Investigating the mechanical and hydraulic characteristics of pervious concrete containing copper slag as coarse aggregate. Constr. Build. Mater. 2019, 197, 130–142. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdul Razak, H. Effect of palm oil clinker incorporation on properties of pervious concrete. Constr. Build. Mater. 2016, 115, 70–77. [Google Scholar] [CrossRef]
- Zarauskas, L.; Skripkiūnas, G.; Girskas, G. Influence of Aggregate Granulometry on Air Content in Concrete Mixture and Freezing—Thawing Resistance of Concrete. Procedia Eng. 2017, 172, 1278–1285. [Google Scholar] [CrossRef]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429079313. [Google Scholar]
- Yeih, W.; Fu, T.C.; Chang, J.J.; Huang, R. Properties of pervious concrete made with air-cooling electric arc furnace slag as aggregates. Constr. Build. Mater. 2015, 93, 737–745. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Yan, C.; Liu, Y. Influence of crushing index on properties of recycled aggregates pervious concrete. Constr. Build. Mater. 2017, 135, 112–118. [Google Scholar] [CrossRef]
- Cadjenovic, B.; Marjanovic, V.; Ljubojev, V.; Milanovic, D. Possibilities of use the copper matte from smelter slag in its direct bakrenca of smelting slag in its direct discharge from furnace. Min. Eng. 2012, 143–148. [Google Scholar] [CrossRef]
- Đorđević, J.; Orešković, M.; Mladenović, G. The possibility of the application of copper slag in asphalt mixtures. J. Road Traffic Eng. 2018, 64. [Google Scholar] [CrossRef]
- Maluckov, B. Waste from mining-metallurgical production of copper and treatment of it. Tehnika 2017, 72, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Stanojlović, R.; Štirbanović, Z.; Sokolović, J. New technological procedure for sustainable processing of mining technological waste. Min. Eng. 2012, 1, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Jevtic, D.; Zakic, D.; Savic, A.; Radevic, A. Statistical analysis of concrete quality testing results. Build. Mater. Struct. 2014, 57, 45–52. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Measurement of the steady and non-steady-state chloride diffusion coefficients in a migration test by means of monitoring the conductivity in the anolyte chamber. Comparison with natural diffusion tests. Cem. Concr. Res. 2001, 31, 1411–1420. [Google Scholar] [CrossRef]
- Killick, D.; Miller, D.; Thondhlana, T.P.; Martinón-Torres, M. Copper mining and smelting technology in the northern Lowveld, South Africa, ca. 1000 CE to ca. 1880 CE. J. Archaeol. Sci. 2016, 75, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Marinković, L. Contribution to Investigations on the Possibility of Copper Blast Furnace Slag from Bor Usage in Pavements Construction; The University of Belgrade, Faculty of Civil Engineering: Belgrade, Serbia, 1989. [Google Scholar]
- Gržetić, I.; Brčeski, I. Degradation of Bor slags as the contamination process. In Proceedings of the Naša ekološka istina ’95, Hotel “Jezero”, Borsko jezero, Bor, Serbia, 31 May–2 June 1995; pp. 95–100. [Google Scholar]
- Urošević, D. Copper Extraction from Smelting Slag by Combined Methods; University of Belgrade, Technical faculty in Bor: Belgrade, Serbia, 2016. [Google Scholar]
- Ray, H.S.; Sridhar, R.; Abraham, K.P. Extraction of Nonferrous Metals; EWP: New Delhi, India, 2014; ISBN 8185095639. [Google Scholar]
- Brzaković, P. Prilog problemu sistematizacije veziva na bazi cementnog klinkera i dodataka nemetaličnog porekla. Građevinski Mater. Konstr. 2002, 45, 23–33. [Google Scholar]
- Dhir Obe, R.K.; de Brito, J.; Mangabhai, R.; Lye, C.Q. Sustainable Construction Materials: Copper Slag; Woodhead Publishing: London, UK, 2016; ISBN 9780081009864. [Google Scholar]
- Ljubojev, V.; Marjanovic, V.; Milanovic, D.; Stankovic, S. Mineralogical characteristics of slag (from the Flotation plant of RTB Bor) granulated in the laboratory conditions. Min. Metall. Eng. Bor. 2015, 1–12. [Google Scholar] [CrossRef]
- Moosberg, H.; Lagerblad, B.; Forssberg, E. The use of by-products from metallurgical and mineral industries as filler in cement-based materials. Waste Manag. Res. 2003, 21, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Crisman, B.; Ossich, G.; Bevilacqua, P.; Roberti, R. Degradation prediction model for friction of road pavements with natural aggregates and steel slags. Appl. Sci. 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Yuan, Q.; Santhanam, M. Erratum to: Test Methods for Chloride Transport in Concrete. In Performance of Cement-Based Materials in Aggressive Aqueous Environments; Springer: Dordrecht, The Netherlands, 2013; p. E1. [Google Scholar]
- Ugur, I.; Demirdag, S.; Yavuz, H. Effect of rock properties on the Los Angeles abrasion and impact test characteristics of the aggregates. Mater. Charact. 2010, 61, 90–96. [Google Scholar] [CrossRef]




| Component | Content (%) | Analytical Method |
|---|---|---|
| SiO2 | 42.06 | G |
| Al2O3 | 3.53 | ICP-AES |
| FeO | 21.93 | V |
| Fe2O3 | 6.84 | C |
| Fe3O4 | 1.22 | A-Fe3O4 |
| CaO | 20.06 | AAS |
| MgO | 0.50 | AAS |
| SO3 | 1.17 | ACS |
| K2O | 0.43 | AES |
| K | 0.36 | C |
| Na2O | 0.15 | AES |
| Na | 0.11 | C |
| TiO2 | 0.28 | ICP-AES |
| Mn2O3 | 0.04 | AAS |
| Cl- | 0.01 | PHOT/SP |
| Cu-ox | 0.95 | AAS |
| P2O5 | 0.09 | ICP-AES |
| Cu | 0.30 | AAS/EG |
| Phase | Hedenbergite | Glassy Matrix | Magnetite | Sulphide Droplets |
|---|---|---|---|---|
| Element | wt% | |||
| O | 38.57 | 36.40 | 28.80 | |
| Na | 0.28 | |||
| Mg | 0.58 | 0.02 | 0.06 | |
| Al | 1.56 | 4.18 | 1.27 | |
| Si | 21.18 | 17.93 | ||
| P | 0.13 | |||
| S | 32.89 | |||
| K | 0.90 | |||
| Ca | 15.17 | 4.65 | 0.44 | |
| Ti | 0.13 | 0.19 | 1.16 | |
| V | 0.20 | |||
| Cr | 0.02 | |||
| Mn | 0.03 | 0.02 | ||
| Fe | 22.73 | 33.31 | 67.98 | 19.50 |
| Cu | 47.61 | |||
| Zn | 0.05 | 0.09 | 0.07 | |
| As | 0.07 | |||
| Ba | 1.83 | |||
| Σ | 100.00 | 100.00 | 100.00 | 100.00 |
| vol% | 82.35 | 13.94 | 2.34 | 1.37 |
| n * | 10 | 8 | 4 | 7 |
| Test Methods | Standard | Fraction (mm) | Determined Values | Categorised EN 12620 | ||
|---|---|---|---|---|---|---|
| CSA | RA | CSA | RA | |||
| Shape index | EN 933-4 | 8/11 | 5 | SI 15 | ||
| 10/14 | 16 | SI 20 | ||||
| Resistance to fragmentation | SRPS B.B8.045 (ASTM C 131) | grading B | 10 | 27 | - | - |
| Resistance to wear | EN 1097-1 | 8/11 | 4 | MDE10 | ||
| 10/14 | 10 | MDE10 | ||||
| Polished stone value | EN 1097-8 | standardized | 43 | -* | PSV44 | - |
| Particle density | EN 1097-6 | 8/11 | ρa 3.40 | ρa 3.40 | ||
| ρrd 3.33 | ρrd 3.33 | |||||
| ρssd 3.36 | ρssd 3.36 | |||||
| 10/14 | ρa 2.66 | ρa 2.66 | ||||
| ρrd 2.58 | ρrd 2.58 | |||||
| ρssd 2.61 | ρssd 2.61 | |||||
| Water absorption | EN 1097-6 | 8/11 | 0.6 | WA24 1 | ||
| 10/14 | 1.2 | WA24 2 | ||||
| Magnesium sulphate test | EN 1367-2 | 10/14 | 7 | 12 | MS18 | MS18 |
| Mixture | Passing (%) through Sieve Opening (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 11.2 | 16 | 22.4 | 31.5 | |
| Control | 1.0 | 4.0 | 18.4 | 28.3 | 33.8 | 39.9 | 54.8 | 61.2 | 78.0 | 90.0 | 99.6 |
| 20% III + 50% IV | 1.0 | 4.0 | 18.4 | 28.3 | 33.8 | 39.9 | 55.4 | 62.6 | 77.0 | 87.4 | 99.8 |
| 50% III + 50% IV | 1.0 | 4.0 | 18.4 | 28.3 | 33.8 | 39.9 | 55.6 | 64.1 | 76.9 | 87.4 | 99.8 |
| Mixture | Water | River Aggregate (RA) | CSA Aggregate | Cement | W/CM * | ||||
|---|---|---|---|---|---|---|---|---|---|
| mv | (0/4) | (4/8) | (8/16) | (16/31.5) | (8/16) | (16/31.5) | mc | ω | |
| (kg/m3) | (kg/m3) | (kg/m3) | (kg/m3) | (kg/m3) | (kg/m3) | (kg/m3) | (kg/m3) | (–) | |
| Control | 212 | 732 | 274 | 343 | 428 | 398 | 0.533 | ||
| 20% III + 50% IV | 265 | 143 | 100 | 294 | |||||
| 50% III + 50% IV | 171 | 214 | 235 | 294 | |||||
| Mixture | Flow Table Test (cm) | Density (kg/m3) | Air Content (%) | Categorized EN 12350-5 |
|---|---|---|---|---|
| Control | 33.5 | 2331 | 1.9 | F1 |
| 20% III + 50% IV | 32.0 | 2431 | 1.7 | F1 |
| 50% III + 50% IV | 28.5 | 2443 | 1.7 | F1 |
| Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|
| Mixture | Average Water Penetration Depth (mm) | After 1 Day | After 7 Days | After 28 Days | Density (kg/m3) |
| Control | 83 | 16.7 | 28.0 | 36.3 | 2303 |
| 20% III + 50% IV | 129 | 20.1 | 34.1 | 40.8 | 2421 |
| 50% III + 50% IV | 106 | 18.6 | 30.6 | 40.1 | 2462 |
| Standard deviation | 1.7 | 3.1 | 2.4 | ||
| Silicate Degree | MO/SiO2 | Slag | |
|---|---|---|---|
| Classification | <1 | >2 | basic |
| >1 | <2 | acid | |
| 2 | 2 | neutral | |
| 2020 (pyroxene copper slag) | 2.1 | 1 | acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipović, S.; Đokić, O.; Radević, A.; Zakić, D. Copper Slag of Pyroxene Composition as a Partial Replacement of Natural Aggregate for Concrete Production. Minerals 2021, 11, 439. https://doi.org/10.3390/min11050439
Filipović S, Đokić O, Radević A, Zakić D. Copper Slag of Pyroxene Composition as a Partial Replacement of Natural Aggregate for Concrete Production. Minerals. 2021; 11(5):439. https://doi.org/10.3390/min11050439
Chicago/Turabian StyleFilipović, Sandra, Olivera Đokić, Aleksandar Radević, and Dimitrije Zakić. 2021. "Copper Slag of Pyroxene Composition as a Partial Replacement of Natural Aggregate for Concrete Production" Minerals 11, no. 5: 439. https://doi.org/10.3390/min11050439









