Petrography, Mineralogy, and Geochemistry of Thermally Altered Coal in the Tashan Coal Mine, Datong Coalfield, China
Abstract
:1. Introduction
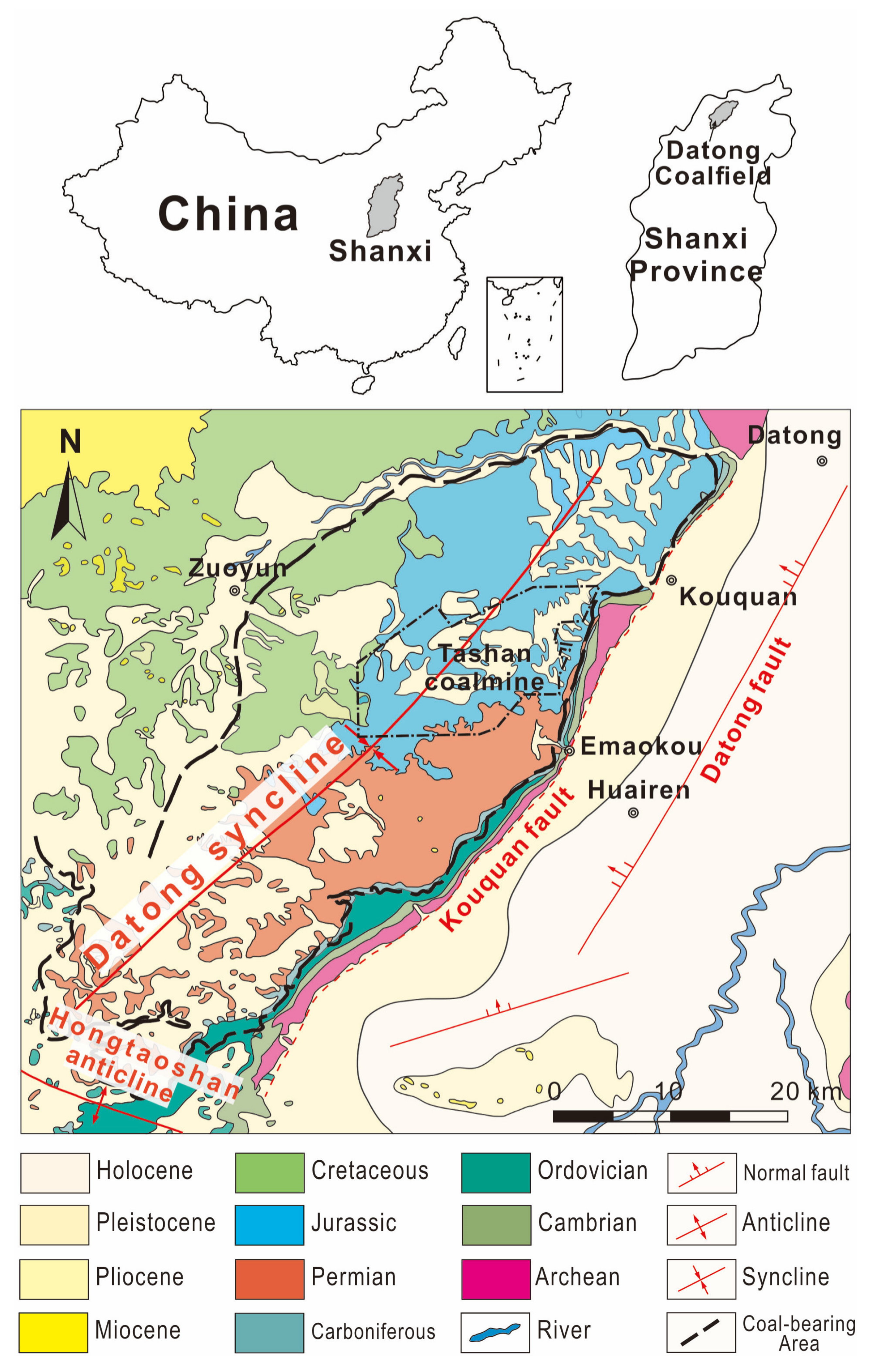
2. Geological Setting
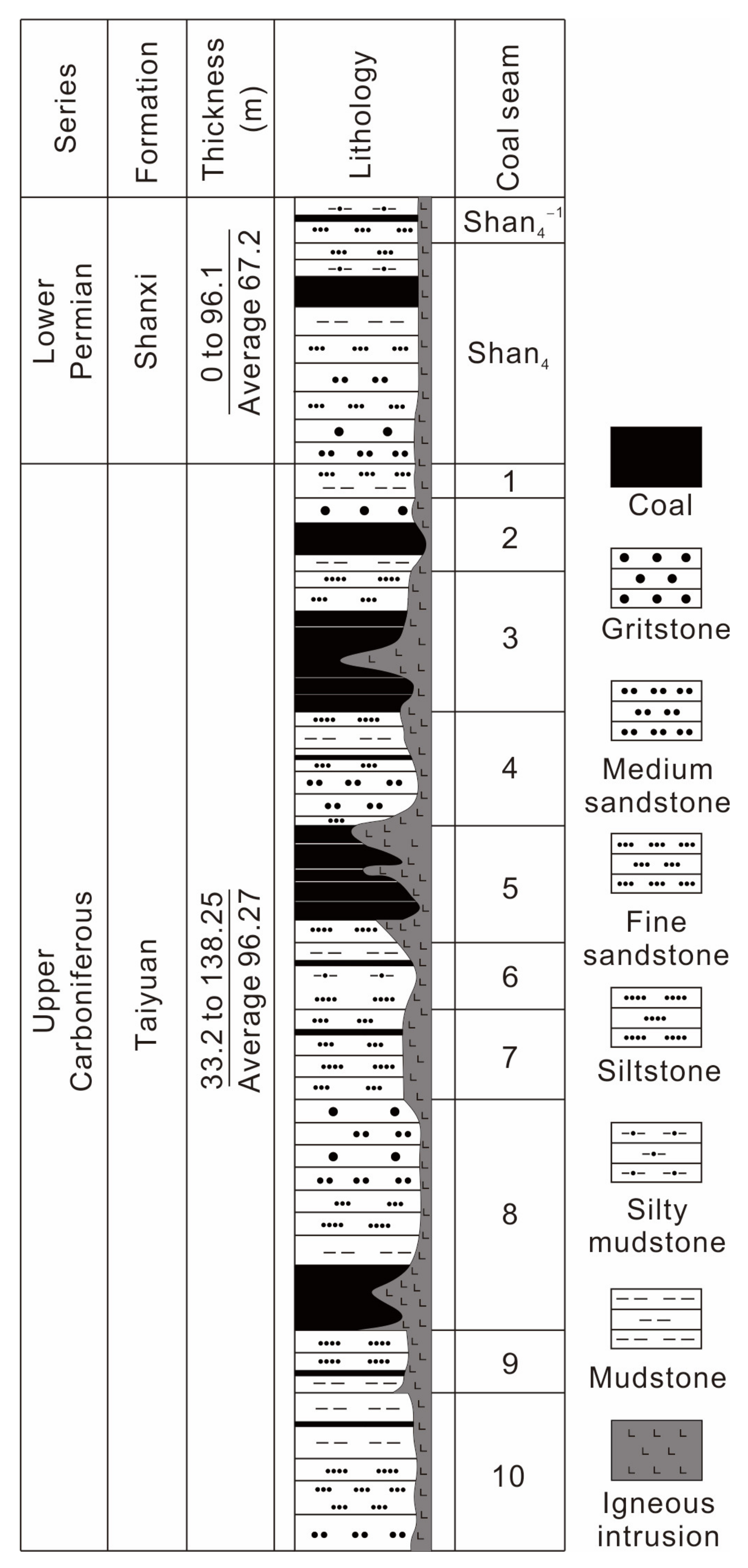
2.1. Coal-Bearing Strata
2.2. Igneous Intrusion
3. Materials and Methods
3.1. Sampling
3.2. Methods
4. Results and Discussion
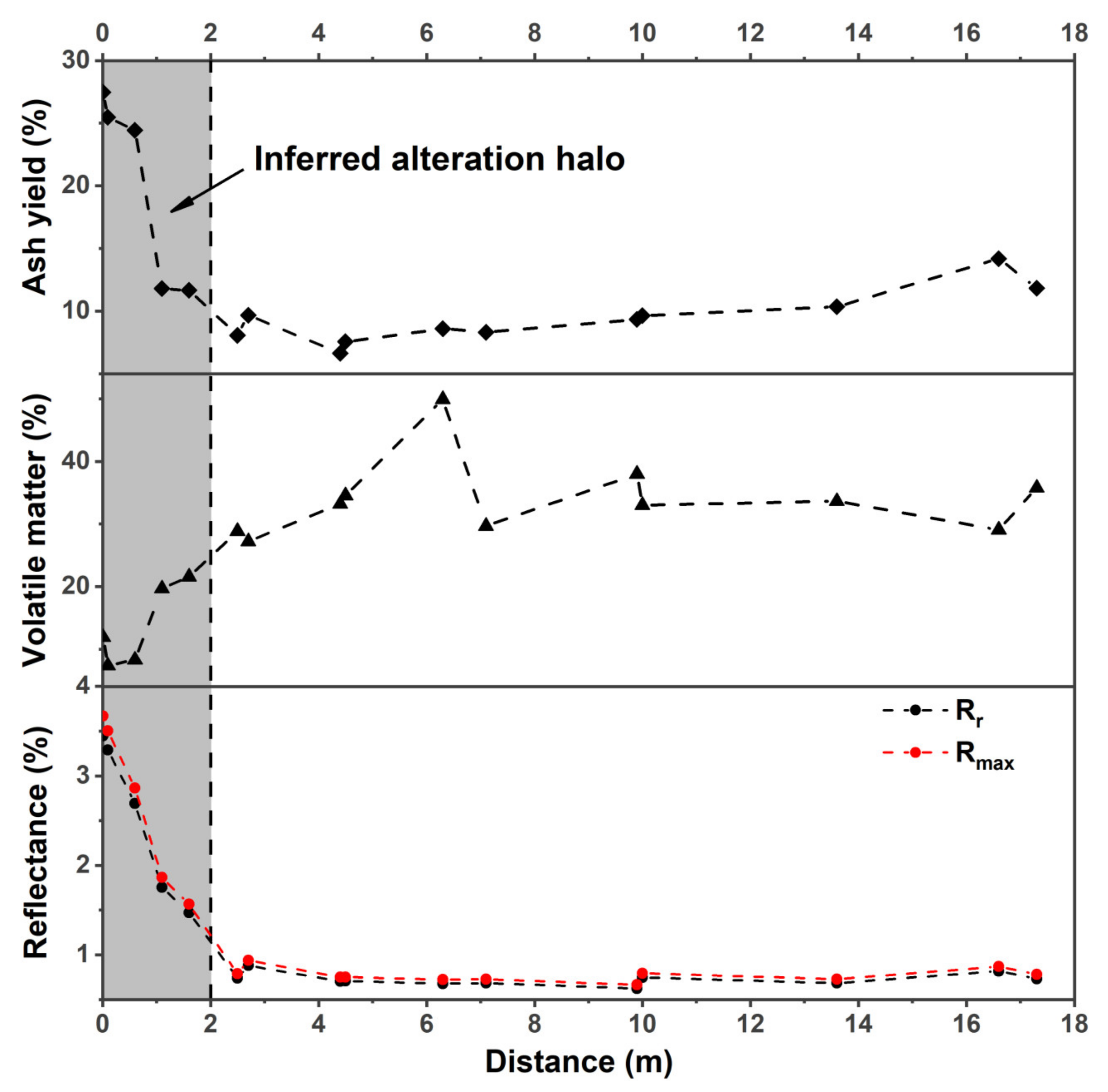
4.1. Identification and Characterization of the Alteration Halo
4.1.1. Vitrinite Reflectance
4.1.2. Proximate Analysis
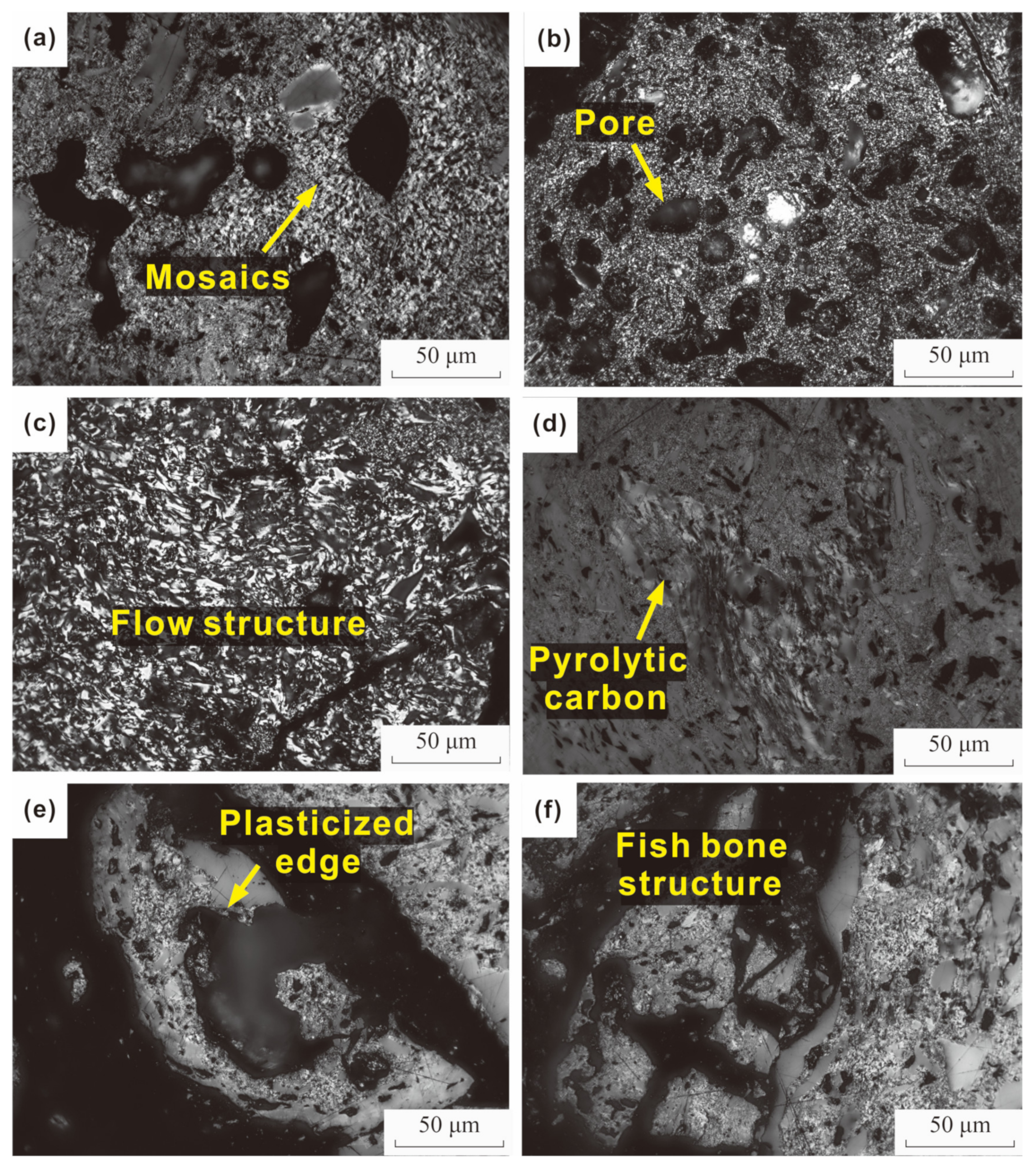
4.1.3. Petrographic Characteristics
4.1.4. Characterization of the Alteration Halo
4.2. Mineralogical Characteristics
4.2.1. Mineral Composition
4.2.2. Occurrence and Origin of Mineral Matter
- 1.
- Kaolinite, Muscovite, and Tobelite
- 2.
- Quartz
- 3.
- Carbonate minerals
4.3. Geochemical Characteristics
4.3.1. Major Elements in the Tashan Coal
4.3.2. Trace Elements in the Tashan Coal
4.3.3. Modes of Occurrence of Elements
- 1.
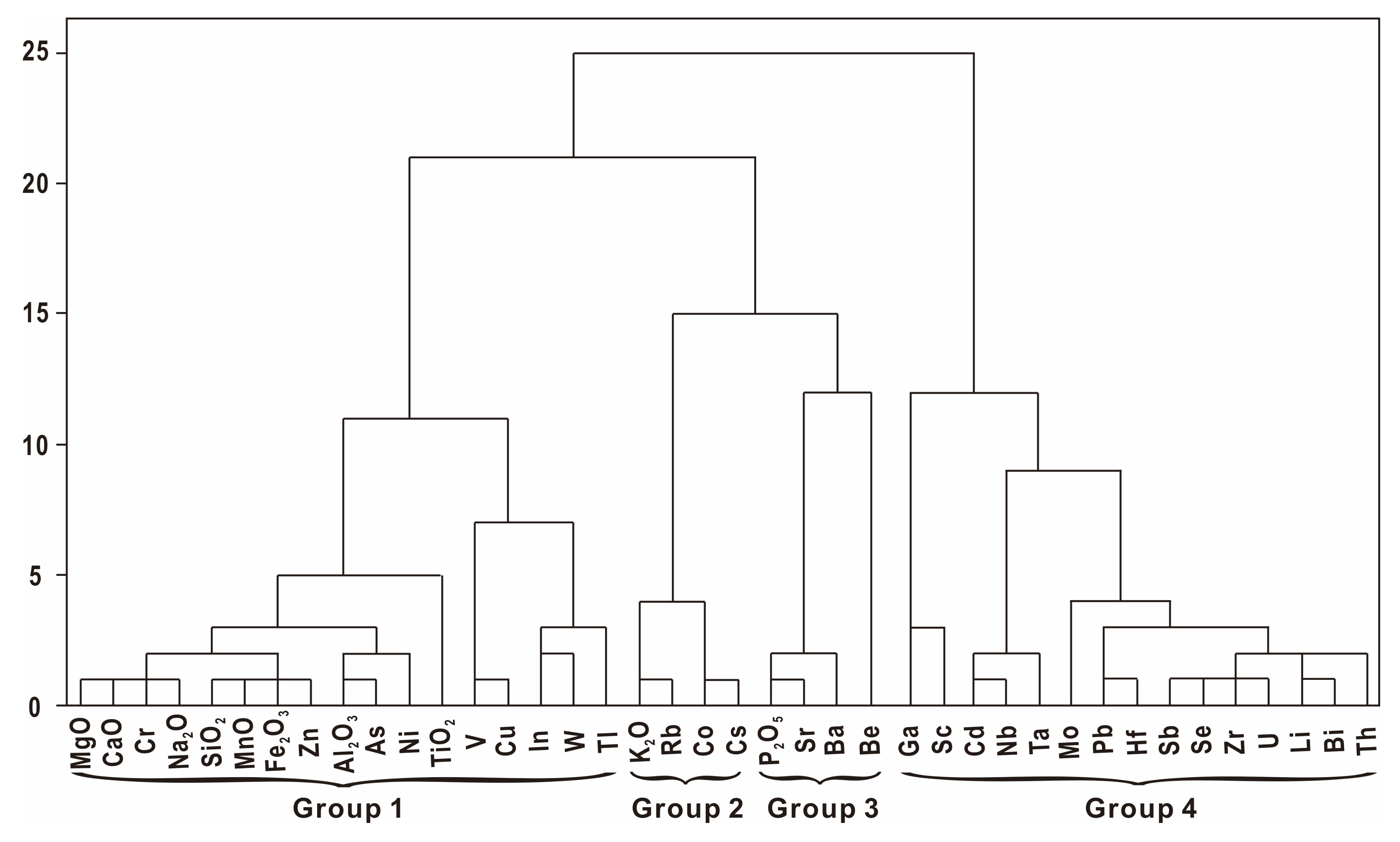
- Correlation and hierarchical cluster analysis
- 2.
- SEM-EDS Analysis
4.3.4. Strontium in the Tashan Coal
4.3.5. Element Sources
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorpe, A.; Senftle, F.; Finkelman, R.; Dulong, F.; Bostick, N. Change in the magnetic properties of bituminous coal intruded by an igneous dike, Dutch Creek Mine, Pitkin County, Colorado. Int. J. Coal Geol. 1998, 36, 243–258. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Bostick, N.H.; Dulong, F.T.; Senftle, F.E.; Thorpe, A.N. Influence of an igneous intrusion on the inorganic geochemistry of a bituminous coal from Pitkin County, Colorado. Int. J. Coal Geol. 1998, 36, 223–241. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Schimmelmann, A. Changes in optical properties, chemistry, and micropore and mesopore characteristics of bituminous coal at the contact with dikes in the Illinois Basin. Int. J. Coal Geol. 2009, 77, 310–319. [Google Scholar] [CrossRef]
- Snyman, C.; Barclay, J. The coalification of South African coal. Int. J. Coal Geol. 1989, 13, 375–390. [Google Scholar] [CrossRef]
- Gröcke, D.R.; Rimmer, S.M.; Yoksoulian, L.E.; Cairncross, B.; Tsikos, H.; van Hunen, J. No evidence for thermogenic methane release in coal from the Karoo-Ferrar large igneous province. Earth. Planet. Sc. Lett. 2009, 277, 204–212. [Google Scholar] [CrossRef]
- Ward, C.R.; Spears, D.; Booth, C.A.; Staton, I.; Gurba, L.W. Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int. J. Coal Geol. 1999, 40, 281–308. [Google Scholar] [CrossRef]
- Gurba, L.W.; Ward, C.R. Elemental composition of coal macerals in relation to vitrinite reflectance, Gunnedah Basin, Australia, as determined by electron microprobe analysis. Int. J. Coal Geol. 2000, 44, 127–147. [Google Scholar] [CrossRef]
- Barker, C.E.; Bone, Y.; Lewan, M.D. Fluid inclusion and vitrinite-reflectance geothermometry compared to heat-flow models of maximum paleotemperature next to dikes, western onshore Gippsland Basin, Australia. Int. J. Coal Geol. 1998, 37, 73–111. [Google Scholar] [CrossRef]
- Moura, H.; Suárez-Ruiz, I.; Marques, M.; Ribeiro, J.; Cunha, P.; Flores, D. Influence of magmatic fluids on the organic and inorganic fractions of coals from the Peñarroya-Belmez-Espiel Basin (Spain). Int. J. Coal Geol. 2021, 235, 103679. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, M.P.; Sharma, M.; Srivastava, S.K. Microstructures and microtextures of natural cokes: A case study of heat-affected coking coals from the Jharia coalfield, India. Int. J. Coal Geol. 2007, 71, 153–175. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, M.; Singh, M.P. Genesis of natural cokes: Some Indian examples. Int. J. Coal Geol. 2008, 75, 40–48. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, M.; Singh, M.P. SEM and reflected light petrography: A case study on natural cokes from seam XIV, Jharia coalfield, India. Fuel 2013, 112, 502–512. [Google Scholar] [CrossRef]
- Arora, A.; Dutta, S.; Gogoi, B.; Banerjee, S. The effects of igneous dike intrusion on organic geochemistry of black shale and its implications: Late Jurassic Jhuran Formation, India. Int. J. Coal Geol. 2017, 178, 84–99. [Google Scholar] [CrossRef]
- Karayigit, A.; Whateley, M. Properties of a lacustrine subbituminous (k1) seam, with special reference to the contact metamorphism, Soma-Turkey. Int. J. Coal Geol. 1997, 34, 131–155. [Google Scholar] [CrossRef]
- Karayigit, A.I. Thermal effects of a basaltic intrusion on the Soma lignite bed in West Turkey. Energ. Source 1998, 20, 55–66. [Google Scholar] [CrossRef]
- Karayiğit, A.İ.; Mastalerz, M.; Oskay, R.G.; Buzkan, İ. Bituminous coal seams from underground mines in the Zonguldak Basin (NW Turkey): Insights from mineralogy, coal petrography, Rock-Eval pyrolysis, and meso-and microporosity. Int. J. Coal Geol. 2018, 199, 91–112. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Lopez-Soler, A.; Plana, F.; Fernandez-Turiel, J.; Zeng, R.; Xu, W.; Zhuang, X.; Spiro, B. Geological controls on the mineral matter and trace elements of coals from the Fuxin basin, Liaoning Province, northeast China. Int. J. Coal Geol. 1997, 34, 89–109. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Effects of igneous intrusions on coal petrology, pore-fracture and coalbed methane characteristics in Hongyang, Handan and Huaibei coalfields, North China. Int. J. Coal Geol. 2012, 96, 72–81. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.; Jiang, Y.; Jiang, C. Transformation of minerals at the boundary of magma-coal contact zone: Case study from Wolonghu Coal Mine, Huaibei Coalfield, China. Int. J. Coal Sci. Technol. 2020, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Zhou, G.; Wang, P.; Wang, R.; Zhao, L.; Chou, C.-L. Behavior of minerals and trace elements during natural coking: A case study of an intruded bituminous coal in the Shuoli mine, Anhui Province, China. Energ. Fuel. 2015, 29, 4100–4113. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Y.; Ji, M.; Duan, H.; Wu, C.; Shi, Q.; Zhang, X.; Wang, Z. Influence of lamprophyre sills on coal metamorphism, coalbed gas composition and coalbed gas occurrence in the Tongxin Minefield, Datong Coalfield, China. Int. J. Coal Geol. 2020, 217, 103286. [Google Scholar] [CrossRef]
- Golab, A.N.; Hutton, A.C.; French, D. Petrography, carbonate mineralogy and geochemistry of thermally altered coal in Permian coal measures, Hunter Valley, Australia. Int. J. Coal Geol. 2007, 70, 150–165. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Cheng, Y.-P.; Yin, G.-Z.; Guo, P.-K.; Mou, J.-H. The effects of magma intrusion on localized stress distribution and its implications for coal mine outburst hazards. Eng. Geol. 2017, 218, 12–21. [Google Scholar] [CrossRef]
- Crelling, J.C.; Dutcher, R.R. A petrologic study of a thermally altered coal from the Purgatoire River Valley of Colorado. Geol. Soc. Am. Bull. 1968, 79, 1375–1386. [Google Scholar] [CrossRef]
- Goodarzi, F.; Cameron, A.R. Organic petrology and elemental distribution in thermally altered coals from Telkwa, British Columbia. Energ. Source. 1990, 12, 315–343. [Google Scholar] [CrossRef]
- Cooper, J.R.; Crelling, J.C.; Rimmer, S.M.; Whittington, A.G. Coal metamorphism by igneous intrusion in the Raton Basin, CO and NM: Implications for generation of volatiles. Int. J. Coal Geol. 2007, 71, 15–27. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Ren, D.; Wang, X.; Li, D.; Zhao, L. Geochemical and mineralogical characteristics in Songzao in Late Permian. Sci. China (Ser. D Earth Sci.) 2007, 37, 353–362. (In Chinese) [Google Scholar] [CrossRef]
- Stewart, A.K.; Massey, M.; Padgett, P.L.; Rimmer, S.M.; Hower, J.C. Influence of a basic intrusion on the vitrinite reflectance and chemistry of the Springfield (No. 5) coal, Harrisburg, Illinois. Int. J. Coal Geol. 2005, 63, 58–67. [Google Scholar] [CrossRef]
- Saghafi, A.; Pinetown, K.; Grobler, P.; Van Heerden, J. CO2 storage potential of South African coals and gas entrapment enhancement due to igneous intrusions. Int. J. Coal Geol. 2008, 73, 74–87. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Zhang, X.; Zhao, Y.; Zhang, H. A study of chemical structural evolution of thermally altered coal and its effect on graphitization. Fuel 2021, 283, 119295. [Google Scholar] [CrossRef]
- Li, K.; Rimmer, S.M.; Liu, Q.; Zhang, Y. Micro-Raman spectroscopy of microscopically distinguishable components of naturally graphitized coals from central Hunan Province, China. Energ. Fuel. 2019, 33, 1037–1048. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Rimmer, S.M.; Huggett, W.W.; Zhang, S. Investigation of the carbon structure of naturally graphitized coals from Central Hunan, China, by density-gradient centrifugation, X-ray diffraction, and high-resolution transmission electron microscopy. Int. J. Coal Geol. 2020, 232, 103628. [Google Scholar] [CrossRef]
- Rimmer, S.M.; Yoksoulian, L.E.; Hower, J.C. Anatomy of an intruded coal, I: Effect of contact metamorphism on whole-coal geochemistry, Springfield (No. 5) (Pennsylvanian) coal, Illinois Basin. Int. J. Coal Geol. 2009, 79, 74–82. [Google Scholar] [CrossRef]
- Hower, J.C.; O'Keefe, J.M.; Valentim, B.; Guedes, A. Contrasts in maceral textures in progressive metamorphism versus near-surface hydrothermal metamorphism. Int. J. Coal Geol. 2021, 103840. [Google Scholar] [CrossRef]
- Hower, J.C.; Rimmer, S.M.; Mastalerz, M.; Wagner, N.J. Notes on the mechanisms of coal metamorphism in the Pennsylvania Anthracite Fields. Int. J. Coal Geol. 2019, 202, 161–170. [Google Scholar] [CrossRef]
- Ward, C.R.; Warbrooke, P.R.; Roberts, F.I. Geochemical and mineralogical changes in a coal seam due to contact metamorphism, Sydney Basin, New South Wales, Australia. Int. J. Coal Geol. 1989, 11, 105–125. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Li, H.; Wu, B. Mineralogical and geochemical responses of coal to igneous intrusion in the Pansan Coal Mine of the Huainan coalfield, Anhui, China. Int. J. Coal Geol. 2014, 124, 11–35. [Google Scholar] [CrossRef]
- Li, B.; Zhuang, X.; Querol, X.; Moreno, N.; Córdoba, P.; Li, J.; Zhou, J.; Ma, X.; Liu, S.; Shangguan, Y. The mode of occurrence and origin of minerals in the Early Permian high-rank coals of the Jimunai depression, Xinjiang Uygur Autonomous Region, NW China. Int. J. Coal Geol. 2019, 205, 58–74. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Dai, S.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Yang, M.; Liu, G.; Sun, R.; Chou, C.-L.; Zheng, L. Characterization of intrusive rocks and REE geochemistry of coals from the Zhuji Coal Mine, Huainan Coalfield, Anhui, China. Int. J. Coal Geol. 2012, 94, 283–295. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Graham, I.T.; Li, X.; Zhang, B. New insights into the lowest Xuanwei Formation in eastern Yunnan Province, SW China: Implications for Emeishan large igneous province felsic tuff deposition and the cause of the end-Guadalupian mass extinction. Lithos 2016, 264, 375–391. [Google Scholar] [CrossRef]
- Dai, S.; Guo, W.; Nechaev, V.P.; French, D.; Ward, C.R.; Spiro, B.F.; Finkelman, R.B. Modes of occurrence and origin of mineral matter in the Palaeogene coal (No. 19-2) from the Hunchun Coalfield, Jilin Province, China. Int. J. Coal Geol. 2018, 189, 94–110. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D. Effects of magmatic intrusion on mineralogy and geochemistry of coals from the Fengfeng−Handan Coalfield, Hebei, China. Energ. Fuel. 2007, 21, 1663–1673. [Google Scholar] [CrossRef]
- Golab, A.N.; Carr, P.F. Changes in geochemistry and mineralogy of thermally altered coal, Upper Hunter Valley, Australia. Int. J. Coal Geol. 2004, 57, 197–210. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, C.; Ren, D.; Chou, C.-L.; Liu, J.; Zeng, R.; Wang, Z.; Zhao, F.; Ge, Y. Distribution of potentially hazardous trace elements in coals from Shanxi province, China. Fuel 2004, 83, 129–135. [Google Scholar] [CrossRef]
- Hu, Q.F.; Zhang, G.D.; Weng, Y.P. Lamprophyre intrusive regularity in Weijiagou minefield, Datong coalfield and their influence on coal seams. Shanxi Geol. 1990, 5, 343–352, (in Chinese with an English abstract). [Google Scholar]
- Zhang, Y.S.; Hao, L.S. Some features of the inorganic matter in contact metamorphic coal, Datong Coalfield. Shanxi Min. Inst. Learn. J. 1994, 12, 126–132, (in Chinese with an English abstract). [Google Scholar]
- Shao, J.; Zhang, Y.; Zhang, L.; Mu, B.; Wang, P.; Guo, F. Early Mesozoic dike swarms of carbonatites and lamprophyres in Datong area. Acta Petrol. Sin. 2003, 19, 93–104, (in Chinese with an English abstract). [Google Scholar] [CrossRef]
- Zhang, F.Q. Lamprophyre invasion’s effects on coal seam and its qualities in the Tashan Minefield, Datong Coalfield. Shanxi Coal 2007, 27, 17–19, (in Chinese with an English abstract). [Google Scholar] [CrossRef]
- Niu, X.; Chen, B.; Feng, G.; Liu, F.; Yang, J. Origin of lamprophyres from the northern margin of the North China Craton: Implications for mantle metasomatism. J. Geol. Soc. London 2017, 174, 353–364. [Google Scholar] [CrossRef]
- Liu, D.N. The Coupling Relationship of Coal Metamorphism and Sedimentary Tectonic Magmatic Activities for Datong Double Period Coal-Bearing Basin; Taiyuan University of Technology: Taiyuan, China, 2015; (in Chinese with an English abstract). [Google Scholar]
- Song, X.; Li, K.; Ma, H.; Liu, D.; Zhao, J.; Zhou, J. Characteristics of an Altered Diabase Dike in a Coal Seam: A Case Study from the Datong Coalfield, Shanxi, China. Geofluids 2020, 3, 1–14. [Google Scholar] [CrossRef]
- GB/T 482-2008, Sampling of Coal Seams; Bureau of Quality and Technical Supervision of China: Beijing, China, 2009. (In Chinese)
- ASTM Standard D3173M-17a, Standard Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM Standard D3174-12 e1, Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM Standard D3175-20, Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2020.
- ISO Standard 7404-2, Methods for the Petrographic Analysis of Coals—part 2: Methods of Preparing Coal Samples; International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO Standard 7404-5, Methods for the Petrographic Analysis of Coals—part 5: Methods of Determining Microscopically the Reflectance of Vitrinite; International Organization for Standardization: Geneva, Switzerland, 2009.
- International Committee for Coal and Organic Petrology (ICCP), The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [CrossRef]
- International Committee for Coal and Organic Petrology (ICCP), The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.; Misz-Kennan, M.; Rodrigues, S.; Hentschel, A.; Hamor-Vido, M. Classification of liptinite–ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Misz-Kennan, M.; Kus, J.; Flores, D.; Avila, C.; Büçkün, Z.; Choudhury, N.; Christanis, K.; Joubert, J.; Kalaitzidis, S.; Karayigit, A. Development of a petrographic classification system for organic particles affected by self-heating in coal waste. (An ICCP Classification System, Self-heating Working Group–Commission III). Int. J. Coal Geol. 2020, 220, 103411. [Google Scholar] [CrossRef]
- GB/T 14506-2010, Methods for Chemical Analysis of Silicate Rocks; Bureau of Quality and Technical Supervision of China: Beijing, China, 2010. (In Chinese)
- ISO Standard 11760, Classification of Coals; International Organization for Standardization: Geneva, Switzerland, 2018.
- ASTM Standard D388-19a, Standard Classification of Coals by Rank; ASTM International: West Conshohocken, PA, USA, 2019.
- Taylor, G.H.; Teichmüller, M.; Davis, A.; Diessel, C.; Littke, R.; Robert, P. Organic Petrology; Schweizerbart Science Publishers: Stuttgart, Germany, 1998. [Google Scholar]
- Goodarzi, F.; Gentzis, T.; Grasby, S.; Dewing, K. Influence of igneous intrusions on thermal maturity and optical texture: Comparison between a bituminous marl and a coal seam of the same maturity. Int. J. Coal Geol. 2018, 198, 183–197. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Eble, C.F.; Wang, Y.; Li, P. Study on petrographic characteristics of devolatilization char/coke related to coal rank and coal maceral. Int. J. Coal Geol. 2020, 227, 103504. [Google Scholar] [CrossRef]
- Liu, J.; Spiro, B.F.; Dai, S.; French, D.; Graham, I.T.; Wang, X.; Zhao, L.; Zhao, J.; Zeng, R. Strontium isotopes in high-and low-Ge coals from the Shengli Coalfield, Inner Mongolia, northern China: New indicators for Ge source. Int. J. Coal Geol. 2021, 233, 103642. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Susilawati, R.; Ward, C.R. Metamorphism of mineral matter in coal from the Bukit Asam deposit, south Sumatra, Indonesia. Int. J. Coal Geol. 2006, 68, 171–195. [Google Scholar] [CrossRef]
- Dai, S.; Ji, D.; Ward, C.R.; French, D.; Hower, J.C.; Yan, X.; Wei, Q. Mississippian anthracites in Guangxi Province, southern China: Petrological, mineralogical, and rare earth element evidence for high-temperature solutions. Int. J. Coal Geol. 2018, 197, 84–114. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I.T.; Dai, S.; Yang, C.; Xie, P.; Zhang, S. Origin of a kaolinite-NH4-illite-pyrophyllite-chlorite assemblage in a marine-influenced anthracite and associated strata from the Jincheng Coalfield, Qinshui Basin, Northern China. Int. J. Coal Geol. 2018, 185, 61–78. [Google Scholar] [CrossRef]
- Permana, A.K.; Ward, C.R.; Li, Z.; Gurba, L.W. Distribution and origin of minerals in high-rank coals of the South Walker Creek area, Bowen Basin, Australia. Int. J. Coal Geol. 2013, 116, 185–207. [Google Scholar] [CrossRef]
- Dai, S.; Zou, J.; Jiang, Y.; Ward, C.R.; Wang, X.; Li, T.; Xue, W.; Liu, S.; Tian, H.; Sun, X. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Adaohai Mine, Daqingshan Coalfield, Inner Mongolia, China: Modes of occurrence and origin of diaspore, gorceixite, and ammonian illite. Int. J. Coal Geol. 2012, 94, 250–270. [Google Scholar] [CrossRef]
- Daniels, E.J.; Altaner, S.P. Inorganic nitrogen in anthracite from eastern Pennsylvania, USA. Int. J. Coal Geol. 1993, 22, 21–35. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Jia, S.; Ward, C.R.; Hower, J.C.; Yan, X.; French, D. Enrichment of U-Re-V-Cr-Se and rare earth elements in the Late Permian coals of the Moxinpo Coalfield, Chongqing, China: Genetic implications from geochemical and mineralogical data. Ore Geol. Rev. 2017, 80, 1–17. [Google Scholar] [CrossRef]
- Golab, A.N.; Carr, P.F.; Palamara, D.R. Influence of localised igneous activity on cleat dawsonite formation in Late Permian coal measures, Upper Hunter Valley, Australia. Int. J. Coal Geol. 2006, 66, 296–304. [Google Scholar] [CrossRef]
- Dawson, G.; Golding, S.; Esterle, J.; Massarotto, P. Occurrence of minerals within fractures and matrix of selected Bowen and Ruhr Basin coals. Int. J. Coal Geol. 2012, 94, 150–166. [Google Scholar] [CrossRef]
- Hower, J.C.; Williams, D.A.; Eble, C.F.; Sakulpitakphon, T.; Moecher, D.P. Brecciated and mineralized coals in Union County, Western Kentucky coal field. Int. J. Coal Geol. 2001, 47, 223–234. [Google Scholar] [CrossRef]
- Kisch, H.; Taylor, G. Metamorphism and alteration near an intrusive-coal contact. Econ. Geol. 1966, 61, 343–361. [Google Scholar] [CrossRef]
- Rimmer, S.M.; Crelling, J.C.; Yoksoulian, L.E. An occurrence of coked bitumen, Raton formation, Purgatoire River valley, Colorado, USA. Int. J. Coal Geol. 2015, 141, 63–73. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, F.; Dai, S.; Zhang, J.; Luo, K. Geochemistry of Trace Elements in Coal; Science Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Tang, X.; Huang, W. Trace Elements in Chinese Coal; Commercial Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Ketris, M.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Deposita. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Finkelman, R.B. Trace and minor elements in coal. In Organic Geochemistry; Springer: Berlin/Heidelberg, Germany, 1993; pp. 593–607. [Google Scholar] [CrossRef]
- Kortenski, J.; Sotirov, A. Trace and major element content and distribution in Neogene lignite from the Sofia Basin, Bulgaria. Int. J. Coal Geol. 2002, 52, 63–82. [Google Scholar] [CrossRef]
- Eskanazy, G.; Finkelman, R.B.; Chattarjee, S. Some considerations concerning the use of correlation coefficients and cluster analysis in interpreting coal geochemistry data. Int. J. Coal Geol. 2010, 83, 491–493. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of Occurrence of Trace Elements in Coal. USGS Open-File Report 1980, 81–99. Available online: https://www.proquest.com/dissertations-theses/modes-occurrence-trace-elements-coal/docview/302996545/se-2?accountid=42709 (accessed on 15 October 2017).
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Querol, X.; Klika, Z.; Weiss, Z.; Finkelman, R.; Alastuey, A.; Juan, R.; López-Soler, A.; Plana, F.; Kolker, A.; Chenery, S. Determination of element affinities by density fractionation of bulk coal samples. Fuel 2001, 80, 83–96. [Google Scholar] [CrossRef]
- Hower, J.C.; Robertson, J.D. Clausthalite in coal. Int. J. Coal Geol. 2003, 53, 219–225. [Google Scholar] [CrossRef]
- Çelik, Y.; Karayigit, A.I.; Oskay, R.G.; Kayseri-Özer, M.S.; Christanis, K.; Hower, J.C.; Querol, X. A multidisciplinary study and palaeoenvironmental interpretation of middle Miocene Keles lignite (Harmancık Basin, NW Turkey), with emphasis on syngenetic zeolite formation. Int. J. Coal Geol. 2021, 237, 103691. [Google Scholar] [CrossRef]
- Hower, J.C.; Campbell, J.I.; Teesdale, W.J.; Nejedly, Z.; Robertson, J.D. Scanning proton microprobe analysis of mercury and other trace elements in Fe-sulfides from a Kentucky coal. Int. J. Coal Geol. 2008, 75, 88–92. [Google Scholar] [CrossRef]
- Swaine, D.J. Trace Elements in Coal; Butterworth-Heinemann: Oxford, UK, 1990. [Google Scholar]
- Goodarzi, F.; Cameron, A. Organic petrology of thermally altered coals from Telkwa, British Columbia. In Contributions to Canadian Coal Geoscience; Geological Survey of Canada: Ottawa, ON, Canada, 1989; Volume 89, pp. 96–103. [Google Scholar]


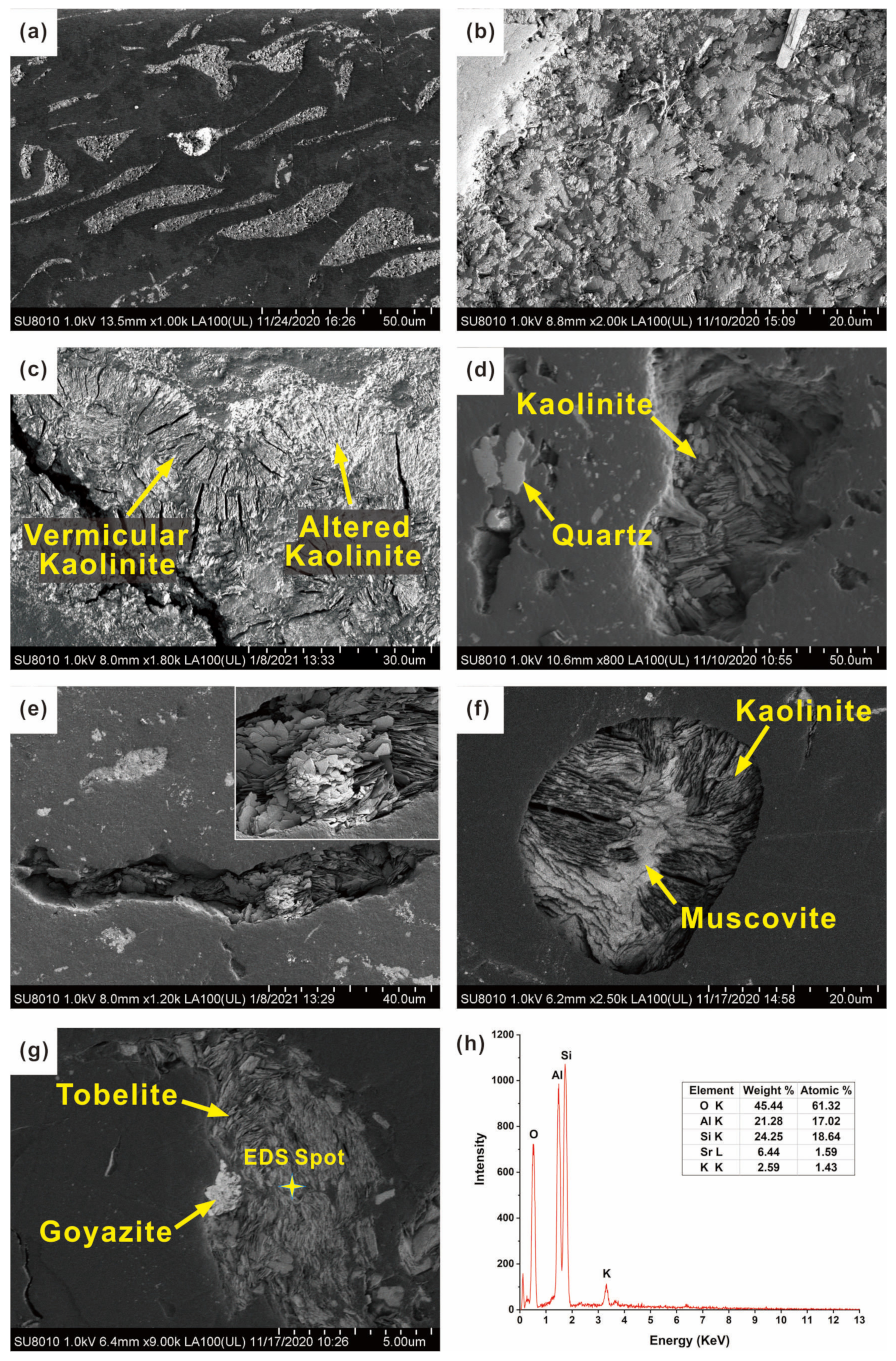

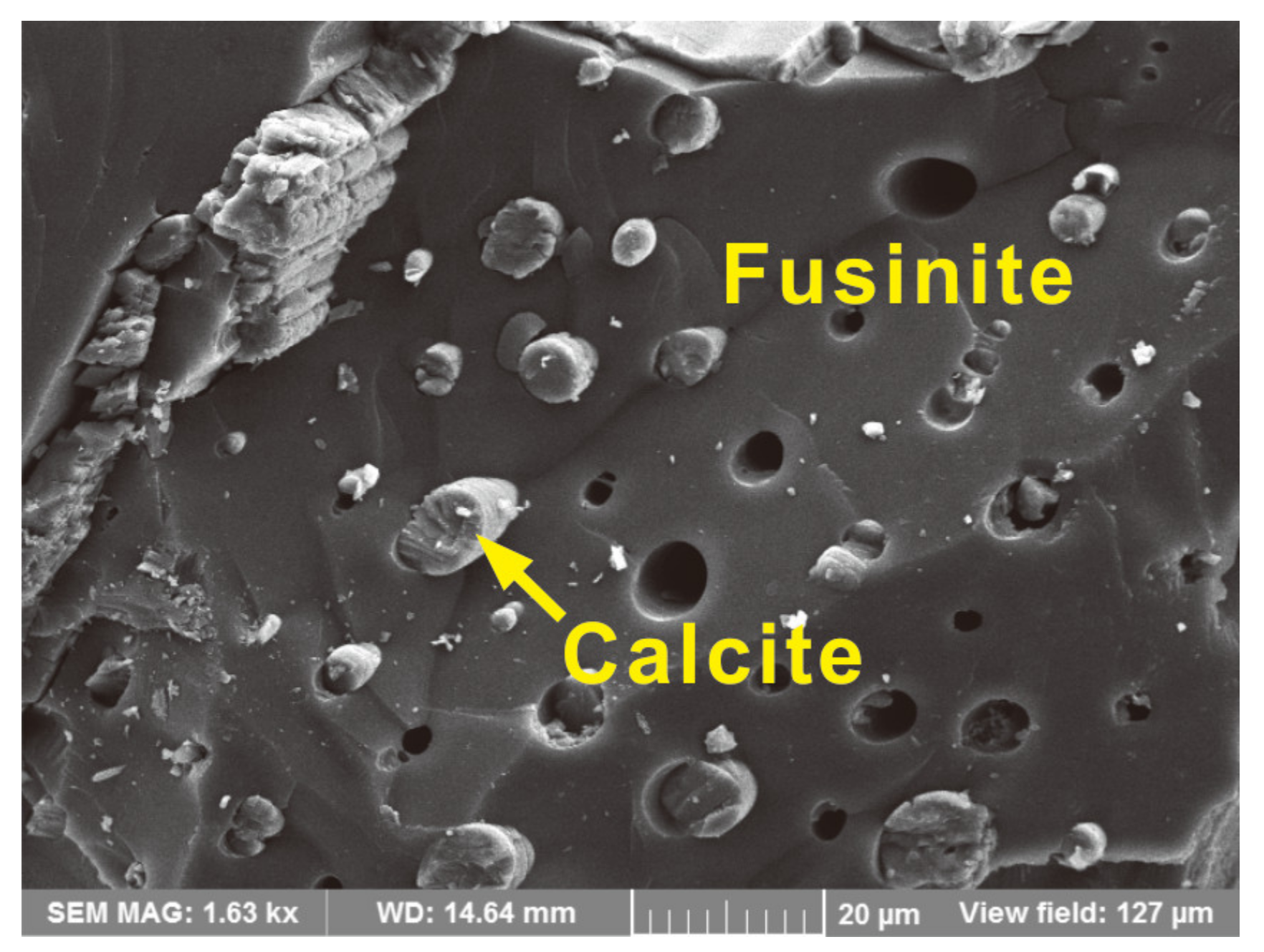
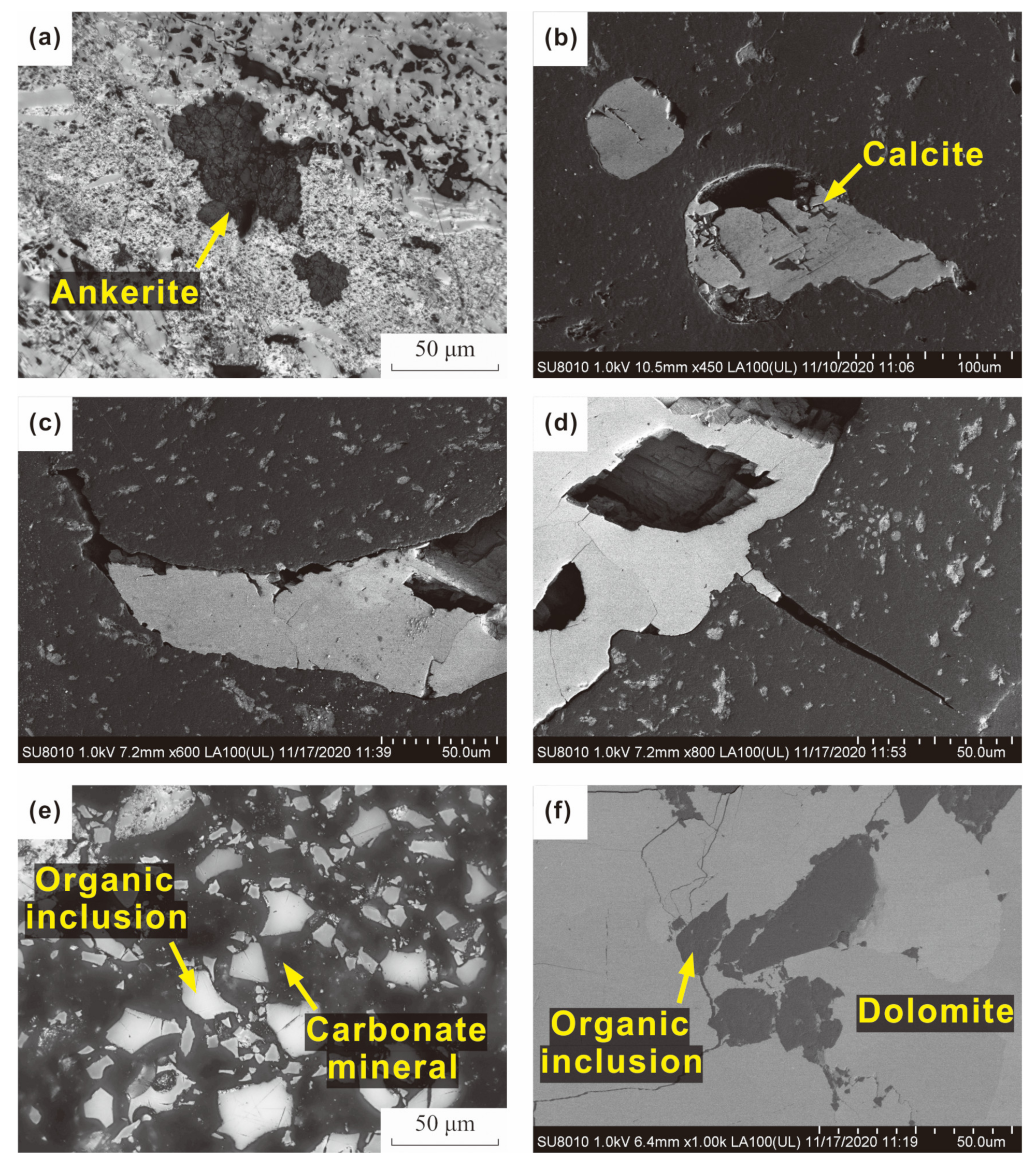
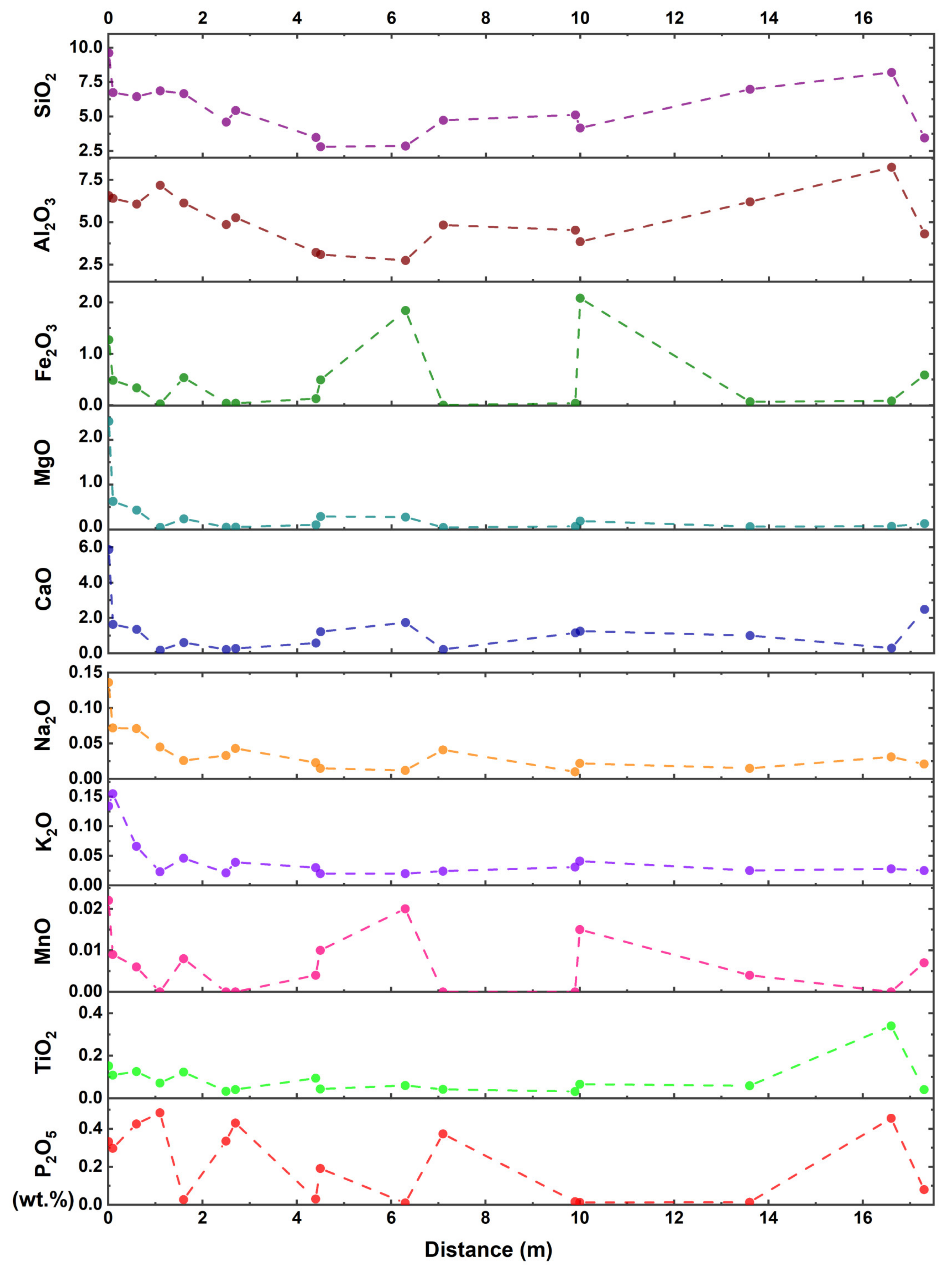



| Sample | Distance (m) | Rr | Rmax | Mad | Ad | VMdaf | FCdaf |
|---|---|---|---|---|---|---|---|
| T1 | Contact zone | 3.45 | 3.67 | 4.1 | 27.5 | 11.9 | 88.1 |
| T2 | 0.1 | 3.29 | 3.50 | 4.8 | 25.5 | 7.4 | 92.6 |
| T3 | 0.6 | 2.69 | 2.86 | 2.0 | 24.4 | 8.3 | 91.7 |
| T4 | 1.1 | 1.75 | 1.87 | 1.1 | 11.8 | 19.7 | 80.3 |
| T5 | 1.6 | 1.47 | 1.57 | 1.2 | 11.7 | 21.6 | 78.4 |
| T6 | 2.5 | 0.74 | 0.79 | 1.8 | 8.1 | 28.9 | 71.2 |
| T7 | 2.7 | 0.88 | 0.94 | 1.4 | 9.7 | 27.2 | 72.8 |
| T8 | 4.4 | 0.70 | 0.75 | 1.8 | 6.6 | 33.2 | 66.8 |
| T9 | 4.5 | 0.71 | 0.75 | 1.9 | 7.6 | 34.6 | 65.5 |
| T10 | 6.3 | 0.68 | 0.72 | 1.9 | 8.6 | 49.9 | 50.1 |
| T11 | 7.1 | 0.68 | 0.73 | 1.9 | 8.3 | 29.7 | 70.3 |
| T12 | 9.9 | 0.63 | 0.67 | 1.6 | 9.3 | 38.0 | 62.0 |
| T13 | 10 | 0.75 | 0.79 | 1.7 | 9.6 | 33.0 | 67.0 |
| T14 | 13.6 | 0.68 | 0.73 | 1.8 | 10.3 | 33.6 | 66.4 |
| T15 | 16.6 | 0.82 | 0.87 | 1.8 | 14.2 | 29.1 | 70.9 |
| T16 | 17.3 | 0.73 | 0.78 | 1.2 | 11.8 | 35.8 | 64.2 |
| Sample | Non-Altered Organic Particles | Altered Organic Particles | Newly Formed Organic Particles | |||
|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | Porous | Massive | Pyrolytic Carbon | |
| T1 | 23.7 | 19.7 | - | 24.0 | 32.1 | 0.5 |
| T2 | 21.4 | 16.2 | - | 18.6 | 42.6 | 1.2 |
| T3 | 15.5 | 18.3 | - | 15.1 | 49.7 | 1.4 |
| T4 | 19.8 | 12.3 | 0.3 | 4.4 | 62.8 | 0.4 |
| T5 | 60.5 | 14.5 | 0.4 | 1.3 | 22.5 | 0.8 |
| Sample No. | T | CT | CD | CG | G | VD | TV | SF | F | Mac | Mic | FG | ID | TI | SP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T6 | 15.4 | 9.2 | 36.1 | 2.0 | 3.6 | 1.6 | 67.9 | 7.9 | 8.9 | 1.3 | 4.3 | - | 2.3 | 24.6 | 7.5 |
| T7 | 18.8 | 18.8 | 30.7 | - | 1.0 | 69.3 | 9.4 | 10.4 | 2.0 | 5.9 | 0.5 | 0.5 | 28.7 | 2.0 | |
| T8 | 10.8 | 12.6 | 33.9 | - | 0.7 | 0.7 | 58.8 | 12.6 | 7.2 | 2.5 | 7.9 | - | 2.2 | 32.5 | 8.7 |
| T9 | 15.0 | 12.3 | 33.3 | 0.3 | 0.6 | 2.4 | 64.0 | 15.3 | 13.2 | 1.5 | 2.1 | 0.3 | 0.9 | 33.3 | 2.7 |
| T10 | 17.0 | 13.8 | 36.4 | 0.4 | 1.8 | 2.5 | 71.7 | 11.3 | 8.5 | 3.2 | 1.4 | - | 0.7 | 25.1 | 3.2 |
| T11 | 11.7 | 10.8 | 28.6 | - | 1.5 | 1.5 | 54.2 | 15.4 | 10.8 | 1.5 | 5.5 | - | 3.4 | 36.6 | 9.2 |
| T12 | 21.0 | 8.2 | 22.7 | 2.7 | 3.1 | 1.0 | 58.8 | 17.5 | 10.0 | 1.0 | 4.8 | - | 2.4 | 35.7 | 5.5 |
| T13 | 19.8 | 5.1 | 36.3 | 0.4 | 1.1 | 4.0 | 66.7 | 9.9 | 7.3 | 1.8 | 3.7 | 0.4 | 1.5 | 24.5 | 8.8 |
| T14 | 25.7 | 13.8 | 23.8 | 1.1 | 1.1 | 1.1 | 66.7 | 11.9 | 11.1 | 1.1 | 1.9 | - | 2.7 | 28.7 | 4.6 |
| T15 | 10.8 | 2.4 | 50.0 | 2.0 | 3.0 | 2.0 | 70.3 | 8.1 | 7.4 | 4.4 | - | 6.1 | 26.0 | 3.7 | |
| T16 | 28.1 | 11.7 | 32.4 | 0.3 | - | 1.9 | 74.4 | 13.9 | 5.9 | 0.9 | 2.2 | - | 0.6 | 23.5 | 2.2 |
| Av. | 17.6 | 10.8 | 33.1 | 1.2 | 1.8 | 1.9 | 65.7 | 12.1 | 9.1 | 1.9 | 4.0 | - | 2.1 | 29.0 | 5.3 |
| Sample | Description Term | Nature | |
|---|---|---|---|
| ISO 11760-2018 a | ASTM D388-19a b | ||
| T1 | Anthracite B, moderately high ash | Semianthracite | Thermally-altered |
| T2 | Anthracite B, moderately high ash | Anthracite | Thermally-altered |
| T3 | Anthracite C, moderately high ash | Semianthracite | Thermally-altered |
| T4 | Bituminous B, medium ash | Low volatile bituminous | Thermally-altered |
| T5 | Bituminous B, medium ash | Low volatile bituminous | Thermally-altered |
| T6 | Bituminous C, low ash, moderately high vitrinite | Medium volatile bituminous | Unaltered |
| T7 | Bituminous C, low ash, moderately high vitrinite | Medium volatile bituminous | Unaltered |
| T8 | Bituminous C, low ash, medium vitrinite | High volatile bituminous | Unaltered |
| T9 | Bituminous C, low ash, moderately high vitrinite | High volatile bituminous | Unaltered |
| T10 | Bituminous C, low ash, moderately high vitrinite | High volatile bituminous | Unaltered |
| T11 | Bituminous C, low ash, medium vitrinite | High volatile bituminous | Unaltered |
| T12 | Bituminous C, low ash, medium vitrinite | High volatile bituminous | Unaltered |
| T13 | Bituminous C, low ash, moderately high vitrinite | High volatile bituminous | Unaltered |
| T14 | Bituminous C, medium ash, moderately high vitrinite | High volatile bituminous | Unaltered |
| T15 | Bituminous C, medium ash, moderately high vitrinite | Medium volatile bituminous | Unaltered |
| T16 | Bituminous C, medium ash, moderately high vitrinite | High volatile bituminous | Unaltered |
| Sample | Distance (m) | Nature | Minerals |
|---|---|---|---|
| T1 | Contact | Thermally-altered | Dolomite, ankerite, muscovite, kaolinite, quartz, calcite |
| T2 | 0.1 | Thermally-altered | Kaolinite, dolomite, ankerite, anhydrite, muscovite, quartz |
| T3 | 0.6 | Thermally-altered | Dolomite, muscovite, kaolinite, quartz, ankerite |
| T4 | 1.1 | Thermally-altered | Kaolinite, muscovite, calcite, tobelite |
| T5 | 1.6 | Thermally-altered | Kaolinite, dolomite, ankerite, tobelite |
| T6 | 2.5 | Unaltered | Kaolinite, quartz |
| T7 | 2.7 | Unaltered | Kaolinite, quartz |
| T8 | 4.4 | Unaltered | Kaolinite, calcite, dolomite |
| T9 | 4.5 | Unaltered | Kaolinite, dolomite, calcite, quartz |
| T10 | 6.3 | Unaltered | Kaolinite, calcite, quartz, dolomite |
| T11 | 7.1 | Unaltered | Kaolinite, quartz, analcime |
| T12 | 9.9 | Unaltered | Kaolinite, calcite |
| T13 | 10 | Unaltered | Kaolinite, calcite, quartz, diopside, ankerite |
| T14 | 13.6 | Unaltered | Quartz, kaolinite, calcite, dolomite |
| T15 | 16.6 | Unaltered | Kaolinite, dolomite, calcite |
| T16 | 17.3 | Unaltered | Calcite, Kaolinite, boehmite, dolomite, quartz |
| Sample No. | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | MnO | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 9.61 | 6.56 | 1.27 | 2.41 | 5.86 | 0.14 | 0.13 | 0.022 | 0.15 | 0.332 |
| T2 | 6.73 | 6.40 | 0.48 | 0.63 | 1.63 | 0.07 | 0.16 | 0.009 | 0.11 | 0.297 |
| T3 | 6.44 | 6.07 | 0.33 | 0.43 | 1.35 | 0.07 | 0.07 | 0.006 | 0.13 | 0.425 |
| T4 | 6.86 | 7.17 | 0.03 | 0.05 | 0.18 | 0.05 | 0.02 | bdl | 0.07 | 0.484 |
| T5 | 6.66 | 6.13 | 0.53 | 0.24 | 0.61 | 0.03 | 0.05 | 0.008 | 0.12 | 0.027 |
| T6 | 4.59 | 4.86 | 0.04 | 0.05 | 0.21 | 0.03 | 0.02 | bdl | 0.03 | 0.335 |
| T7 | 5.43 | 5.26 | 0.04 | 0.05 | 0.27 | 0.04 | 0.04 | bdl | 0.04 | 0.430 |
| T8 | 3.47 | 3.22 | 0.13 | 0.10 | 0.58 | 0.02 | 0.03 | 0.004 | 0.10 | 0.030 |
| T9 | 2.80 | 3.09 | 0.49 | 0.29 | 1.22 | 0.02 | 0.02 | 0.010 | 0.04 | 0.191 |
| T10 | 2.85 | 2.74 | 1.84 | 0.27 | 1.74 | 0.01 | 0.02 | 0.020 | 0.06 | 0.009 |
| T11 | 4.72 | 4.83 | bdl | 0.04 | 0.22 | 0.04 | 0.02 | bdl | 0.04 | 0.373 |
| T12 | 5.11 | 4.53 | 0.04 | 0.07 | 1.15 | 0.01 | 0.03 | bdl | 0.03 | 0.016 |
| T13 | 4.16 | 3.85 | 2.08 | 0.19 | 1.25 | 0.02 | 0.04 | 0.015 | 0.07 | 0.012 |
| T14 | 6.97 | 6.20 | 0.06 | 0.06 | 1.00 | 0.02 | 0.03 | 0.004 | 0.06 | 0.013 |
| T15 | 8.20 | 8.24 | 0.08 | 0.07 | 0.29 | 0.03 | 0.03 | bdl | 0.34 | 0.455 |
| T16 | 3.44 | 4.31 | 0.59 | 0.13 | 2.48 | 0.02 | 0.03 | 0.007 | 0.04 | 0.080 |
| Chinese coal | 8.47 | 5.98 | 4.85 | 0.22 | 1.23 | 0.16 | 0.19 | 0.015 | 0.33 | 0.092 |
| Av. thermally altered | 7.26 | 6.47 | 0.53 | 0.75 | 1.93 | 0.07 | 0.09 | 0.011 | 0.12 | 0.313 |
| Av. unaltered | 4.70 | 4.65 | 0.54 | 0.12 | 0.95 | 0.02 | 0.03 | 0.010 | 0.08 | 0.18 |
| CC. thermally altered | 0.86 | 1.08 | 0.11 | 3.41 | 1.57 | 0.44 | 0.45 | 0.75 | 0.35 | 3.40 |
| CC. unaltered | 0.56 | 0.78 | 0.11 | 0.55 | 0.77 | 0.15 | 0.15 | 0.67 | 0.23 | 1.92 |
| Sample No. | Li | Be | V | Cr | Co | Ni | Cu | Zn | Ga | Rb | Sr | Mo | Cd | In | Sb |
| T1 | 33 | 1.1 | 22 | 38 | 1.7 | 6.2 | 37 | 25 | 14 | 3.4 | 1649 | 1.2 | 0.04 | 0.05 | 0.24 |
| T2 | 35 | 1.8 | 24 | 14 | 2.4 | 4.5 | 37 | 17 | 24 | 5.1 | 1452 | 1.8 | 0.03 | 0.04 | 0.45 |
| T3 | 46 | 1.8 | 16 | 13 | 1.7 | 3.2 | 18 | 14 | 13 | 1.6 | 2318 | 1.4 | 0.04 | 0.03 | 0.24 |
| T4 | 59 | 1.8 | 14 | 5.7 | 1.0 | 2.0 | 33 | 11 | 9.2 | 0.41 | 3067 | 0.62 | 0.01 | 0.03 | 0.46 |
| T5 | 121 | 1.3 | 21 | 5.6 | 1.6 | 2.4 | 35 | 18 | 21 | 0.97 | 85 | 2.2 | 0.04 | 0.05 | 0.92 |
| T6 | 37 | 1.5 | 17 | 5.1 | 1.2 | 2.2 | 31 | 13 | 10 | 0.29 | 1839 | 1.1 | 0.02 | 0.02 | 0.33 |
| T7 | 39 | 1.6 | 12 | 4.8 | 1.4 | 2.6 | 14 | 9.3 | 11 | 0.38 | 2618 | 0.72 | 0.01 | 0.02 | 0.27 |
| T8 | 27 | 1.6 | 19 | 7.4 | 2.2 | 7.1 | 31 | 16 | 25 | 0.66 | 108 | 1.8 | 0.08 | 0.10 | 0.76 |
| T9 | 23 | 1.5 | 11 | 5.4 | 2.5 | 3.8 | 30 | 18 | 21 | 0.41 | 57 | 2.7 | 0.03 | 0.02 | 0.42 |
| T10 | 27 | 0.98 | 19 | 6.4 | 2.8 | 4.0 | 33 | 20 | 33 | 0.37 | 24 | 2.2 | 0.06 | 0.07 | 1.2 |
| T11 | 37 | 1.8 | 8.7 | 4.8 | 1.6 | 3.6 | 35 | 13 | 12 | 0.60 | 2110 | 0.77 | 0.02 | 0.02 | 0.38 |
| T12 | 34 | 1.7 | 11 | 4.1 | 3.7 | 7.4 | 31 | 13 | 27 | 0.62 | 36 | 3.1 | 0.03 | 0.03 | 0.88 |
| T13 | 43 | 1.9 | 11 | 6.1 | 1.4 | 2.5 | 32 | 24 | 12 | 0.89 | 36 | 1.1 | 0.08 | 0.09 | 0.33 |
| T14 | 62 | 1.6 | 13 | 4.5 | 6.9 | 13 | 31 | 15 | 21 | 0.49 | 30 | 1.6 | 0.03 | 0.05 | 0.56 |
| T15 | 60 | 2.8 | 23 | 8.2 | 1.6 | 10 | 32 | 17 | 12 | 0.55 | 2729 | 1.0 | 0.08 | 0.14 | 0.37 |
| T16 | 49 | 0.81 | 14 | 4.7 | 2.3 | 4.8 | 29 | 15 | 24 | 0.46 | 485 | 2.7 | 0.06 | 0.02 | 0.51 |
| Av. thermally altered | 59 | 1.5 | 19 | 15 | 1.7 | 3.7 | 32 | 17 | 16 | 2.3 | 1714 | 1.4 | 0.03 | 0.04 | 0.46 |
| Av. unaltered | 40 | 1.6 | 15 | 5.6 | 2.5 | 5.6 | 30 | 16 | 19 | 0.52 | 916 | 1.7 | 0.05 | 0.05 | 0.54 |
| Ratio | 1.5 | 0.94 | 1.3 | 2.7 | 0.67 | 0.66 | 1.1 | 1.1 | 0.8 | 4.4 | 1.9 | 0.83 | 0.6 | 0.8 | 0.85 |
| Sample No. | Cs | Ba | W | Tl | Pb | Bi | Th | U | Nb | Ta | Zr | Hf | Se | As | Sc |
| T1 | 0.36 | 184 | 1.0 | 0.35 | 32 | 0.16 | 7.4 | 2.5 | 5.2 | 0.40 | 63 | 2.2 | 1.3 | 0.94 | 4.2 |
| T2 | 0.99 | 138 | 0.59 | 0.28 | 28 | 0.19 | 6.3 | 3.8 | 3.6 | 0.23 | 68 | 2.4 | 2.1 | 0.66 | 4.6 |
| T3 | 0.23 | 173 | 0.47 | 0.05 | 22 | 0.21 | 6.1 | 1.6 | 4.0 | 0.32 | 54 | 1.5 | 1.4 | 0.40 | 3.9 |
| T4 | 0.04 | 171 | 0.21 | 0.16 | 20 | 0.16 | 6.2 | 1.5 | 1.6 | 0.24 | 49 | 1.9 | 2.4 | bdl | 3.1 |
| T5 | 0.12 | 28 | 1.03 | 0.40 | 44 | 0.39 | 13 | 6.5 | 6.4 | 0.42 | 110 | 3.7 | 4.6 | 0.51 | 4.6 |
| T6 | 0.01 | 93 | 0.16 | 0.10 | 19 | 0.08 | 2.0 | 1.1 | 1.4 | 0.08 | 26 | 1.0 | 2.5 | bdl | 1.6 |
| T7 | 0.01 | 134 | 0.17 | 0.17 | 13 | 0.10 | 2.5 | 1.1 | 1.1 | 0.11 | 29 | 1.1 | 1.3 | bdl | 1.9 |
| T8 | 0.04 | 42 | 0.71 | 0.22 | 49 | 0.39 | 9.2 | 6.1 | 3.9 | 0.40 | 148 | 4.2 | 2.8 | bdl | 5.1 |
| T9 | 0.02 | 56 | 0.24 | 0.50 | 17 | 0.10 | 3.3 | 0.82 | 1.0 | 0.12 | 43 | 1.4 | 1.7 | 0.43 | 2.1 |
| T10 | 0.02 | 13 | 0.17 | 0.24 | 20 | 0.16 | 6.4 | 6.3 | 2.7 | 0.15 | 147 | 4.1 | 2.4 | 0.32 | 6.5 |
| T11 | 0.01 | 133 | 0.23 | 0.03 | 15 | 0.13 | 3.1 | 1.6 | 1.2 | 0.11 | 37 | 1.1 | 1.6 | bdl | 2.8 |
| T12 | 0.01 | 13 | 0.41 | 0.12 | 34 | 0.12 | 2.3 | 1.8 | 2.2 | 0.10 | 49 | 1.6 | 2.5 | bdl | 2.1 |
| T13 | 0.03 | 12 | 0.18 | 0.25 | 25 | 0.43 | 6.5 | 4.5 | 4.2 | 0.23 | 122 | 3.2 | 3.1 | 0.30 | 6.3 |
| T14 | 0.03 | 17 | 0.27 | 0.11 | 34 | 0.13 | 2.2 | 2.2 | 3.1 | 0.17 | 57 | 1.9 | 3.9 | bdl | 3.7 |
| T15 | 0.03 | 156 | 0.97 | 0.13 | 45 | 0.83 | 8.9 | 4.5 | 8.6 | 0.75 | 146 | 4.1 | 3.8 | bdl | 10 |
| T16 | 0.03 | 34 | 0.95 | 0.28 | 21 | 0.12 | 2.6 | 0.86 | 1.2 | 0.12 | 44 | 1.5 | 2.5 | 0.25 | 2.2 |
| Av. thermally altered | 0.35 | 139 | 0.66 | 0.25 | 29 | 0.22 | 7.8 | 3.2 | 4.2 | 0.32 | 69 | 2.3 | 2.3 | 0.63 | 4.1 |
| Av. unaltered | 0.02 | 64 | 0.41 | 0.20 | 27 | 0.24 | 4.5 | 2.8 | 2.8 | 0.21 | 77 | 2.3 | 2.6 | 0.32 | 4.0 |
| Ratio | 17.5 | 2.17 | 1.6 | 1.25 | 1.1 | 0.92 | 1.73 | 1.1 | 1.5 | 1.52 | 0.89 | 1 | 0.88 | 2.0 | 1.0 |
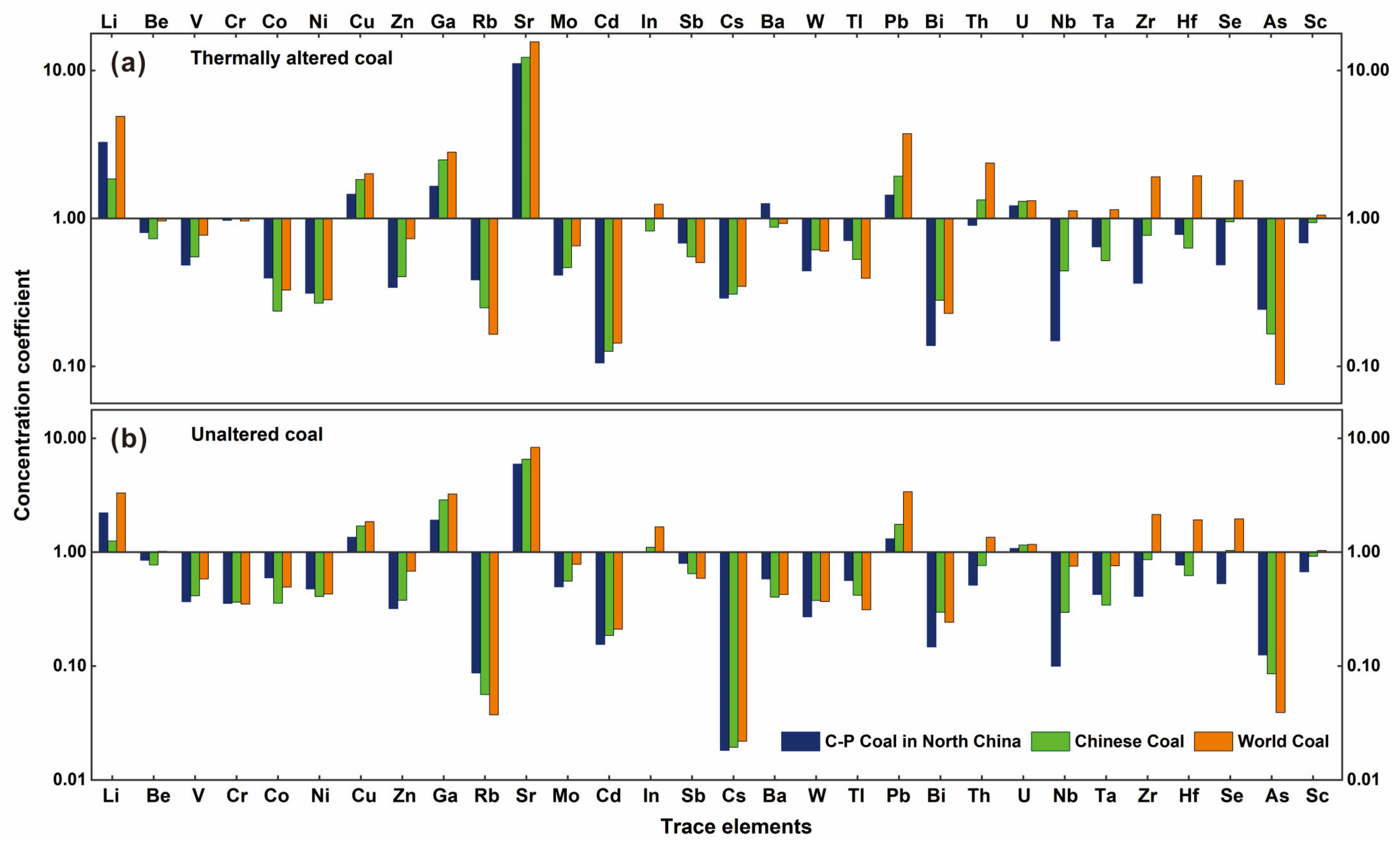
| Element | C–P coal in North China a | Chinese Coal b | World Coal c | Thermally Altered Coal d | Unaltered Coal e | ||||
|---|---|---|---|---|---|---|---|---|---|
| CC1 | CC2 | CC3 | CC1’ | CC2’ | CC3’ | ||||
| Li | 18 | 31.8 | 12 | 3.3 | 1.9 | 4.9 | 2.2 | 1.3 | 3.3 |
| Be | 1.92 | 2.11 | 1.6 | 0.8 | 0.7 | 1.0 | 0.9 | 0.8 | 1.0 |
| V | 39.79 | 35.1 | 25 | 0.5 | 0.6 | 0.8 | 0.4 | 0.4 | 0.6 |
| Cr | 15.81 | 15.4 | 16 | 1.0 | 1.0 | 1.0 | 0.4 | 0.4 | 0.4 |
| Co | 4.24 | 7.08 | 5.1 | 0.4 | 0.2 | 0.3 | 0.6 | 0.4 | 0.5 |
| Ni | 11.75 | 13.7 | 13 | 0.3 | 0.3 | 0.3 | 0.5 | 0.4 | 0.4 |
| Cu | 21.97 | 17.5 | 16 | 1.5 | 1.8 | 2.0 | 1.4 | 1.7 | 1.9 |
| Zn | 48.99 | 41.4 | 23 | 0.3 | 0.4 | 0.7 | 0.3 | 0.4 | 0.7 |
| Ga | 9.88 | 6.55 | 5.8 | 1.7 | 2.5 | 2.8 | 1.9 | 2.9 | 3.3 |
| Rb | 6 | 9.25 | 14 | 0.4 | 0.3 | 0.2 | 0.1 | 0.06 | 0.04 |
| Sr | 154 | 140 | 110 | 11.1 | 12.2 | 15.6 | 6.0 | 6.5 | 8.3 |
| Mo | 3.47 | 3.08 | 2.2 | 0.4 | 0.5 | 0.7 | 0.5 | 0.6 | 0.8 |
| Cd | 0.3 | 0.25 | 0.22 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 |
| In | Nd | 0.047 | 0.031 | Nd | 0.8 | 1.3 | Nd | 1.1 | 1.7 |
| Sb | 0.68 | 0.84 | 0.92 | 0.7 | 0.6 | 0.5 | 0.8 | 0.6 | 0.6 |
| Cs | 1.2 | 1.13 | 1 | 0.3 | 0.3 | 0.4 | 0.02 | 0.02 | 0.02 |
| Ba | 110 | 159 | 150 | 1.3 | 0.9 | 0.9 | 0.6 | 0.4 | 0.4 |
| W | 1.5 | 1.08 | 1.1 | 0.4 | 0.6 | 0.6 | 0.3 | 0.4 | 0.4 |
| Tl | 0.35 | 0.47 | 0.63 | 0.7 | 0.5 | 0.4 | 0.6 | 0.4 | 0.3 |
| Pb | 20.28 | 15.1 | 7.8 | 1.4 | 1.9 | 3.7 | 1.3 | 1.8 | 3.4 |
| Bi | 1.6* | 0.79 | 0.97 | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 |
| Th | 8.7 | 5.84 | 3.3 | 0.9 | 1.3 | 2.4 | 0.5 | 0.8 | 1.4 |
| U | 2.6 | 2.43 | 2.4 | 1.2 | 1.3 | 1.3 | 1.1 | 1.2 | 1.2 |
| Nb | 28 | 9.44 | 3.7 | 0.2 | 0.4 | 1.1 | 0.1 | 0.3 | 0.8 |
| Ta | 0.5 | 0.62 | 0.28 | 0.6 | 0.5 | 1.2 | 0.4 | 0.3 | 0.8 |
| Zr | 188.28 | 89.5 | 36 | 0.4 | 0.8 | 1.9 | 0.4 | 0.9 | 2.1 |
| Hf | 3 | 3.71 | 1.2 | 0.8 | 0.6 | 1.9 | 0.8 | 0.6 | 1.9 |
| Se | 4.84* | 2.47 | 1.3 | 0.5 | 1.0 | 1.8 | 0.5 | 1.0 | 2.0 |
| As | 2.59* | 3.79 | 8.3 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.04 |
| Sc | 6* | 4.38 | 3.9 | 0.7 | 0.9 | 1.1 | 0.7 | 0.9 | 1.0 |
| Correlation with Ash Yield | ||
| Group 1 | rash= | MgO (0.594), CaO (0.623), Cr (0.697), Na2O (0.835), SiO2 (0.457), MnO (0.449), Fe2O3 (0.348), Zn (0.239), Al2O3 (0.592), As (0.481), Ni (0.790), TiO2 (0.284), V (0.002), Cu (-0.087), In (−0.103), W (−0.453), Tl (−0.423) |
| Group 2 | rash= | K2O (0.751), Rb (0.676), Co (0.379), Cs (0.516) |
| Group 3 | rash= | P2O5 (0.907), Sr (0.867), Ba (0.967), Be (0.200) |
| Group 4 | rash= | Ga (−0.394), Sc (−0.479), Cd (−0.725), Nb (−0.763), Ta (−0.516), Mo (−0.876), Pb (−0.810), Hf (−0.840), Sb (−0.948), Se (−0.965), Zr (−0.944), U (−0.882), Li (−0.996), Bi (−1.00), Th (−0.945) |
| Correlation with Aluminosilicate | ||
| rSi-Al > 0.7 | MnO, Fe2O3, MgO, CaO, Zn, Cr, As, TiO2, Na2O, Ni, In, K2O, Rb, Cu, V | |
| rSi-Al 0.5–0.69 | W, Cs | |
| rSi-Al 0.35–0.49 | Ba, Ta, Tl | |
| Correlation with Carbonate | ||
| rCa > 0.7 | MgO (0.999), Cr (0.995), SiO2 (0.980), MnO (0.971), Na2O (0.949), Fe2O3 (0.945), Ni (0.920), As (0.909), Zn (0.887), TiO2 (0.852), Al2O3 (0.835) | |
| rCa 0.5–0.69 | In (0.668), Ba (0.628), K2O (0.557) | |
| Correlation with Phosphate | ||
| rp > 0.5 | Sr (0.996), Ba (0.958), Na2O (0.646) | |
| Correlation Coefficient between Selected Elements | ||
| SiO2-Al2O3 = 0.820, Na2O-K2O = 0.659, MnO-Fe2O3 = 0.994, TiO2-SiO2 = 0.881, Co-K2O = 0.709, Rb- K2O = 0.977, Sr-Ba = 0.936, Zr-Hf = 0.972, Li-Se = 0.947, V-Cu = 0.966, Co-Cs = 0.972, Cd-Nb = 0.969, Pb-Hf = 0.970, Li-Bi = 0.995 | ||
| Mineral | O | Si | Al | Fe | Ca | Mg | K | P | Sr | Sb | Zr | Nb | Se | W | Pb | Mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaolinite (n = 19) | Min | 15.36 | 4.04 | 2.60 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 58.04 | 38.45 | 28.74 | bdl | 9.30 | bdl | 2.24 | bdl | 23.46 | 3.54 | 12.80 | bdl | bdl | bdl | bdl | bdl | |
| Average | 44.43 | 18.33 | 18.87 | bdl | 1.11 | bdl | 0.12 | bdl | 3.13 | 0.19 | 0.67 | bdl | bdl | bdl | bdl | bdl | |
| Illite (n = 3) | Min | 44.52 | 19.47 | 20.79 | bdl | bdl | bdl | 2.01 | bdl | 6.34 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 50.24 | 24.44 | 21.94 | bdl | bdl | bdl | 2.56 | bdl | 6.87 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
| Average | 47.02 | 22.60 | 21.45 | bdl | bdl | bdl | 2.33 | bdl | 6.60 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
| Dolomite (n = 3) | Min | 46.75 | bdl | bdl | bdl | 18.47 | 11.76 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 57.62 | bdl | bdl | bdl | 27.20 | 15.38 | bdl | bdl | bdl | 10.38 | bdl | bdl | bdl | bdl | bdl | bdl | |
| Average | 51.33 | bdl | bdl | bdl | 21.50 | 13.23 | bdl | bdl | bdl | 5.85 | bdl | bdl | bdl | bdl | bdl | bdl | |
| Ankerite (n = 14) | Min | 18.16 | bdl | bdl | 3.10 | 3.51 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 59.37 | bdl | 9.69 | 29.54 | 28.00 | 15.86 | bdl | bdl | bdl | 14.88 | 1.94 | 2.60 | 5.32 | 4.33 | 0.30 | 42.02 | |
| Average | 45.82 | bdl | 0.94 | 8.85 | 19.02 | 9.83 | bdl | bdl | bdl | 2.39 | 0.14 | 0.37 | 0.38 | 0.31 | 0.02 | 6.47 | |
| Quartz (n = 5) | Min | 53.13 | 43.95 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 56.05 | 46.87 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
| Average | 54.65 | 45.35 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
| Goyazite (n = 2) | Min | 27.80 | 3.57 | 3.82 | bdl | bdl | bdl | bdl | 3.34 | 2.73 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Max | 30.25 | 4.10 | 5.87 | bdl | bdl | bdl | bdl | 4.60 | 3.86 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
| Average | 29.03 | 3.84 | 4.85 | bdl | bdl | bdl | bdl | 3.97 | 3.30 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Ma, H.; Saalidong, B.M.; Li, K. Petrography, Mineralogy, and Geochemistry of Thermally Altered Coal in the Tashan Coal Mine, Datong Coalfield, China. Minerals 2021, 11, 1024. https://doi.org/10.3390/min11091024
Song X, Ma H, Saalidong BM, Li K. Petrography, Mineralogy, and Geochemistry of Thermally Altered Coal in the Tashan Coal Mine, Datong Coalfield, China. Minerals. 2021; 11(9):1024. https://doi.org/10.3390/min11091024
Chicago/Turabian StyleSong, Xiaoxia, Hongtao Ma, Benjamin M. Saalidong, and Kaijie Li. 2021. "Petrography, Mineralogy, and Geochemistry of Thermally Altered Coal in the Tashan Coal Mine, Datong Coalfield, China" Minerals 11, no. 9: 1024. https://doi.org/10.3390/min11091024






