Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment
Abstract
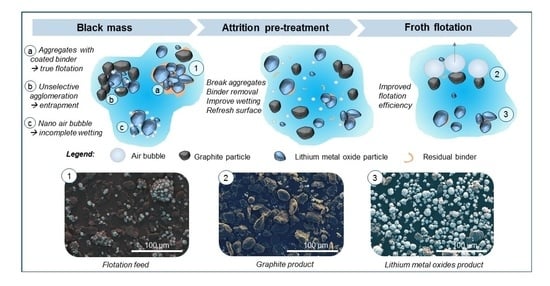
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Froth Flotation
- No attrition pre-treatment (I);
- Attrition pre-treatment (II); and
- Attrition pre-treatment with kerosene addition (III).
2.3. Characterization
3. Results and Discussion
3.1. Flotation Trends of Spent Black Mass
3.2. Possible Flotation Mechanisms as Result of Black Mass Pre-Treatment
3.2.1. Effect of Attrition on the PSD
3.2.2. Effect of Residual Binder on LMO Recovery
3.2.3. Black Mass and Particle Entrainment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jenu, S.; Deviatkin, I.; Hentunen, A.; Myllysilta, M.; Viik, S.; Pihlatie, M. Reducing the climate change impacts of lithium-ion batteries by their cautious management through integration of stress factors and life cycle assessment. J. Energy Storage 2020, 27, 101023. [Google Scholar] [CrossRef]
- Global Battery Alliance. A Vision for a Sustainable Battery Value Chain in 2030; Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation; Report from World Economic Forum: Geneva, Switzerland, 2019. [Google Scholar]
- Moradi, B.; Botte, G.G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 2016, 46, 123–148. [Google Scholar] [CrossRef]
- Porvali, A.; Aaltonen, M.; Ojanen, S.; Velazquez-Martinez, O.; Eronen, E.; Liu, F.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour. Conserv. Recycl. 2019, 142, 257–266. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [Green Version]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef] [Green Version]
- Halleux, V. New EU Regulatory Framework for Batteries—Setting Sustainability Requirements; European Parliamentary Research Service: Brussels, Belgium, 2021. [Google Scholar]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent deveelopments including silicon (oxide) composites. Sustain. Energy and Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Gaines, L.; Sullivan, J.L.; Burnham, A. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling. In Proceedings of the 90th Annual Meeting of the Transportation Research Board, Washington, DC, USA, 23–27 January 2011. [Google Scholar]
- Blengini, G.A.; Nuss, P.; Dewulf, J.; Nita, V.; Peirò, L.T.; Vidal-Legaz, B.; Latunussa, C.; Mancini, L.; Blagoeva, D.; Pennington, D.; et al. EU methodology for critical raw materials assessment: Policy needs and proposed solutions for incremental improvements. Resour. Policy 2017, 53, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Damm, S.; Qizhong, Z. Supply and Demand of Natural Graphite—DERA Rohstoffinformationen; Federal Institute for Geosciences and Natural Resources (BGR), German Mineral Resources Agency (DERA): Berlin, Germany, 2021. [Google Scholar]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling chain for spent lithium-ion batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- Kim, Y.; Matsuda, M.; Shibayama, A.; Fujita, T. Recovery of LiCoO2 from Wasted Lithium Ion Batteries by using Mineral Processing Technology. Resour. Process. 2003, 51, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Zhan, R.; Oldenburg, Z.; Pan, L. Recovery of active cathode materials from lithium-ion batteries using froth flotation. Sustain. Mater. Technol. 2018, 17, e00062. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A sustainable process for the recovery of anode and cathode materials derived from spent lithium-ion batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Zhan, R.; Dhindsa, K.S.; Pan, L.; Han, T. Electrochemical Performance of Recycled Cathode Active Materials Using Froth Flotation-based Separation Process. J. Electrochem. Soc. 2020, 167, 020504. [Google Scholar] [CrossRef]
- Gaines, L.; Dai, Q.; Vaughey, J.T.; Gillard, S. Direct recycling R&D at the recell center. Recycling 2021, 6, 31. [Google Scholar]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- De Meatza, I.; Bengoechea, M.; Eguia-Barrio, I.; Tedjar, F.; Cognard, J.; Vieira Carvalho, D.; Moretti, A.; Passerini, S. Enhancing lithium-ion battery recycling: Evaluation of graphite and carbon recovered from aged cells for the production of ‘new’ negative electrodes. In Proceedings of the 7th Transport Research Arena TRA 2018, Vienna, Austria, 16–19 April 2018. [Google Scholar]
- Wills, B.A.; Finch, J.A. Chapter 12—Froth Flotation. In Wills’ Mineral Processing Technology, 8th ed.; Wills, B.A., Finch, J.A., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 265–380. [Google Scholar]
- Chelgani, S.C.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A Review of Graphite Beneficiation Techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Pur. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Li, H.; Xie, W.; Zhang, T. Effect of the secondary product of semi-solid phase Fenton on the flotability of electrode material from spent lithium-ion battery. Powder Technol. 2017, 315, 139–146. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.; Zhang, J.; Zhang, J.; Xing, J.; Hu, D.; Peng, Y.; Zhou, J.; Liu, Q.; Gu, H.; et al. Understanding and application of an electroplating sludge-derived catalyst with an active texture for improved NO reduction. Sci. Total Environ. 2018, 631–632, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, L.; Yuan, X.; He, Y.; Wang, H.; He, J. Recycling of electrode materials from spent lithium-ion battery by pyrolysis-assisted flotation. J. Environ. Chem. Eng. 2021, 9, 106777. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K.; Bradshaw, D. A Review of Entrainment: Mechanisms, Contributing Factors and Modelling in Flotation. Miner. Eng. 2015, 70, 77–91. [Google Scholar] [CrossRef]
- Smith, P.G.; Warren, L.J. Entrainment of Particles into Flotation Froths. Miner. Process. Extr. Met. Rev. 2007, 5, 123–145. [Google Scholar] [CrossRef]
- Kursun, H. Effect of fine particles’ entrainment on conventional and column flotation. Part. Sci. Technol. 2014, 32, 251–256. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Wang, D.; Han, H.; Fu, Y.; Sun, H.; Chu, F.; Yao, J. A new model for the degree of entrainment in froth flotation based on mineral particle characteristics. Powder Technol. 2019, 354, 358–368. [Google Scholar] [CrossRef]
- Hoang, D.H.; Kupka, N.; Peuker, U.A.; Rudolph, M. Flotation study of fine grained carbonaceous sedimentary apatite ore – Challenges in process mineralogy and impact of hydrodynamics. Miner. Eng. 2018, 121, 196–204. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. Effect of high intensity conditioning on the flotation of a nickel ore. Part 1: Size-by-size analysis. Miner. Eng. 1999, 12, 1185–1200. [Google Scholar] [CrossRef]
- Lu, X.; Forssberg, E. Technical note flotation selectivity and upgrading of Woxna fine graphite concentrate. Miner. Eng. 2001, 14, 1541–1543. [Google Scholar] [CrossRef]
- Sun, W.; Deng, M.J.; Hu, Y.H. Fine particle aggregating and flotation behavior induced by high intensity conditioning of a CO2 saturation slurry. Min. Sci. Technol. 2009, 19, 483–488. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, G.; Peng, Y.; Chen, Y.; Ma, G. How Does High Intensity Conditioning Affect Flotation Performance? Int. J. Coal Prep. Util. 2019, 39, 302–316. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Shao, H.; Hu, Y.; Alheshibri, M.; Li, B.; Ni, C.; Peng, Y.; Xie, G. Effects of emulsified kerosene nanodroplets on the entrainment of gangue materials and selectivity index in aphanitic graphite flotation. Miner. Eng. 2020, 158, 106592. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dańczak, A.; Taskinen, P.; Serna-Guerrero, R.; Jokilaakso, A. Integrating Flotation and Pyrometallurgy for Recovering Graphite and Valuable Metals from Battery Scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Gugala, E.; Blannin, R.; Bachmann, K.; Serna-Guerrero, R.; Rudolph, M. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 2021, 169, 106924. [Google Scholar] [CrossRef]
- Johnson, N.W. The Flotation Behaviour of Some Chalcopyrite Ores. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1972. [Google Scholar]
- Feng, D.; Aldrich, C. Effect of preconditioning on the flotation of coal. Chem. Eng. Commun. 2005, 192, 972–983. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Li, D.; Zhang, C.; Yan, X.; Wang, L.; Zhang, H. Enhancement of coal flotation using impact flow conditioning pulp. J. Clean. Prod. 2020, 267, 122124. [Google Scholar] [CrossRef]
- Sheng, Y.; Fell, C.R.; Son, Y.K.; Metz, B.M.; Jiang, J.; Church, C.B. Effect of Calendering on Electrode Wettability in Lithium-Ion Batteries. Front. Energy Res. 2014, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, S.; Liu, N.; Chen, Y.; Xi, Y.; Li, S.; Jie, Y.; Hu, F. Study on Vacuum Pyrolysis Process of Cathode Sheets from Spent Lithium-ion Batteries. In Minerals, Metals and Materials Series; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 421–435. [Google Scholar]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Widijatmoko, S.D.; Gu, F.; Wang, Z.; Hall, P. Selective liberation in dry milled spent lithium-ion batteries. Sustain. Mater. Technol. 2020, 23, e00134. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Frey, C. Processing of Natural Flake Graphite for Lithium-Ion-Batteries. In Proceedings of the EMPRC 2018, Essen, Germany, 15–26 June 2018; pp. 203–210. [Google Scholar]
- Mundszinger, M.; Farsi, S.; Rapp, M.; Golla-Schindler, U.; Kaiser, U.; Wachtler, M. Morphology and texture of spheroidized natural and synthetic graphites. Carbon N. Y. 2017, 111, 764–773. [Google Scholar] [CrossRef]
- Zhan, R.; Payne, T.; Leftwich, T.; Perrine, K.; Pan, L. De-agglomeration of cathode composites for direct recycling of Li-ion batteries. Waste Manag. 2020, 105, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-Ultrasonic-Assisted Flotation Technology for Recovering Graphite and LiCoO2 from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K. Entrainment in froth flotation: The degree of entrainment and its contributing factors. Powder Technol. 2015, 288, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Lu, S. Theory and Applications of Hydrophobic Flocculation Technology. In Developments in Mineral Processing, Proceedings of the XXI International Mineral Processing Congress, Rome, Italy, 23–27 July 2000; Massacci, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 13, pp. C5-31–C5-38. [Google Scholar]
- Guan, W.; Sha, J.; Liu, P.; Peng, Y.; Xie, G. Effect of stirring time on oil agglomeration of fine coal. J. South. Afr. Inst. Min. Metall. 2018, 118, 89–94. [Google Scholar] [CrossRef]










| Size (µm) | Model Black Mass | Spent Black Mass | ||
|---|---|---|---|---|
| Without Pre-Treatment | After Pre-Treatment | Without Pre-Treatment | After Pre-Treatment | |
| D10 | 10.7 ± 0.6 | 10.8 ± 0.6 | 2.7 ± 0.1 | 2.1 ± 0.1 |
| D50 | 18.0 ± 0.2 | 17.9 ± 0.2 | 16.9 ± 0.1 | 14.1 ± 0.3 |
| D90 | 27.1 ± 0.6 | 26.9 ± 0.1 | 53.1 ± 1.3 | 45.9 ± 1.5 |
| Conditioning | No Attrition (I) | Attrition (II) | Attrition with Kerosene (III) | |||
|---|---|---|---|---|---|---|
| Spent Black mass | ||||||
| Cumulative time (s) | R/W LMOs | ENT Tracer | R/W LMOs | ENT Tracer | R/W LMOs | ENT Tracer |
| 18 | 3.30 | 0.71 | 1.86 | 0.59 | 2.34 | 1.22 |
| 36 | 3.15 | 0.69 | 2.42 | 0.73 | 2.25 | 1.01 |
| 54 | 2.99 | 0.64 | 2.28 | 0.67 | 2.25 | 0.93 |
| 90 | 3.00 | 0.64 | 2.15 | 0.60 | 2.25 | 0.86 |
| 390 | 2.44 | 0.55 | 1.47 | 0.46 | 1.84 | 0.67 |
| Model Black mass | ||||||
| Cumulative time (s) | R/W LMOs | ENT Tracer | R/W LMOs | ENT Tracer | R/W LMOs | ENT Tracer |
| 18 | 1.10 | 0.47 | 0.61 | 0.51 | 0.90 | 0.80 |
| 36 | 1.01 | 0.42 | 0.53 | 0.46 | 0.83 | 0.74 |
| 54 | 0.99 | 0.41 | 0.51 | 0.44 | 0.78 | 0.70 |
| 90 | 0.95 | 0.39 | 0.46 | 0.42 | 0.74 | 0.68 |
| 390 | 0.67 | 0.29 | 0.30 | 0.28 | 0.58 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderbruggen, A.; Salces, A.; Ferreira, A.; Rudolph, M.; Serna-Guerrero, R. Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment. Minerals 2022, 12, 72. https://doi.org/10.3390/min12010072
Vanderbruggen A, Salces A, Ferreira A, Rudolph M, Serna-Guerrero R. Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment. Minerals. 2022; 12(1):72. https://doi.org/10.3390/min12010072
Chicago/Turabian StyleVanderbruggen, Anna, Aliza Salces, Alexandra Ferreira, Martin Rudolph, and Rodrigo Serna-Guerrero. 2022. "Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment" Minerals 12, no. 1: 72. https://doi.org/10.3390/min12010072