A Review on Froth Washing in Flotation
Abstract
:1. Introduction
1.1. Methods That Alter Froth Residence Time
1.2. Methods That Reduce Turbulence in the Pulp
1.3. Froth Washing
2. Froth Phase Sub-Processes
2.1. Brief Overview of Froth Behavior
2.2. Transporting Gangue Minerals into the Froth
2.3. Managing Entrainment Recovery
2.4. Froth Washing
2.4.1. Mechanism of Froth Washing
2.4.2. Impact of Froth Washing on Flotation Performance
2.4.3. Impact on Grade
2.4.4. Impact on Recovery
2.4.5. Impact on Froth Mobility and Stability
2.5. Factors That Affect the Efficiency of Froth Washing
2.5.1. Impact of Wash-Water Quality
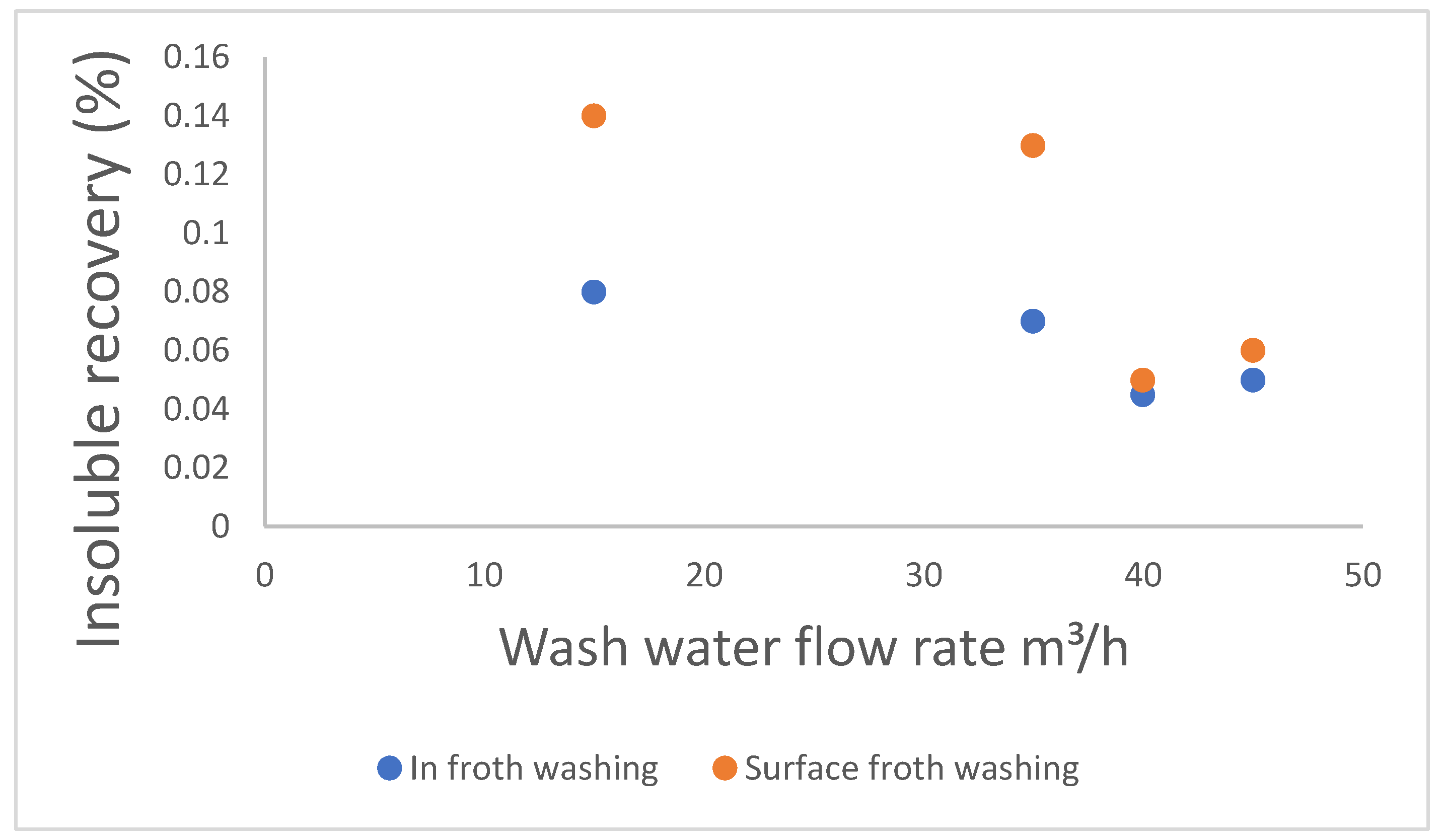
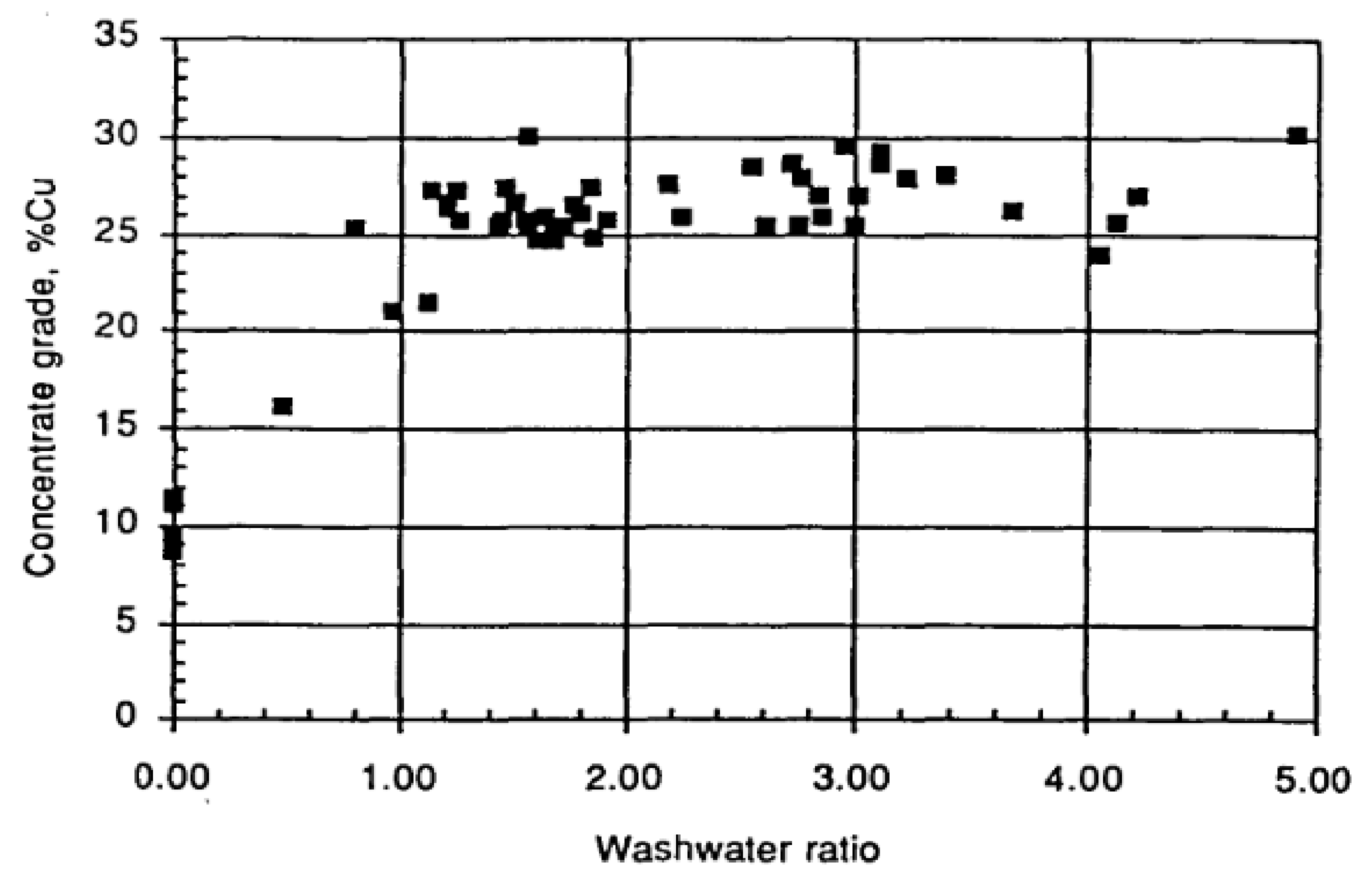
2.5.2. Impact of Wash-Water Rate
2.5.3. Impact of the Type of Froth Washing, Mechanism of Delivery and Area Covered by Wash Water
3. Wash-Water Distribution Systems
3.1. Surface Froth Washing Distributors
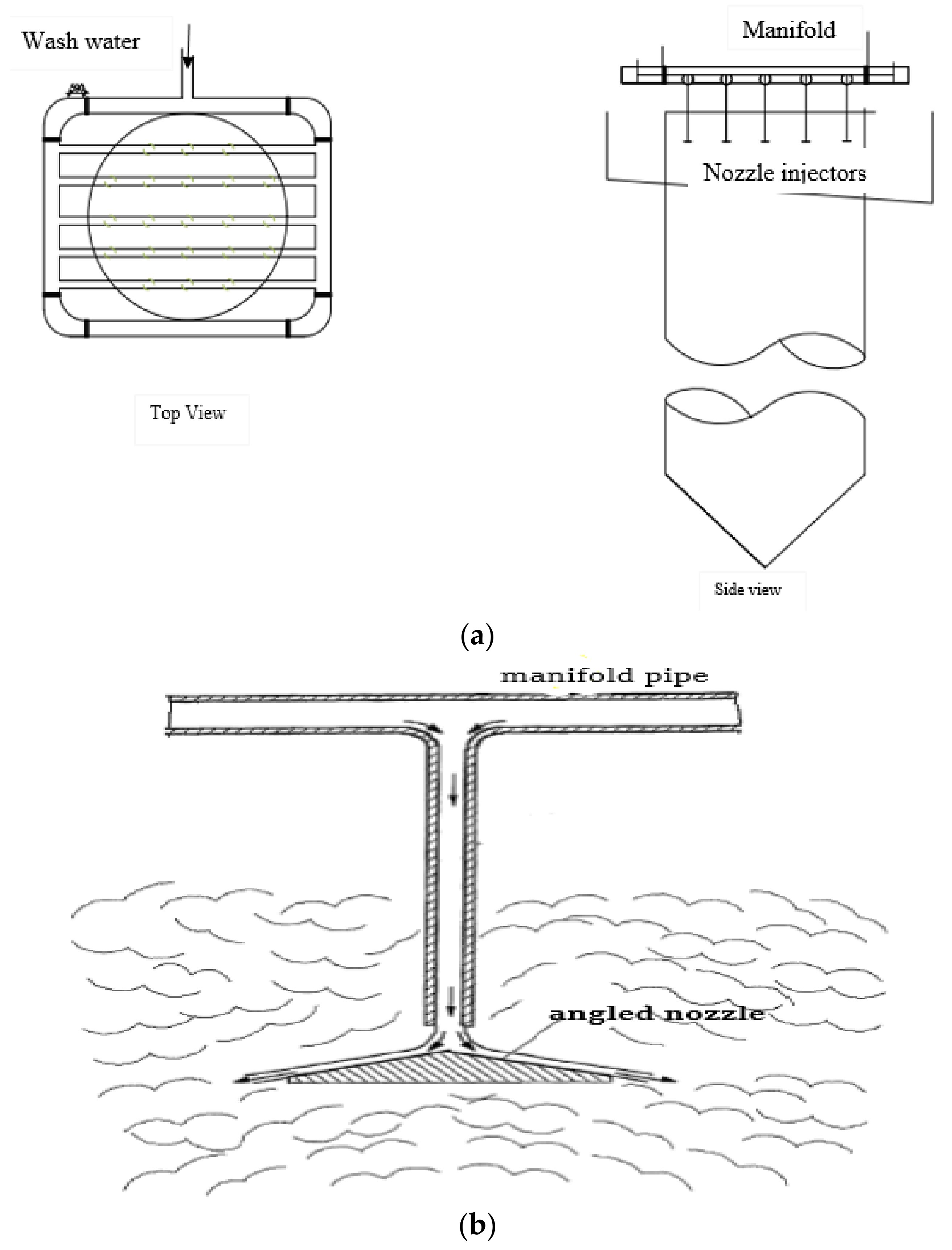
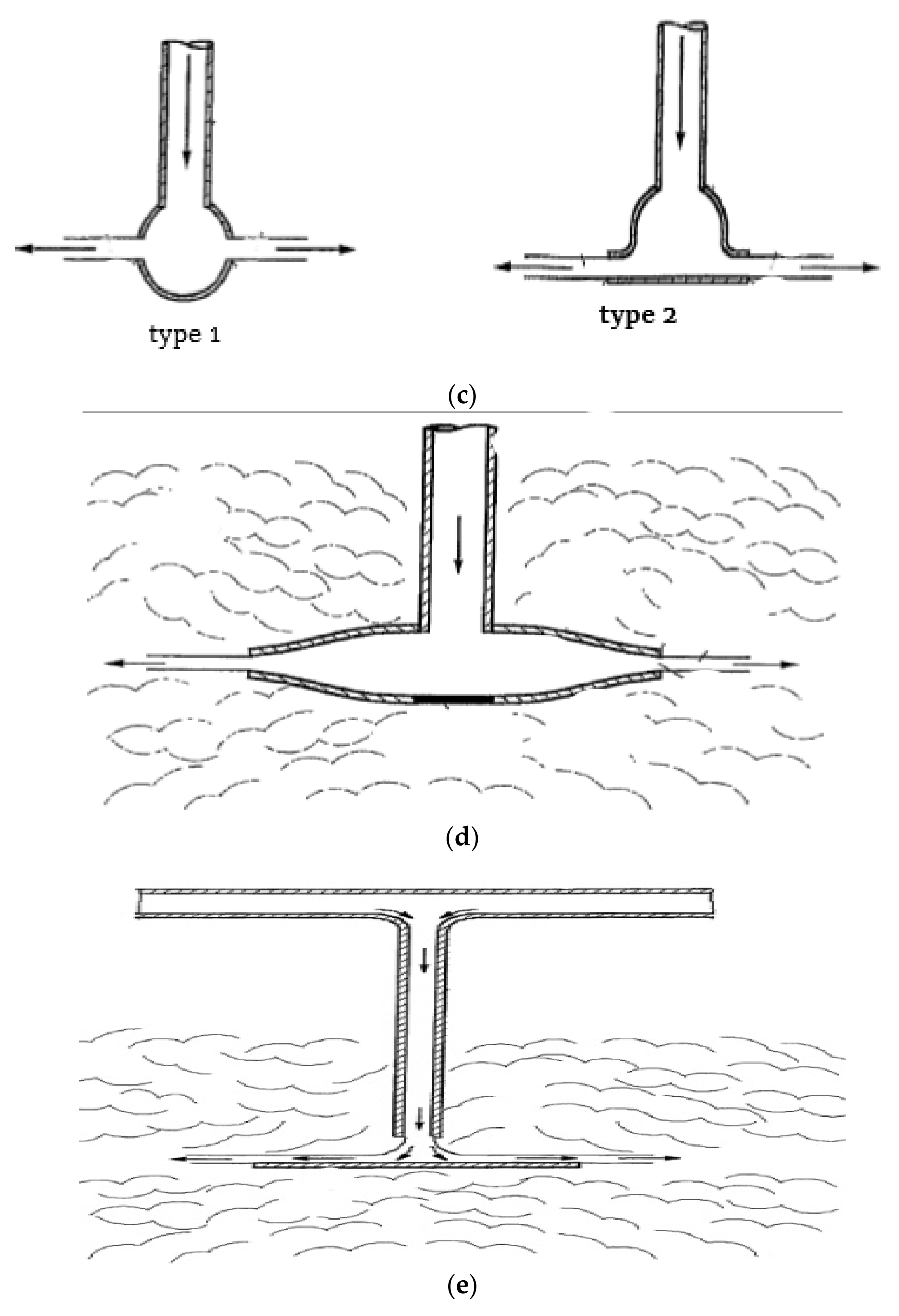
3.1.1. Wash-Water Distributor Nozzles
3.1.2. Showers and Perforated Pipes
3.1.3. Wash-Water Boxes and Wash-Water Trays
3.1.4. Lip Washing
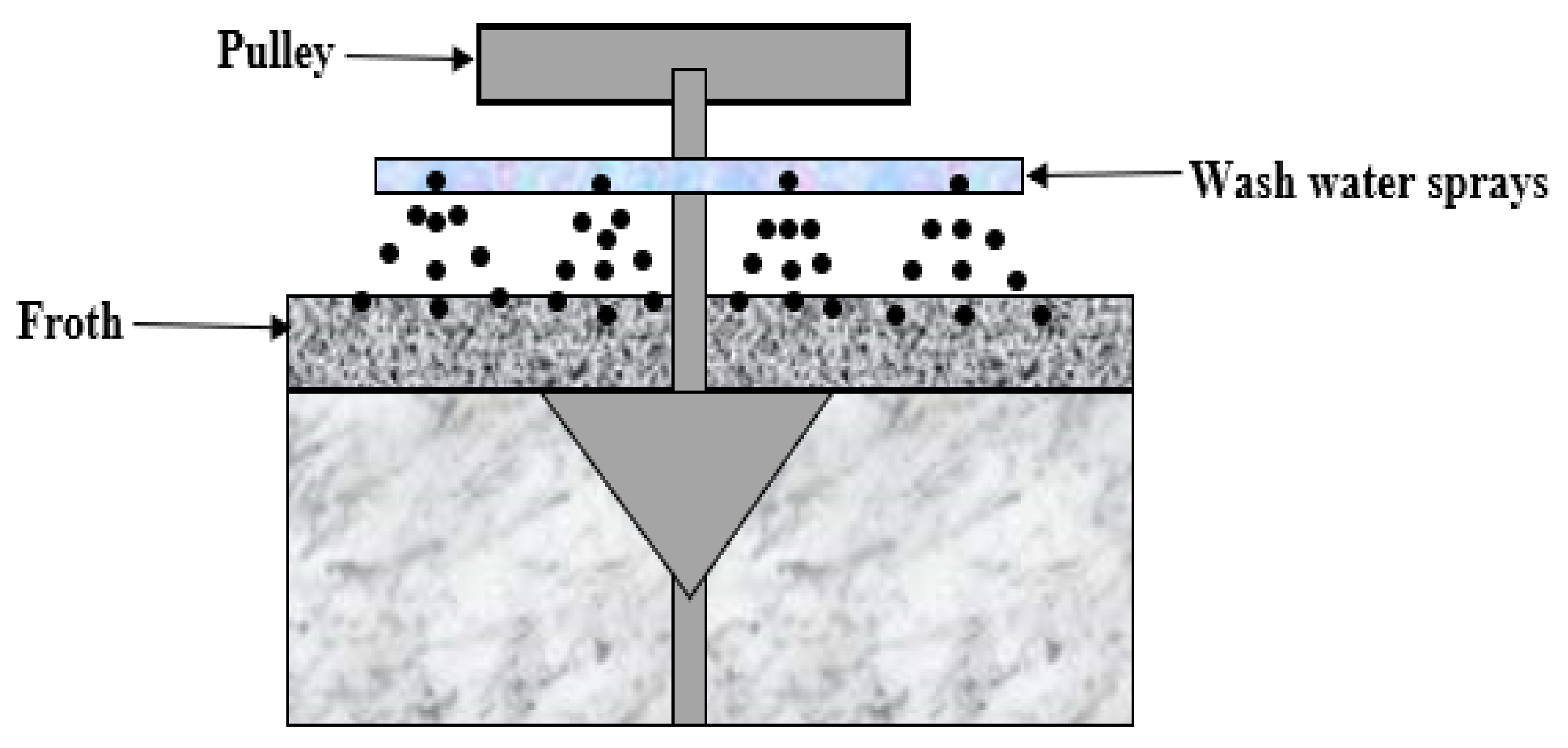
3.2. In-Froth Washing Distributors
Plunging and Submerged Nozzles
3.3. Under-Froth Washing
3.4. Industrial Applications of Froth Washing
3.4.1. Froth Washing in Column Cells
3.4.2. Froth Washing in Mechanical Cells
3.4.3. Froth Washing in Jameson Cells
3.5. Conclusion on Froth Washing
3.5.1. Froth Washing Description
3.5.2. Applications in Industry, including Flotation Cell Types
3.5.3. Impact on Fundamentals That Govern Froth Phase Sub-Processes, Including Contradictions
3.5.4. Wash-Water Quality and Application Rates, Areas
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wills, B.A.; Napier-Munn, T.J. An introduction to the practical aspects of ore treatment and mineral recovery. In Wills’ Mineral Processing Technology; Elsevier Science & Technology Books: London, UK, 2006; pp. 267–352. [Google Scholar]
- Finch, J. Column Flotation; Pergamon Press plc: South Croydon, UK, 1991; p. 180. [Google Scholar]
- Ata, S. Phenomena in the froth phase of flotation—A review. Int. J. Miner. Process. 2012, 102, 1–12. [Google Scholar] [CrossRef]
- Jowett, A. Flotation kinetics. Gangue mineral contamination of froth. Brit. Chem. Eng. 1966, 11, 330–333. [Google Scholar]
- Pease, J.D.; Curry, D.C.; Young, M.F. Designing flotation circuits for high fines recovery. Miner. Eng. 2006, 19, 831–840. [Google Scholar] [CrossRef]
- Moys, M.H. A study of a plug-flow model for flotation froth behaviour. Int. J. Miner. Process. 1978, 5, 21–38. [Google Scholar] [CrossRef]
- Yianatos, J.B.; Finch, J.A.; Laplante, A.R. Apparent hindered settling in a gas-liquid-solid counter current column. Int. J. Miner. Process. 1986, 18, 155–165. [Google Scholar] [CrossRef]
- Smith, P.G.; Warren, L.J. Entrainment of particles into flotation froths. Miner. Process. Extr. Metall. Rev. 1989, 5, 123–145. [Google Scholar] [CrossRef]
- Gaudin, A.M. Flotation; McGraw-Hill: New York, NY, USA, 1957. [Google Scholar]
- Cutting, G.W.; Devenish, M. A steady-state model of froth flotation structures. In Proceedings of the AIME Annual Meeting, New York, NY, USA, 24 February 1975; Volume 20. [Google Scholar]
- Engelbrecht, J.A.; Woodburn, E.T. The effect of froth height, aeration rate and gas precipitation on flotation. S. Afr. Inst. Min. Metall. 1975, 76, 125–132. [Google Scholar]
- Moys, M.H. A Study of Processes Occurring in Flotation Froths. Ph.D. Thesis, University of Natal, Durban, South Africa, 1979. [Google Scholar]
- Cutting, G.W.; Watson, D.; Whitehead, A.; Barber, S.P. Froth structure in continuous flotation cells: Relation to the prediction of plant performance from laboratory data using process models. Int. J. Miner. Process. 1981, 7, 347–369. [Google Scholar] [CrossRef]
- Hanumanth, G.S.; Williams, D.J. An experimental study of the effects of froth height on the flotation of China clay. Powder Technol. 1990, 60, 131–144. [Google Scholar] [CrossRef]
- Zheng, X.; Johnson, N.W.; Franzidis, J.P. Modelling of entrainment in industrial flotation cells: Water recovery and degree of entrainment. Miner. Eng. 2006, 19, 1191–1203. [Google Scholar] [CrossRef]
- Harris, A.; Venkatesan, L.; Greyling, M. A practical approach to plant-scale flotation optimization. J. S. Afr. Inst. Min. Metall. 2013, 113, 263–272. [Google Scholar]
- Wang, L. Entrainment of Fine Particles in Froth Flotation. Ph.D. Thesis, The University of Queensland, Sustainable Mineral Institute, St. Lucia, Australia, 2017. [Google Scholar]
- Wang, L.; Xing, Y.; Wang, J. Mechanism of the combined effects of air rate and froth depth on entrainment factor in copper flotation. Physicochem. Probl. Miner. Process. 2020, 56, 43–53. [Google Scholar] [CrossRef]
- Nelson, M.G.; Lelinski, D. Hydrodynamic design of self-aerating flotation machines. Miner. Eng. 2000, 13, 991–998. [Google Scholar] [CrossRef]
- Nguyen, A.; Schulze, H.J. Colloidal Science of Flotation; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Bhondayi, C. A Study of Flotation Froth Phase Behaviour. Ph.D. Thesis, University of the Witwatersrand, Faculty of Engineering and the Built Environment, School of Chemical and Metallurgical Engineering, Johannesburg, South Africa, 2014. [Google Scholar]
- Gorain, B.K.; Franzidis, J.P.; Manlapig, E.V. Flotation Cell Design: Application of Fundamental Principles; Julius Kruttschnitt Mineral Research Centre, Indooroopilly: Queensland, Australia, 2000. [Google Scholar] [CrossRef]
- Schubert, H. On the turbulence-controlled micro-processes in flotation machines. Int. J. Miner. Process. 1999, 56, 257–276. [Google Scholar] [CrossRef]
- Razmjooei, S.; Abdollahy, M.; Khalesi, M.R.; Mohseni, M. The effect of cross section of mechanical flotation cells on the height of turbulent and quiescent zones. Int. J. Min. Sci. 2017, 3, 89–92. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Eisele, T.C. Use of horizontal baffles to reduce axial mixing in coal flotation columns. In Proceedings of the 12th International Coal Preparation Congress, Cracow, Poland, 23–27 May 1994; pp. 1241–1249. [Google Scholar]
- Cilek, E.C. The effect of hydrodynamic conditions on true flotation and entrainment in flotation of a complex sulphide ore. Int. J. Miner. Process. 2009, 90, 35–44. [Google Scholar] [CrossRef]
- Finch, J.A.; Yianatos, J.; Dobby, G. Column froths. Miner. Process. Extr. Metall. Rev. 1989, 5, 281–305. [Google Scholar] [CrossRef]
- Kaya, M. Froth Washing in Mechanical Flotation Cells. Ph.D. Thesis, McGill University, Montréal, QC, Canada, 1989. [Google Scholar]
- McKeon, T.J. An In-Plant Evaluation of Froth Washing on Conventional Flotation Cells for Coal. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2001. [Google Scholar]
- Jameson, G.J. Method and Apparatus for Froth Washing in Flotation. U.S. Patent 7,770,736, 10 August 2010. [Google Scholar]
- Ata, S. The role of frother on the detachment of particles from bubbles. Miner. Eng. 2011, 24, 476–478. [Google Scholar] [CrossRef]
- Bennie, D.I. An Investigation of Froth Effects in Scavenging Flotation of Platinum from UG-2 ore. Ph.D. Thesis, The University of KwaZulu-Natal, Durban, South Africa, 2013. [Google Scholar]
- Dobby, G.S.; Kosick, A. Underfroth Washing. U.S. Patent US 2017/0215756A1, 24 May 2017. [Google Scholar]
- Evans, G.M.; Atkinson, B. The Jameson Cell. In Flotation Science and Engineering; Matis, K.A., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 331–363. [Google Scholar]
- Laskowski, J. Flotation machines. Dev. Miner. Process. 2001, 14, 225–262. [Google Scholar] [CrossRef]
- Sripada, S.R.; Ahmed, N.; Jameson, G.J. Froth washing in flotation. In Proceedings of the Chemeca 89, 17th Australian Conference on Chemical Engineering, Gold Coast, QLD, Australia, 1 January 1989. [Google Scholar]
- Mao, W.P.; Sripada, S.R.; Ahmed, N.; Jameson, G.J. Froth washing in the flotation of coal. In Proceedings of the 19th Australasian Chemical Engineering Conference (Chemeca 91), Newcastle, Australia, 18–20 September 1991. [Google Scholar]
- Ireland, P.; Cunningham, R.; Jameson, G.J. The behaviour of wash water injected into a froth. Int. J. Miner. Process. 2007, 84, 99–107. [Google Scholar] [CrossRef]
- Parga, J.R.; Valenzuela, J.L.; Aguayo, S. Bacís flotation cell for gold-and silver-beard pyrite recovery. Min. Metall. Explor. 2009, 26, 25–29. [Google Scholar] [CrossRef]
- Clean Process Technologies. CleanProTech. 2014. Available online: http://www.cleanprotech.com.au/washwater.html (accessed on 22 September 2021).
- Klassen, V.I.; Mokrousov, V.A. An Introduction to the Theory of Flotation; Butterworth: London, UK, 1963. [Google Scholar]
- Cilliers, J. Understanding froth behaviour with CFD. In Proceedings of the Fifth International Conference on CFD in the Process Industries, CSIRO, Melbourne, Australia, 13 December 2006; pp. 13–15. [Google Scholar]
- Kennedy, D.L. Redesign of Industrial Column Flotation Circuits Based on a Simple Residence Time Distribution Model. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2008. [Google Scholar]
- Yianatos, J.B.; Bergh, L.G. Troubleshooting industrial flotation columns. Miner. Eng. 1995, 8, 1593–1605. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier Limited: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- King, R.P. Modeling and Simulation of Mineral Processing Systems; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- Waters, K.E.; Rowson, N.A.; Fan, X.; Parker, D.J.; Cilliers, J.J. Positron emission particle tracking as a method to map the movement of particles in the pulp and froth phases. Miner. Eng. 2008, 21, 877–882. [Google Scholar] [CrossRef]
- Ata, S.; Ahmed, N.; Jameson, G.J. A study of bubble coalescence in flotation froths. Int. J. Miner. Process. 2003, 72, 255–266. [Google Scholar] [CrossRef]
- Ata, S. The detachment of particles from coalescing bubble pairs. J. Colloid Interface Sci. 2009, 338, 558–565. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Ata, S. On the detachment of hydrophobic particles from the froth phase. Miner. Eng. 2016, 95, 113–115. [Google Scholar] [CrossRef]
- Espinosa-Gomez, R.; Finch, J.A.; Yianatos, J.B.; Dobby, G.S. Flotation column carrying capacity: Particle size and density effects. Miner. Eng. 1988, 1, 77–79. [Google Scholar] [CrossRef]
- Johansson, G.; Pugh, R.J. The influence of particle size and hydrophobicity on the stability of mineralized froths. Int. J. Miner. Process. 1992, 34, 1–21. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef]
- Moolman, D.W.; Eksteen, J.J.; Aldrich, C.; Van Deventer, J.S. The significance of flotation froth appearance for machine vision control. Int. J. Miner. Process. 1996, 48, 135–158. [Google Scholar] [CrossRef]
- Wright, B.A. The Development of a Vision-Based Flotation Froth Analysis System. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 1999. [Google Scholar]
- Neethling, S.J.; Cilliers, J.J. The entrainment of gangue into a flotation froth. Int. J. Miner. Process. 2002, 64, 123–134. [Google Scholar] [CrossRef]
- Cutting, G.W.; Barber, S.P.; Newton, S. Effects of froth structure and mobility on the performance and simulation of continuously operated flotation cells. Int. J. Miner. Process. 1986, 16, 43–61. [Google Scholar] [CrossRef]
- Farrokhpay, S. The importance of rheology in mineral flotation: A review. Miner. Eng. 2012, 36, 272–278. [Google Scholar] [CrossRef]
- Shi, F.N.; Zheng, X.F. The rheology of flotation froths. Int. J. Miner. Process. 2003, 69, 115–128. [Google Scholar] [CrossRef]
- Hemmings, C.E. On the Significance of Flotation Froth Liquid Lamella Thickness; Institution of Mining and Metallurgy Transactions: Leeds, UK, 1981; p. 90. [Google Scholar]
- Bascur, O.A.; Herbst, J.A. Dynamic modelling of a flotation cell with a view toward automatic control. In CIM Bulletin; Canadian Inst. Mining Metallurgy Petroleum: Toronto, ON, Canada, 1982; Volume 75, p. 76. [Google Scholar]
- Yianatos, J.B.; Finch, J.A.; Laplante, A.R. Selectivity in column flotation froths. Int. J. Miner. Process. 1988, 23, 279–292. [Google Scholar] [CrossRef]
- Gorain, B.K.; Harris, M.C.; Franzidis, J.P.; Manlapig, E.V. The effect of froth residence time on the kinetics of flotation. Miner. Eng. 1988, 11, 627–638. [Google Scholar] [CrossRef]
- Seaman, D.R.; Manlapig, E.V.; Franzidis, J.P. Selective transport of attached particles across the pulp–froth interface. Miner. Eng. 2006, 19, 841–851. [Google Scholar] [CrossRef]
- Gong, J.; Peng, Y.; Bouajila, A.; Ourriban, M.; Yeung, A.; Liu, Q. Reducing quartz gangue entrainment in sulphide ore flotation by high molecular weight polyethylene oxide. Int. J. Miner. Process. 2010, 97, 44–51. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K. Entrainment in froth flotation: The degree of entrainment and its contributing factors. Powder Technol. 2016, 288, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Valenta, M.M.; Mapheto, H. Application of fundamentals in optimizing platinum concentrator performance. J. South. Afr. Inst. Min. Metall. 2011, 111, 93–99. [Google Scholar]
- Popli, K.; Afacan, A.; Liu, Q.; Prasad, V. Real-time monitoring of entrainment using fundamental models and froth images. Miner. Eng. 2018, 124, 44–62. [Google Scholar] [CrossRef]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Laplante, A.R.; Kaya, M.; Smith, H.W. The effect of froth on flotation kinetics—A mass transfer approach. Miner. Process. Extr. Metall. Rev. 1989, 5, 147–168. [Google Scholar] [CrossRef]
- Ross, V.E. Particle-bubble attachment in flotation froths. Miner. Eng. 1997, 10, 695–706. [Google Scholar] [CrossRef]
- Savassi, O.N.; Alexander, D.J.; Franzidis, J.P.; Manlapig, E.V. An empirical model for entrainment in industrial flotation plants. Miner. Eng. 1998, 11, 243–256. [Google Scholar] [CrossRef]
- Savassi, O.N. A compartment model for the mass transfer inside a conventional flotation cell. Int. J. Miner. Process. 2005, 77, 65–79. [Google Scholar] [CrossRef]
- Lynch, A.J.; Johnson, N.W.; Manlapig, E.V.; Thorne, C.G. Mineral and coal flotation circuits. In Their Simulation and Control; Elsevier Scientific Publishing Company: New York, NY, USA, 1981. [Google Scholar]
- Kirjavainen, V.M. Application of a probability model for the entrainment of hydrophilic particles in froth flotation. Int. J. Miner. Process. 1989, 27, 63–74. [Google Scholar] [CrossRef]
- Maachar, A.; Dobby, G.S. Measurement of feed water recovery and entrainment solids recovery in flotation columns. Can. Metall. Q. 1992, 31, 167–172. [Google Scholar] [CrossRef]
- Johnson, B.N.W. A review of the entrainment mechanism and its modelling in industrial flotation processes. In Proceedings of the Conference Proceedings: Centenary of Flotation Symposium, Brisbane, Australia, 6–9 June 2005; Australasian Institute of Mining and Metallurgy (AusIMM): Brisbane, Australia, 2005. [Google Scholar]
- Akdemir, Ü.; Sönmez, İ. Investigation of coal and ash recovery and entrainment in flotation. Fuel Process. Technol. 2003, 82, 1–9. [Google Scholar] [CrossRef]
- Miller, F.G. The effect of froth sprinkling on coal flotation efficiency. Trans. AIME 1969, 244, 158–167. [Google Scholar]
- Cole, K.E. Bubble Size, Coalescence and Particle Motion in Flowing Foams. Ph.D. Thesis, Imperial College London, London, UK, 2010. [Google Scholar]
- Yianatos, J.B. Column Flotation Froths. Ph.D. Thesis, McGill University, Department of Mining and Metallurgical Engineering, Montréal, QC, Canada, 1987. [Google Scholar]
- Young, M.F.; Barnes, K.E.; Anderson, G.S.; Pease, J.D.; Zinc, X. Jameson Cell: The “comeback” in base metals applications using improved design and flow sheets. In Proceedings of the 38th Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, USA, 17 January 2006; pp. 311–322. [Google Scholar]
- Hacifazlioglu, H.; Sutcu, H. Optimization of some parameters in column flotation and a comparison of conventional cell and column cell in terms of flotation performance. J. Chin. Inst. Chem. Eng. 2007, 38, 287–293. [Google Scholar] [CrossRef]
- Kawatra, S.K. Froth flotation-fundamental principles flotation system. Miner. Eng. 1995, 1–30. [Google Scholar]
- Valkanov, N.; Grigorova, I.; Nishkov, I.; Bodurova, R.; Damianov, M. Minerals liberation management of lead-zinc flotation ore. In Proceedings of the XXIII World Mining Congress, Montreal, QC, Canada, 11–15 August 2013; Available online: https://www.researchgate.net/publication/306273069 (accessed on 20 December 2021).
- Bhugwandeen, Y. An Investigation of the Effects of Water Injection on Froth Flotation. Ph.D. Thesis, Chemical Engineering, University of KwaZulu-Natal, Pinetown, South Africa, 2014. [Google Scholar]
- Vera, M.A.; Mathe, Z.T.; Franzidis, J.P.; Harris, M.C.; Manlapig, E.V.; O’Connor, C.T. The modelling of froth zone recovery in batch and continuously operated laboratory flotation cells. Int. J. Miner. Process. 2002, 64, 135–151. [Google Scholar] [CrossRef]
- Yianatos, J.B.; Moys, M.H.; Contreras, F.; Villanueva, A. Froth recovery of industrial flotation cells. Miner. Eng. 2008, 21, 817–825. [Google Scholar] [CrossRef]
- Falutsu, M. Column flotation froth characteristics—stability of the bubble-particle system. Int. J. Miner. Process. 1994, 40, 225–243. [Google Scholar] [CrossRef]
- Barbian, N.; Hadler, K.; Cilliers, J.J. The froth stability column: Measuring froth stability at an industrial scale. Miner. Eng. 2006, 19, 713–718. [Google Scholar] [CrossRef]
- Hadler, K.; Cilliers, J.J. The relationship between the peak in air recovery and flotation bank performance. Miner. Eng. 2009, 22, 451–455. [Google Scholar] [CrossRef]
- Cowburn, J.; Stone, R.; Bourke, S.; Hill, B. Design developments of the Jameson Cell. In Proceedings of the Centenary of Flotation Symposium, Brisbane, Australia, 5 June 2005; pp. 193–199. [Google Scholar]
- Moudgil, B.M. Correlation between froth viscosity and flotation efficiency. Min. Metall. Explor. 1993, 10, 100–101. [Google Scholar] [CrossRef]
- Moolman, D.W.; Aldrich, C.; Van Deventer, J.S.; Bradshaw, D.J. The interpretation of flotation froth surfaces by using digital image analysis and neural networks. Chem. Eng. Sci. 1995, 50, 3501–3513. [Google Scholar] [CrossRef]
- Bhondayi, C.; Moys, M.H. Effects of gas distribution profile on flotation cell performance: An experimental investigation. Int. J. Miner. Process. 2015, 135, 20–31. [Google Scholar] [CrossRef]
- Mesa, D.; Brito-Parada, P.R. Scale-up in froth flotation: A state-of-the-art review. Sep. Purif. Technol. 2019, 210, 950–962. [Google Scholar] [CrossRef]
- Du Plessis, C. Investigating the Opportunity to Increase Yield by Means of Froth Washing on Mechanical Coal Flotation Cells. Ph.D. Thesis, University of the Witwatersrand, Faculty of Engineering and the Built Environment, School of Chemical and Metallurgical Engineering, Johannesburg, South Africa, 2018. [Google Scholar]
- Johnson, B.N. Issues in maximisation of recycling of water in a mineral processing plant. In Water in Mining; AusIMM: Australasian Institute of Mining and Metallurgy: Brisbane, Australia, 2003; pp. 239–245. [Google Scholar]
- Slatter, K.A.; Plint, N.D.; Cole, M.; Dilsook, V.; De Vaux, D.; Palm, N.; Oostendorp, B. Water management in Anglo Platinum process operations: Effects of water quality on process operations. In Proceedings of the International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009; pp. 19–23. [Google Scholar]
- Michaux, B.; Rudolph, M.; Reuter, M.A. Challenges in predicting the role of water chemistry in flotation through simulation with an emphasis on the influence of electrolytes. Miner. Eng. 2018, 125, 252–264. [Google Scholar] [CrossRef]
- Forssberg, K.S.; Hallin, M.I. Process water recirculation in a lead-zinc plant and other sulphide flotation plants. Chall. Miner. Process. 1989, 452–466. [Google Scholar]
- Rao, S.R.; Finch, J.A. A review of water re-use in flotation. Miner. Eng. 1989, 2, 65–85. [Google Scholar] [CrossRef]
- Rey, M.; Raffinot, P.; Von Michaelis, H. Flotation of ores in sea water. Water Manag. Treat. Min. Metall. Oper. 1966, 6, 3167–3174. [Google Scholar]
- Shackleton, N.J.; Malysiak, V.; Slatter, K.A. Effects of Water Quality and Surface Passivation on Flotation Behaviour. In Proceedings of the Anglo American Metallurgical Symp, Chicago, IL, USA, 1 November 2001. [Google Scholar]
- Schumann, R.; Levay, G.; Dunne, R.; Hart, S. Managing process water quality in base metal sulfide flotation. In Proceedings of the Water in Mining, Brisbane, Australia, 13–15 October 2003; pp. 251–259. [Google Scholar]
- Hoover, M.R. Water chemistry effects in the flotation of sulfide ores—A review and discussion for Molybdenite. Complex Sulphide Ores 1980, 100–112. [Google Scholar]
- Stevenson, P. The wetness of a rising foam. Ind. Eng. Chem. Res. 2006, 45, 803–807. [Google Scholar] [CrossRef]
- Neethling, S.J.; Cilliers, J.J. Simulation of the effect of froth washing on flotation performance. Chem. Eng. Sci. 2001, 56, 6303–6311. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.H. Froth Flotation: A Century of Innovation, 2007th ed.; Fuerstenau, M.C., Jameson, G.J., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Englewood, CO, USA, 2007. [Google Scholar]
- Dobby, G.S. Column Flotation. SGS Technical Paper. Available online: https://www.sgs.com//media/global/documents/technical-documents/sgs-technical-papers.2002-23 (accessed on 20 January 2022).
- Sastri, S.R. Column Flotation: Theory and practice. 1998, pp. 44–63. Available online: https://www.911metallurgist.com/blog/wp-content/uploads/2016/02/Column-Flotation.pdf.html (accessed on 28 October 2021).
- Harbort, G.J.; Jackson, B.R.; Manlapig, E.V. Recent advances in Jameson flotation cell technology. Miner. Eng. 1994, 7, 319–332. [Google Scholar] [CrossRef]
- Jameson Cell Rising to the Challenge. Glencore Technology. 2015. Available online: www.glencoretechnology.com.GLT2623G (accessed on 15 October 2021).









| Species | Concentration (mmol/L) | |
|---|---|---|
| Fresh Water | Tailing’s Water | |
| Ca2+ | 0.207 | 2.17 |
| Mg2+ | 0.118 | 0.45 |
| F− | 0.006 | 0.25 |
| SO42− | 0.005 | 0.67 |
| Advantages of Using Nozzles | Disadvantages of Using Nozzles |
|---|---|
|
|
| Method | Advantages | Disadvantages |
|---|---|---|
| Froth washing in general |
| |
| Washing on top of the froth, i.e., wash-water boxes/trays/pipes and jets |
|
|
| Internal froth washing |
| |
| Lip washing |
| |
| Under-froth washing |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jera, T.M.; Bhondayi, C. A Review on Froth Washing in Flotation. Minerals 2022, 12, 1462. https://doi.org/10.3390/min12111462
Jera TM, Bhondayi C. A Review on Froth Washing in Flotation. Minerals. 2022; 12(11):1462. https://doi.org/10.3390/min12111462
Chicago/Turabian StyleJera, Tawona Martin, and Clayton Bhondayi. 2022. "A Review on Froth Washing in Flotation" Minerals 12, no. 11: 1462. https://doi.org/10.3390/min12111462






