Recovery of Uranium, Thorium, and Other Rare Metals from Eudialyte Concentrate by a Binary Extractant Based on 1,5-bis[2-(hydroxyethoxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and Methyl Trioctylammonium Nitrate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zepf, V. Rare Earth Elements; Springer Theses; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-35457-1. [Google Scholar]
- Moss, R.; Tzimas, E.; Willis, P.; Arendorf, J.; Thompson, P.; Chapman, A.; Morley, N.; Sims, E.; Bryson, R.; Pearson, J.; et al. Critical Metals in the Path towards the Decarbonisation of the EU Energy Sector: Assessing Rare Metals as Supply-Chain Bottlenecks in Low-Carbon Energy Technologies; Publication Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Schulz, K.J.; DeYoung, J.H., Jr.; Seal, R.R., II; Bradly, D.C. Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply: U.S. Geological Survey Professional Paper 1802; U.S. Geological Surve: Reston, VA, USA, 2017.
- Romanova, O.A.; Sirotin, D.V. Metal Industry Development in the Conditions of Formation of New Technological and Institutional Trends. KnE Mater. Sci. 2019, 5, 15. [Google Scholar] [CrossRef]
- Samsonov, N. Global Chains of Supply of Rare-Earth and Rare Metals as High-Tech Raw Materials Within the Framework of International Industrial Cooperation. Spat. Econ. 2018, 3, 43–66. [Google Scholar] [CrossRef]
- Kryukov, V.A.; Yatsenko, V.A.; Kryukov, Y.V. Rare Earth Industry—How to Take Advantage of Opportunities. Min. Ind. Gorn. Promishlennost 2020, 5, 68–84. [Google Scholar] [CrossRef]
- Yahorava, V.; Lakay, E.; Clark, W.; Strauss, J. Hydrothermal Modification of Phosphogypsum to Improve Subsequent Recovery of Rare Earths. In Extraction 2018; Springer: Cham, Switzerland, 2018; pp. 2415–2427. [Google Scholar]
- Jarosiński, A.; Kowalczyk, J.; Mazanek, C. Development of the Polish wasteless technology of apatite phosphogypsum utilization with recovery of rare earths. J. Alloys Compd. 1993, 200, 147–150. [Google Scholar] [CrossRef]
- Moalla, R.; Gargouri, M.; Khmiri, F.; Kamoun, L.; Zairi, M. Phosphogypsum purification for plaster production: A process optimization using full factorial design. Environ. Eng. Res. 2017, 23, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Soukeur, A.; Szymczyk, A.; Berbar, Y.; Amara, M. Extraction of rare earth elements from waste products of phosphate industry. Sep. Purif. Technol. 2021, 256, 117857. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Eggenkamp, H.G.M.; Atanasova, P.; Mundel, F.; Kümmel, S.; Hagen, M.; Wenzel, T.; Markl, G. Review on the Compositional Variation of Eudialyte-Group Minerals in the Ilímaussaq Complex (South Greenland). Minerals 2020, 10, 1011. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Pakhomovsky, Y.A.; Panikorovskii, T.L.; Bazai, A.V.; Yakovenchuk, V.N. Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance. Minerals 2020, 10, 1070. [Google Scholar] [CrossRef]
- Kogarko, L.; Nielsen, T.F.D. Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia. Minerals 2020, 10, 1036. [Google Scholar] [CrossRef]
- Lebedev, V.N. Sulfuric Acid Technology for Processing of Eudialyte Concentrate. Russ. J. Appl. Chem. 2003, 76, 1559–1563. [Google Scholar] [CrossRef]
- Lebedev, V.N.; Shchur, T.E.; Maiorov, D.V.; Popova, L.A.; Serkova, R.P. Specific Features of Acid Decomposition of Eudialyte and Certain Rare-Metal Concentrates from Kola Peninsula. Russ. J. Appl. Chem. 2003, 76, 1191–1196. [Google Scholar] [CrossRef]
- Vaccarezza, V.; Anderson, C. An Overview of Beneficiation and Hydrometallurgical Techniques on Eudialyte Group Minerals. Min. Metall. Explor. 2020, 37, 39–50. [Google Scholar] [CrossRef]
- Chanturia, V.A.; Minenko, V.G.; Koporulina, E.V.; Ryazantseva, M.V.; Samusev, A.L. Influence of Acids on Extraction Efficiency of Zirconium and Rare Earth Metals in Eudialyte Concentrate Leaching. J. Min. Sci. 2019, 55, 984–994. [Google Scholar] [CrossRef]
- Zakharov, V.I.; Maiorov, D.V.; Alishkin, A.R.; Matveev, V.A. Causes of insufficient recovery of zirconium during acidic processing of lovozero eudialyte concentrate. Russ. J. Non-Ferr. Met. 2011, 52, 423–428. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from eudialyte concentrate by suppressing silica gel formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Minenko, V.G.; Samusev, A.L.; Chanturia, E.L.; Koporulina, E.V.; Bunin, I.Z.; Ryazantseva, M.V. The Effect of Energy Impacts on the Acid Leaching of Eudialyte Concentrate. Miner. Process. Extr. Metall. Rev. 2020, 42, 484–495. [Google Scholar] [CrossRef]
- Balinski, A.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Separation of rare earth elements from contaminants and valuable components by in-situ precipitation during the hydrometallurgical processing of eudialyte concentrate. Hydrometallurgy 2020, 194, 105345. [Google Scholar] [CrossRef]
- Balinski, A.; Atanasova, P.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates. Minerals 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Stopic, S.; Gronen, L.; Milivojevic, M.; Obradovic, S.; Friedrich, B. Neural Network Modeling for the Extraction of Rare Earth Elements from Eudialyte Concentrate by Dry Digestion and Leaching. Metals 2018, 8, 267. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Friedrich, B. Recovery of Zr, Hf, Nb from eudialyte residue by sulfuric acid dry digestion and water leaching with H2O2 as a promoter. Hydrometallurgy 2018, 181, 206–214. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P.; Hake, J.-F.; Voßenkaul, D.; Friedrich, B. Environmental Impacts of Rare Earth Mining and Separation Based on Eudialyte: A New European Way. Resources 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The Nomenclature of Eudialyte-Group Minerals. Can. Miner. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Stepenshchikov, D.G.; Kalashnikov, A.O.; Aksenov, S.M. Who Is Who in the Eudialyte Group: A New Algorithm for the Express Allocation of a Mineral Name Based on the Chemical Composition. Minerals 2022, 12, 224. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Rastsvetaeva, R.K.; Mitchell, R.H.; Chakrabarty, A. Crystal structure of manganese-rich variety of eudialyte from Suchina Hill, India, and manganese ordering in eudialyte-group minerals. Crystallogr. Reports 2014, 59, 146–154. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Pruseth, K.L.; Sen, A.K. First report of eudialyte occurrence from the Sushina hill region, Purulia district, West Bengal. J. Geol. Soc. India 2011, 77, 12–16. [Google Scholar] [CrossRef]
- Amores-Casals, S.; Gonçalves, A.O.; Melgarejo, J.-C.; Martí Molist, J. Nb and REE Distribution in the Monte Verde Carbonatite–Alkaline–Agpaitic Complex (Angola). Minerals 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Hammache, Z.; Berbar, Y.; Bensaadi, S.; Trari, M.; Amara, M. Recovery of light rare earth elements by leaching and extraction from phosphate mining waste (Fluorapatite and Carbonate-Fluorapatite). J. Afr. Earth Sci. 2020, 171, 103937. [Google Scholar] [CrossRef]
- Borst, A.M.; Finch, A.A.; Friis, H.; Horsburgh, N.J.; Gamaletsos, P.N.; Goettlicher, J.; Steininger, R.; Geraki, K. Structural state of rare earth elements in eudialyte-group minerals. Mineral. Mag. 2020, 84, 19–34. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. The Principles of Radioactive Waste Management. Safety Series, No. 111-F; IAEA: Vienna, Austria, 1996. [Google Scholar]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Multicoordinate ligands for actinide/lanthanide separations. Chem. Soc. Rev. 2007, 36, 367–377. [Google Scholar] [CrossRef]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef] [PubMed]
- Sharova, E.V.; Artyushin, O.I.; Odinets, I.L. Synthetic routes to carbamoylmethylphosphoryl compounds—Extractants for the processing of spent nuclear fuels. Russ. Chem. Rev. 2014, 83, 95–119. [Google Scholar] [CrossRef]
- Belova, V.V.; Voshkin, A.A.; Kholkin, A.I.; Payrtman, A.K. Solvent extraction of some lanthanides from chloride and nitrate solutions by binary extractants. Hydrometallurgy 2009, 97, 198–203. [Google Scholar] [CrossRef]
- AKHILAMAHESWARI, M. Selective enrichment of U(VI), Th(IV) and La(III) from high acidic streams using a new chelating ion-exchange polymeric matrix. Talanta 2004, 64, 202–209. [Google Scholar] [CrossRef]
- Ivanova, I.S.; Krivorot’ko, E.S.; Ilyukhin, A.B.; Demin, S.V.; Pyatova, E.N.; Baulin, V.E.; Tsivadze, A.Y. Extraction of Rare Earth Elements in 1,1,7-Trihydrododecafluoroheptanol–Water System with Phosphoryl Podands Derived from Diphosphonic Acids. Russ. J. Inorg. Chem. 2019, 64, 666–672. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E.; Tsivadze, A.Y. Extraction of REEs(III), U(VI), and Th(IV) with acidic phosphoryl-containing podands from nitric acid solutions. Radiochemistry 2014, 56, 22–26. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y. Liquid- and Solid-Phase Extraction of Uranium(VI), Thorium(IV), and Rare Earth Elements(III) from Nitric Acid Solutions Using Acid-Type Phosphoryl-Containing Podands. Russ. J. Inorg. Chem. 2019, 64, 536–542. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Matveeva, A.G.; Ivanets, D.V.; Kudryavtsev, E.M.; Grigor’ev, M.S.; Baulin, V.E.; Tsivadze, A.Y. Phosphoryl-containing acidic podands as extractants for recovery of f-elements. Russ. Chem. Bull. 2015, 64, 161–168. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Matveeva, A.G.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, V.E.; Tsivadze, A.Y. Phosphoryl-containing acidic podands as extractants for recovery of f-elements. Russ. Chem. Bull. 2015, 64, 169–175. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y. Extraction of f Elements by Binary Extractants Based on 1,5-Bis[o-(dioxyphosphoryl)phenoxy]-3-Oxapentane Derivatives and Trioctylamine. Russ. J. Inorg. Chem. 2018, 63, 1679–1683. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Volkov, S.N.; Filatov, S.K. RietveldToTensor: Program for Processing Powder X-Ray Diffraction Data under Variable Conditions. Glas. Phys. Chem. 2018, 44, 33–40. [Google Scholar] [CrossRef]
- Chizhevskaya, S.V.; Chekmarev, A.M.; Klimenko, O.M.; Povetkina, M.V.; Sinegribova, O.A.; Cox, M. Non-traditional methods of treating high-silicon ores containing rare elements. In Hydrometallurgy’94; Springer: Dordrecht, The Netherlands, 1994; pp. 219–228. [Google Scholar]
- Safiulina, A.M.; Anan’ev, A.V.; Lizunov, A.V.; Tuiza, M.; Logunov, M.V.; Dvoeglazov, K.N. Experimental Modeling of Technetium(VII) Recovery from Raffinates after Extractive Reprocessing of Spent Nuclear Fuel. Russ. J. Inorg. Chem. 2020, 65, 1928–1934. [Google Scholar] [CrossRef]
- Belova, V.V.; Voshkin, A.A.; Egorova, N.S.; Kholkin, A.I. Solvent extraction of rare earth metals from nitrate solutions with di(2,4,4-trimethylpentyl)phosphinate of methyltrioctylammonium. J. Mol. Liq. 2012, 172, 144–146. [Google Scholar] [CrossRef]
- Belova, V.V.; Voshkin, A.A.; Egorova, N.S.; Khol’kin, A.I. Extraction of rare earth metals from nitrate solutions with a binary extractant based on Cyanex 272. Russ. J. Inorg. Chem. 2010, 55, 629–633. [Google Scholar] [CrossRef]
- Belova, V.V.; Zakhodyaeva, Y.A.; Khol’kin, A.I. Extraction of rare earth metal nitrates with methyltrioctylammonium dialkyl phosphate and dialkylphosphinate. Russ. J. Inorg. Chem. 2015, 60, 526–530. [Google Scholar] [CrossRef]
- Egorova, N.S.; Belova, V.V.; Voshkin, A.A.; Khol’kin, A.I.; Pyartman, A.K.; Keskinov, V.A. Extraction of uranyl nitrate with a binary extractant based on di(2,4,4-trimethylpentyl)phosphinic acid. Theor. Found. Chem. Eng. 2008, 42, 708–713. [Google Scholar] [CrossRef]
- Stepanov, S.I.; Boyarintsev, A.V.; Vazhenkov, M.V.; Myasoedov, B.F.; Nazarov, E.O.; Safiulina, A.M.; Tananaev, I.G.; So, H.V.; Chekmarev, A.M.; Civadze, A.Y. CARBEX process, a new technology of reprocessing of spent nuclear fuel. Russ. J. Gen. Chem. 2011, 81, 1949–1959. [Google Scholar] [CrossRef]

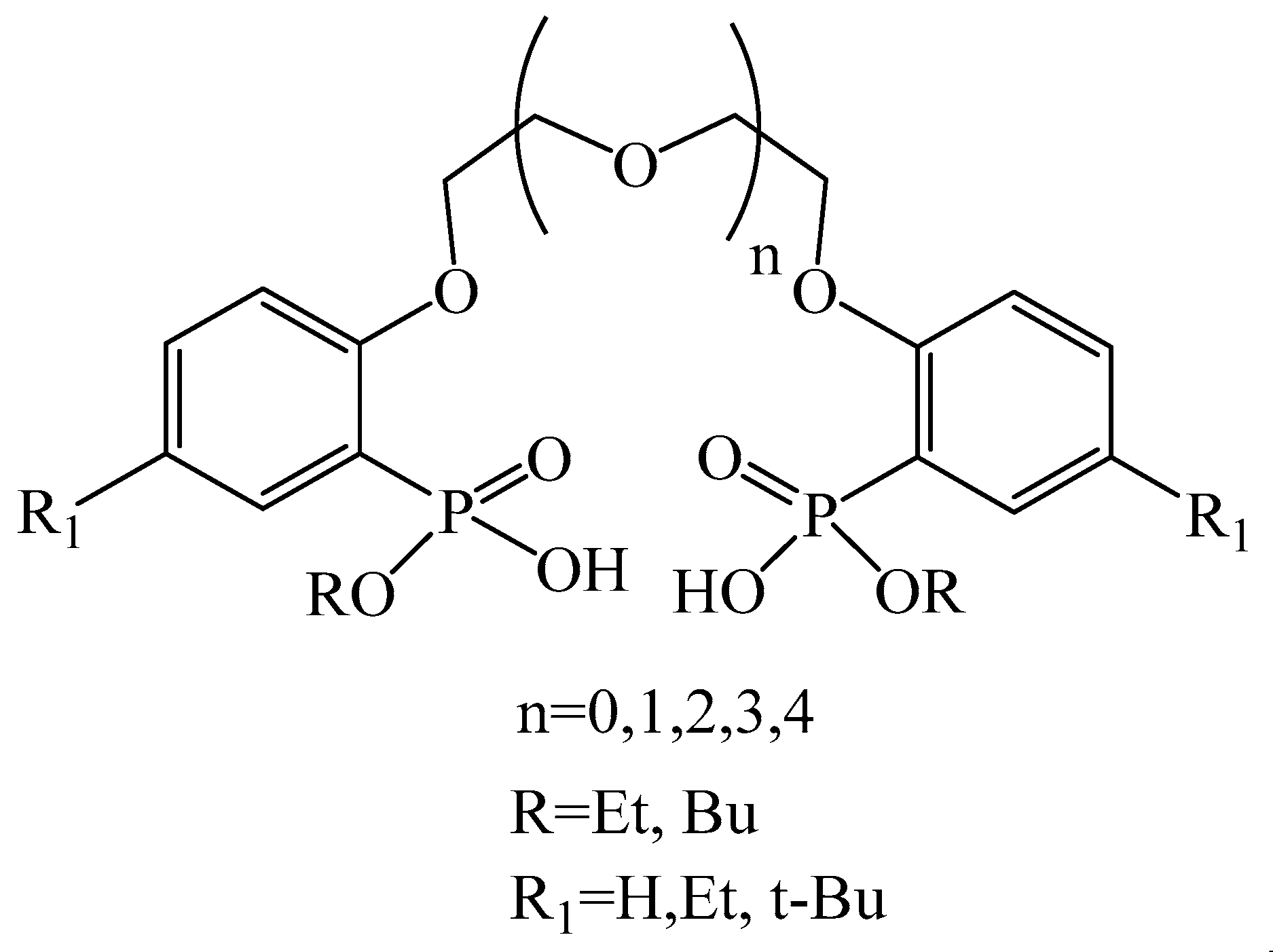
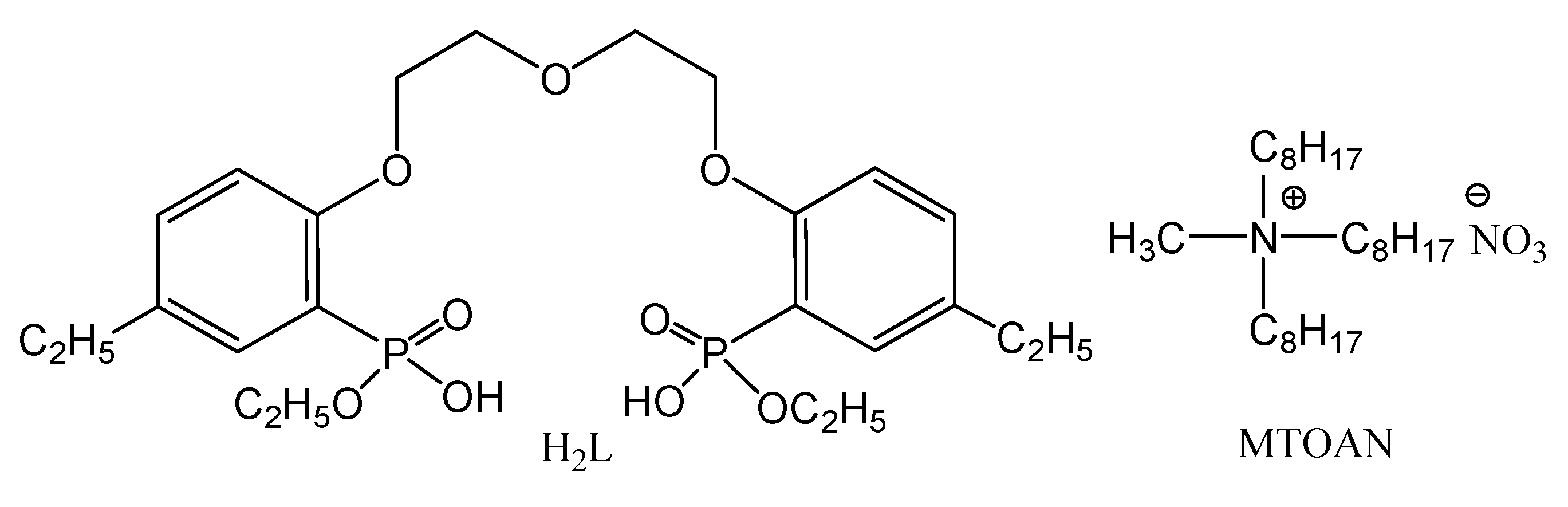


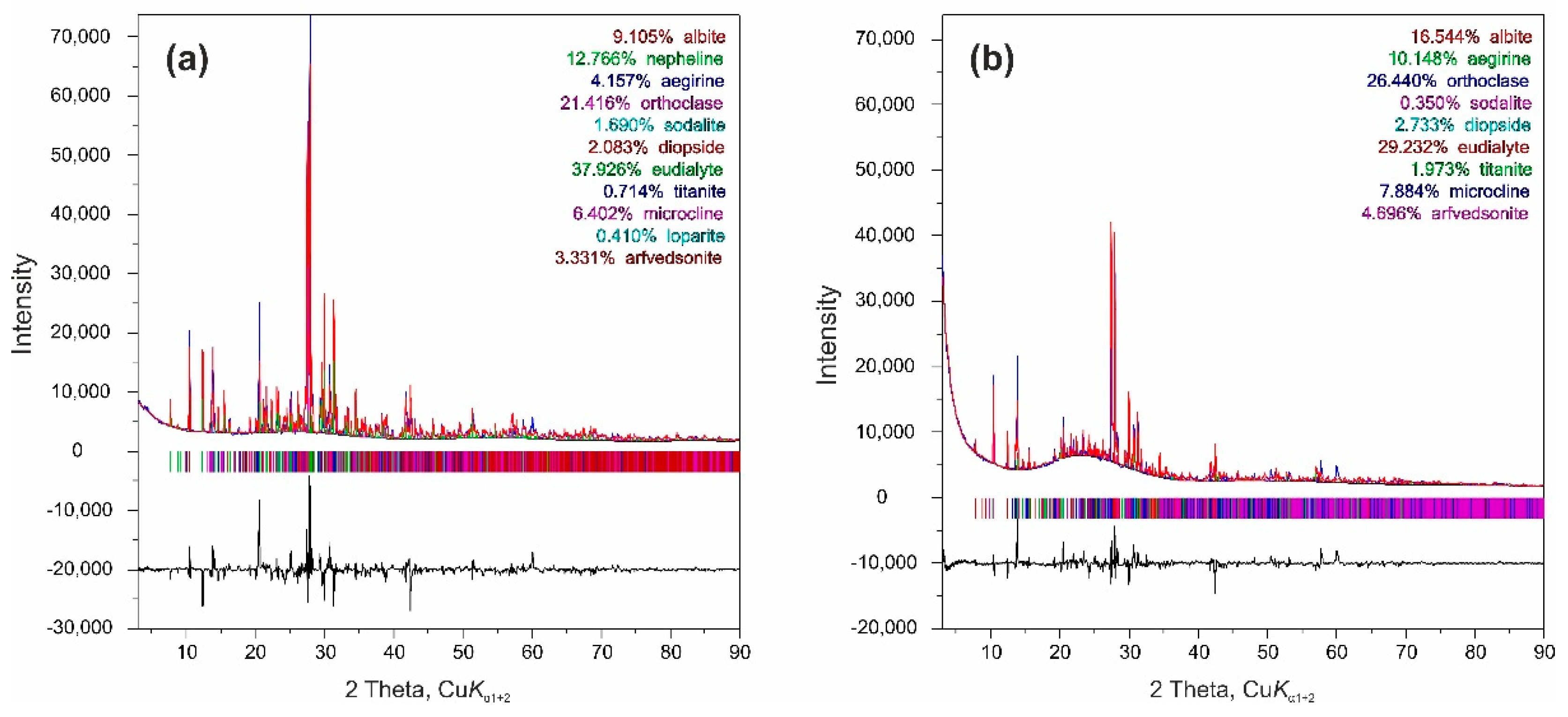

| Ligand Concentration, M | F (U/La) | F (Th/La) |
|---|---|---|
| 0.01 | 4200 | 60,000 |
| 0.001 | 14,200 | 14,333 |
| Element | Si | Al | Fe | Zr | Mn | Ca | Na | K | Ti |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 49.97 | 11.65 | 11.55 | 8.67 | 3.81 | 3.13 | 2.63 | 2.01 | 1.96 |
| Element | Sr | Cl | Nb | Mg | P(V) | Hf | Ba | Mo | Ta |
| wt.% | 1.25 | 0.675 | 0.503 | 0.309 | 0.292 | 0.122 | 0.064 | 0.039 | 0.031 |
| Element | S | Ni | Y, La–Lu | La–Nd | Y, Sm–Lu | Y | |||
| wt.% | 0.023 | 0.017 | 1.22 | 0.77 | 0.45. | 0.403 | |||
| Elements | La–Nd | Ce | Sm–Lu + Y | Y |
|---|---|---|---|---|
| wt.% | 63.2 | 0.40 | 36.8 | 0.40 |
| Element | Detection Limits (DL), mg/L | Content of Elements in the Solution after Eudialyte Leaching, mg/L | Element | Detection Limits (DL), mg/L | Content of Elements in the Solution after Eudialyte Leaching, mg/L |
|---|---|---|---|---|---|
| Li | 0.003 | 0.20 | Sn | 0.006 | 0.22 |
| Be | 0.001 | 0.54 | Sb | 0.003 | 0.056 |
| B | 0.1 | 0.65 | Cs | 0.001 | 0.086 |
| Na | 0.6 | 4153 | Ba | 0.006 | 1.3 |
| Mg | 0.6 | 14.9 | La | 0.001 | 72.9 |
| Al | 0.4 | 2032 | Ce | 0.002 | 159 |
| S | 2 | 33.4 | Pr | 0.0004 | 19.4 |
| K | 0.6 | 510 | Nd | 0.0008 | 78.5 |
| Ca | 0.6 | 1572 | Sm | 0.0007 | 21.6 |
| Sc | 0.01 | 0.63 | Eu | 0.0006 | 7.0 |
| Ti | 0.2 | 29.2 | Gd | 0.0005 | 21.8 |
| Cr | 0.06 | 0.19 | Tb | 0.0005 | 4.0 |
| Mn | 0.02 | 489 | Dy | 0.0007 | 25.3 |
| Fe | 0.7 | 701 | Ho | 0.0006 | 5.3 |
| Co | 0.02 | 0.053 | Er | 0.0003 | 15.0 |
| Ni | 0.06 | 0.17 | Tm | 0.0004 | 2.3 |
| Cu | 0.07 | 0.39 | Yb | 0.0006 | 13.7 |
| Zn | 0.08 | 4.1 | Lu | 0.0004 | 1.8 |
| Rb | 0.01 | 3.3 | Hf | 0.001 | 44.1 |
| Sr | 0.005 | 34.9 | Ta | 0.003 | 0.016 |
| Y | 0.005 | 119 | W | 0.003 | 0.050 |
| Zr | 0.002 | 2107 | Bi | 0.0009 | 0.020 |
| Tl | 0.0003 | 0.012 | Th | 0.0006 | 2.5 |
| Pb | 0.006 | 27.0 | U | 0.0005 | 2.1 |
| Element | Initial Solution, mg/L | Raffinate, mg/L | D | Recovery % | Element | Initial Solution, mg/L | Raffinate, mg/L | D | Recovery, % |
|---|---|---|---|---|---|---|---|---|---|
| Li | 0.055 | 0.055 | 0 | 0 | Sn | 0.048 | 0.009 | 4.51 | 81.9 |
| Be | 0.14 | 0.14 | 0 | 0 | Sb | 0.019 | 0.019 | 0.04 | 3.89 |
| B | 0.43 | 0.43 | 0 | 0 | Cs | 0.023 | 0.023 | 0 | 0 |
| Na | 1083 | 1083 | 0 | 0 | Ba | 0.30 | 0.30 | 0 | 0 |
| Mg | 3.3 | 3.3 | 0 | 0 | La | 18.4 | 18.4 | 0 | 0 |
| Al | 495 | 495 | 0 | 0 | Ce | 39.7 | 39.7 | 0 | 0 |
| S | 8.4 | 8.2 | 0 | 0 | Pr | 4.9 | 4.9 | 0 | 0 |
| K | 119 | 119 | 0 | 0 | Nd | 19.7 | 19.7 | 0 | 0 |
| Ca | 420 | 420 | 0 | 0 | Sm | 5.0 | 5.0 | 0 | 0 |
| Sc | 0.16 | 0.01 | 15.1 | 93.7 | Eu | 1.6 | 1.6 | 0 | 0 |
| Ti | 7.2 | 2.5 | 1.93 | 65.8 | Gd | 5.4 | 5.4 | 0 | 0 |
| Cr | 0.16 | 0.081 | 0.91 | 47.7 | Tb | 0.97 | 0.97 | 0 | 0 |
| Mn | 127 | 127 | 0 | 0 | Dy | 6.2 | 6.2 | 0 | 0 |
| Fe | 186 | 163 | 0.14 | 12.5 | Ho | 1.3 | 1.3 | 0 | 0 |
| Co | 0.033 | 0.02 | 0.67 | 40.1 | Er | 3.6 | 3.6 | 0 | 0 |
| Cu | 0.17 | 0.17 | 0 | 0 | Tm | 0.53 | 0.51 | 0.02 | 2.1 |
| Zn | 1.0 | 1.0 | 0 | 0 | Yb | 3.3 | 3.2 | 0.05 | 4.4 |
| Rb | 0.81 | 0.81 | 0 | 0 | Lu | 0.42 | 0.40 | 0.06 | 5.5 |
| Sr | 8.5 | 8.5 | 0 | 0 | Hf | 10.3 | 0.090 | 114 | 99.1 |
| Y | 29.6 | 29.6 | 0 | 0 | W | 0.009 | 0.006 | 0.53 | 34.7 |
| Zr | 522 | 4.4 | 118 | 99.1 | Tl | 0.003 | 0.0003 | 7.96 | 88.8 |
| Pb | 6.7 | 6.7 | 0 | 0 | Th | 0.56 | 0.0006 | 979 | 99.9 |
| Bi | 0.0043 | 0.0035 | 0.22 | 17.9 | U | 2.0 | 0.025 | 78.3 | 98.7 |
| Contact No. | Organic Phase Volume, mL | O/A Ratio | Aqueous Phase Volume, mL | |
|---|---|---|---|---|
| O | A | |||
| 1 | 25 | 5 | 1 | 5 |
| 2 | 20 | 4 | 1 | 5 |
| 3 | 15 | 3 | 1 | 5 |
| 4 | 10 | 2 | 1 | 5 |
| 5 | 5 | 1 | 1 | 5 |
| 6 | 5 | 1 | 2 | 10 |
| 7 | 5 | 1 | 3 | 15 |
| 8 | 5 | 1 | 4 | 20 |
| 9 | 5 | 1 | 5 | 25 |
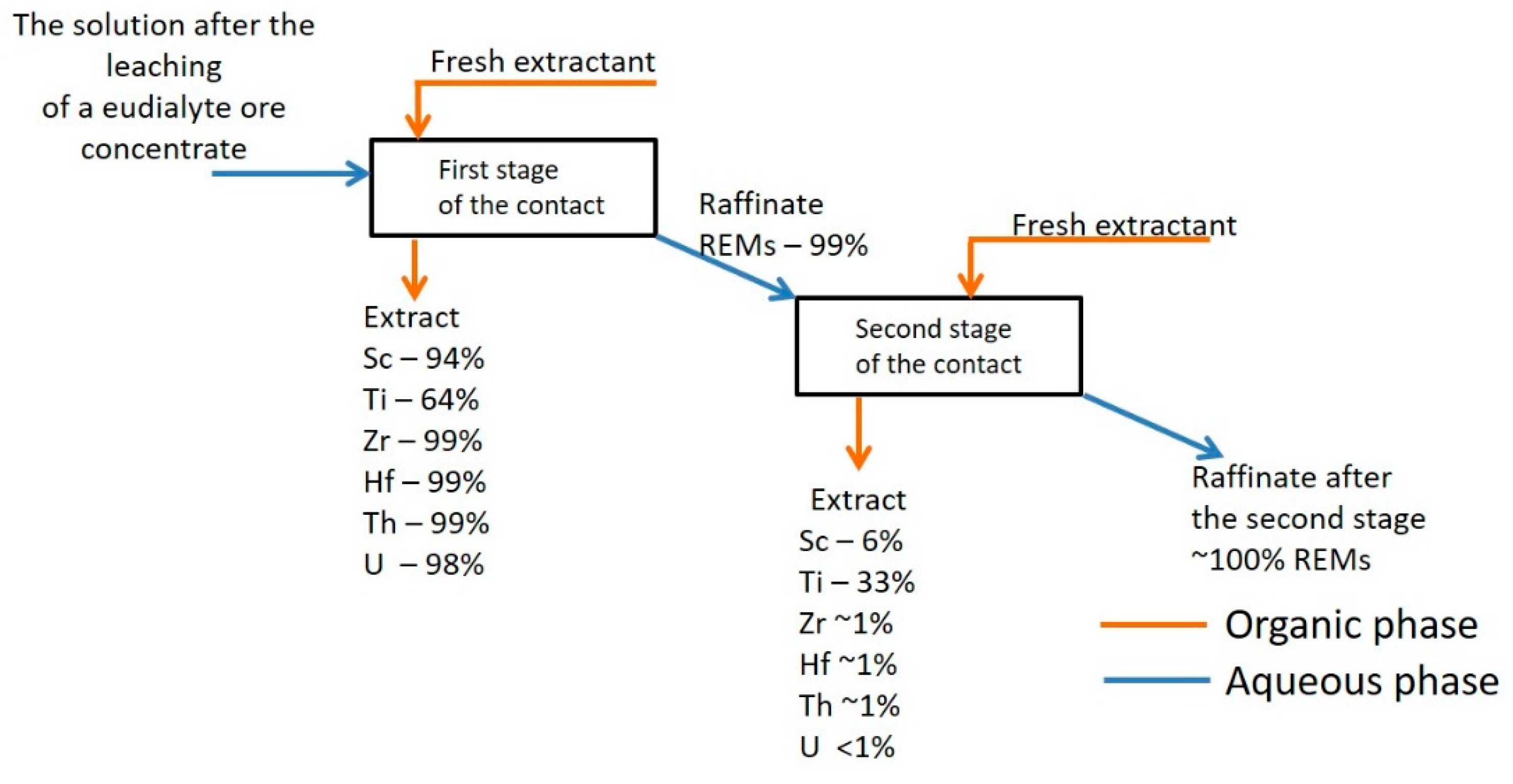
| Element | Initial Solution, mg/L | 1st Stage Extraction Raffinate, mg/L | 1st Stage Extraction Degree, % | 2nd Stage Extraction Raffinate, mg/L | 2nd Stage Extraction Degree, % |
|---|---|---|---|---|---|
| Sc | 0.16 | 0.01 | 93.8 | <DL | ~100 |
| Ti | 7.2 | 2.5 | 65.8 | 0.2 | 97.2 |
| Zr | 522 | 4.4 | 99.2 | 1 | 99.8 |
| Hf | 10.3 | 0.090 | 99.1 | 0.032 | 99.7 |
| Th | 0.56 | 0.0006 | 99.9 | <DL | ~100 |
| U | 2.0 | 0.025 | 98.7 | 0.0033 | 99.8 |
| Element | Initial Solution, mg/L | Ionic Pair (R4N)2L in 1,2-dichloroethane (0.05 M by H2L) | 1.19 M (30%) TBP in 1,2-dichloroethane | ||||
|---|---|---|---|---|---|---|---|
| Raffinate, mg/L | D | % | Raffinate, mg/L | D | % | ||
| Sc | 0.161 | <DL | - | ~100 | 0.160 | 0.004 | 0.4 |
| Ti | 7.20 | 2.46 | 1.9 | 66 | 7.20 | 0 | 0.04 |
| Zr | 522.1 | 4.36 | 118 | 99 | 432 | 0.21 | 17.2 |
| Hf | 10.33 | 0.090 | 114 | 99 | 8.60 | 0.20 | 16.8 |
| Th | 0.564 | <DL | - | ~100 | 0.154 | 2.7 | 72 |
| U | 1.952 | 0.025 | 78.3 | 99 | 0.197 | 8.9 | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safiulina, A.M.; Lizunov, A.V.; Semenov, A.A.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y.; Aksenov, S.M.; Tananaev, I.G. Recovery of Uranium, Thorium, and Other Rare Metals from Eudialyte Concentrate by a Binary Extractant Based on 1,5-bis[2-(hydroxyethoxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and Methyl Trioctylammonium Nitrate. Minerals 2022, 12, 1469. https://doi.org/10.3390/min12111469
Safiulina AM, Lizunov AV, Semenov AA, Baulin DV, Baulin VE, Tsivadze AY, Aksenov SM, Tananaev IG. Recovery of Uranium, Thorium, and Other Rare Metals from Eudialyte Concentrate by a Binary Extractant Based on 1,5-bis[2-(hydroxyethoxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and Methyl Trioctylammonium Nitrate. Minerals. 2022; 12(11):1469. https://doi.org/10.3390/min12111469
Chicago/Turabian StyleSafiulina, Alfiya M., Alexey V. Lizunov, Aleksandr A. Semenov, Dmitriy V. Baulin, Vladimir E. Baulin, Aslan Yu. Tsivadze, Sergey M. Aksenov, and Ivan G. Tananaev. 2022. "Recovery of Uranium, Thorium, and Other Rare Metals from Eudialyte Concentrate by a Binary Extractant Based on 1,5-bis[2-(hydroxyethoxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and Methyl Trioctylammonium Nitrate" Minerals 12, no. 11: 1469. https://doi.org/10.3390/min12111469






