Comprehensive Utilization of Tailings in Quartz Vein-Hosted Gold Deposits
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Basic Characteristics
2.1.1. Materials
2.1.2. Basic Sample Characteristics
2.2. Separation Principles and Experimental Methods
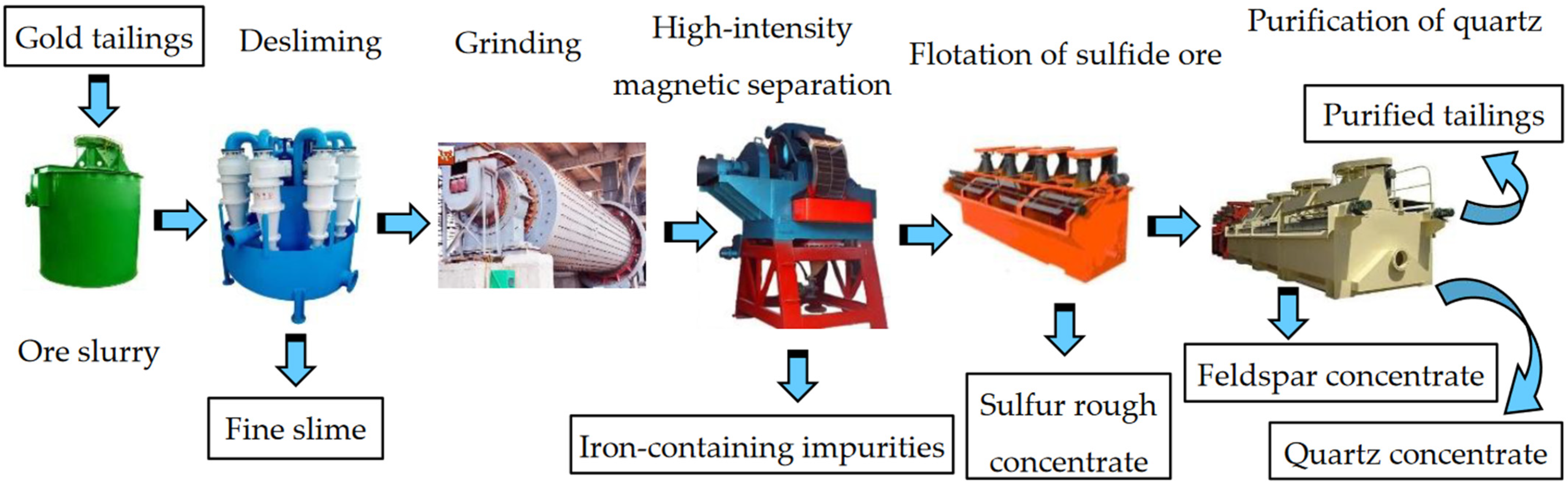
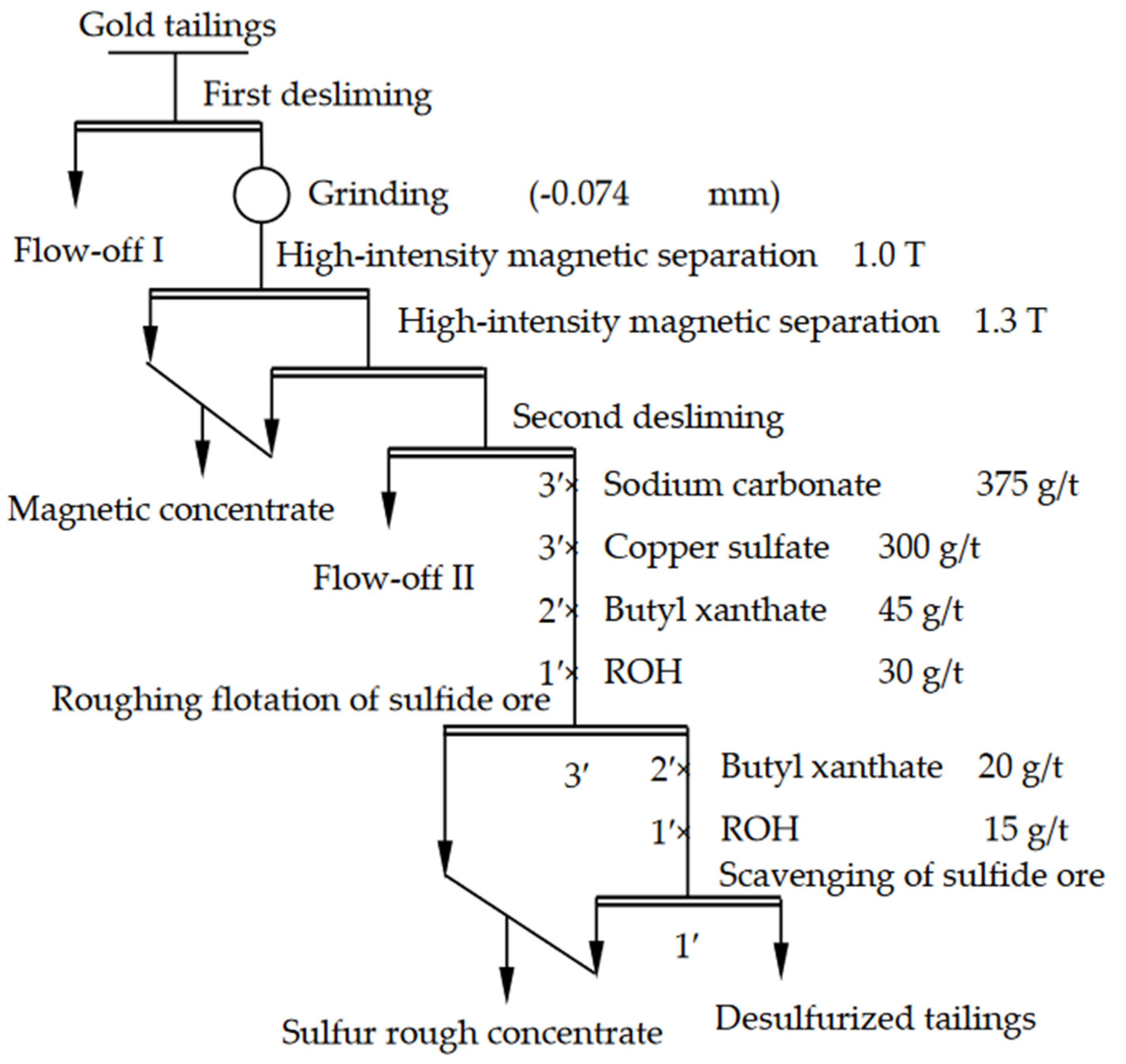
2.2.1. Recovery Process
2.2.2. Experimental Methods and Procedures
2.3. Analysis and Detection Methods
3. Results and Discussion
3.1. Characterization of Gold Tailings
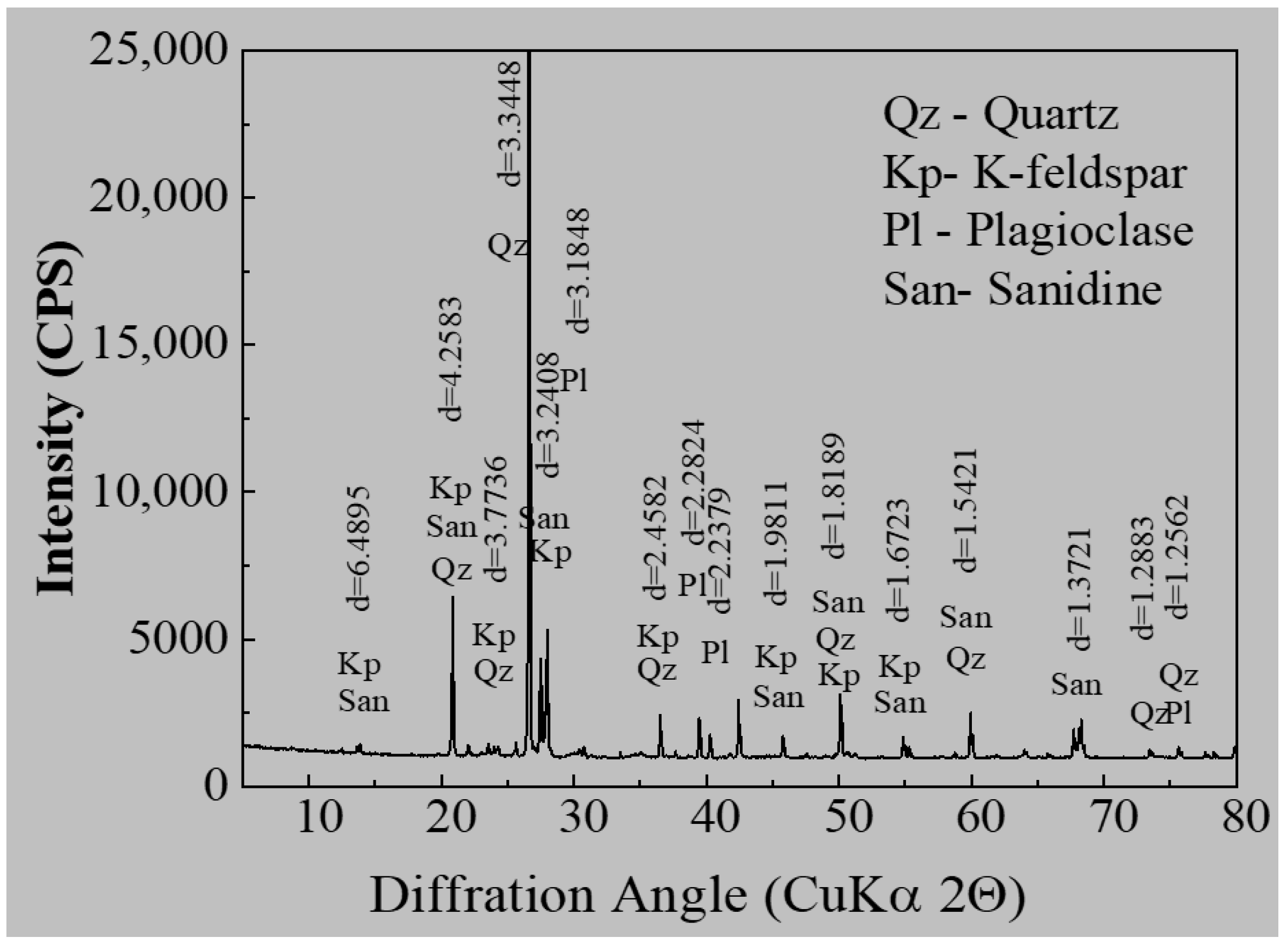
3.1.1. Mineralogical Composition
3.1.2. Mineralogical Characterization
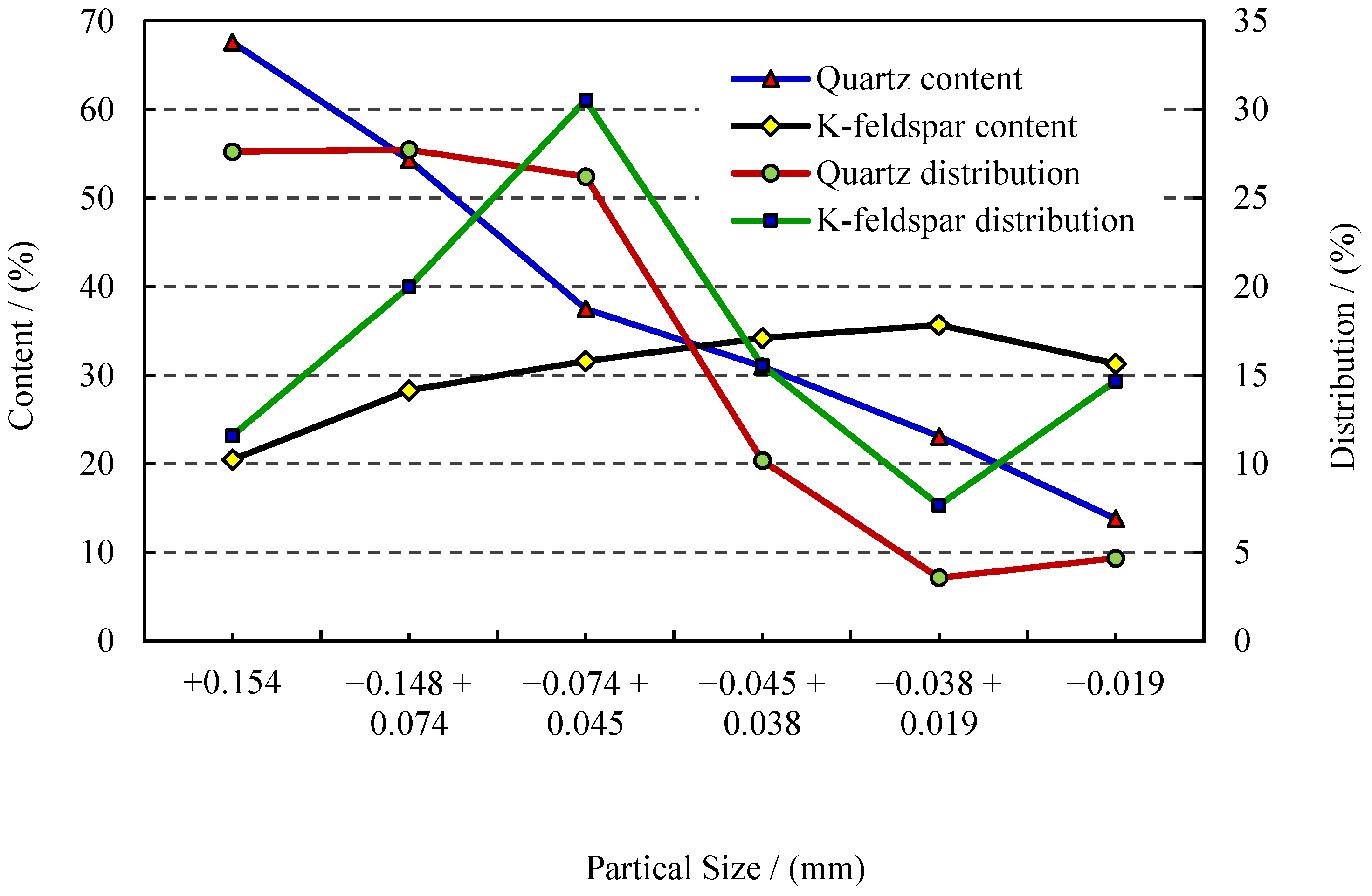
3.1.3. Particle Size Distribution and Mineral Liberation Degree
3.2. Desliming through Hydrocyclone Classification
3.3. Gold Recovery
3.4. Recovery of Quartz and Feldspar
3.4.1. Removal of Impurities
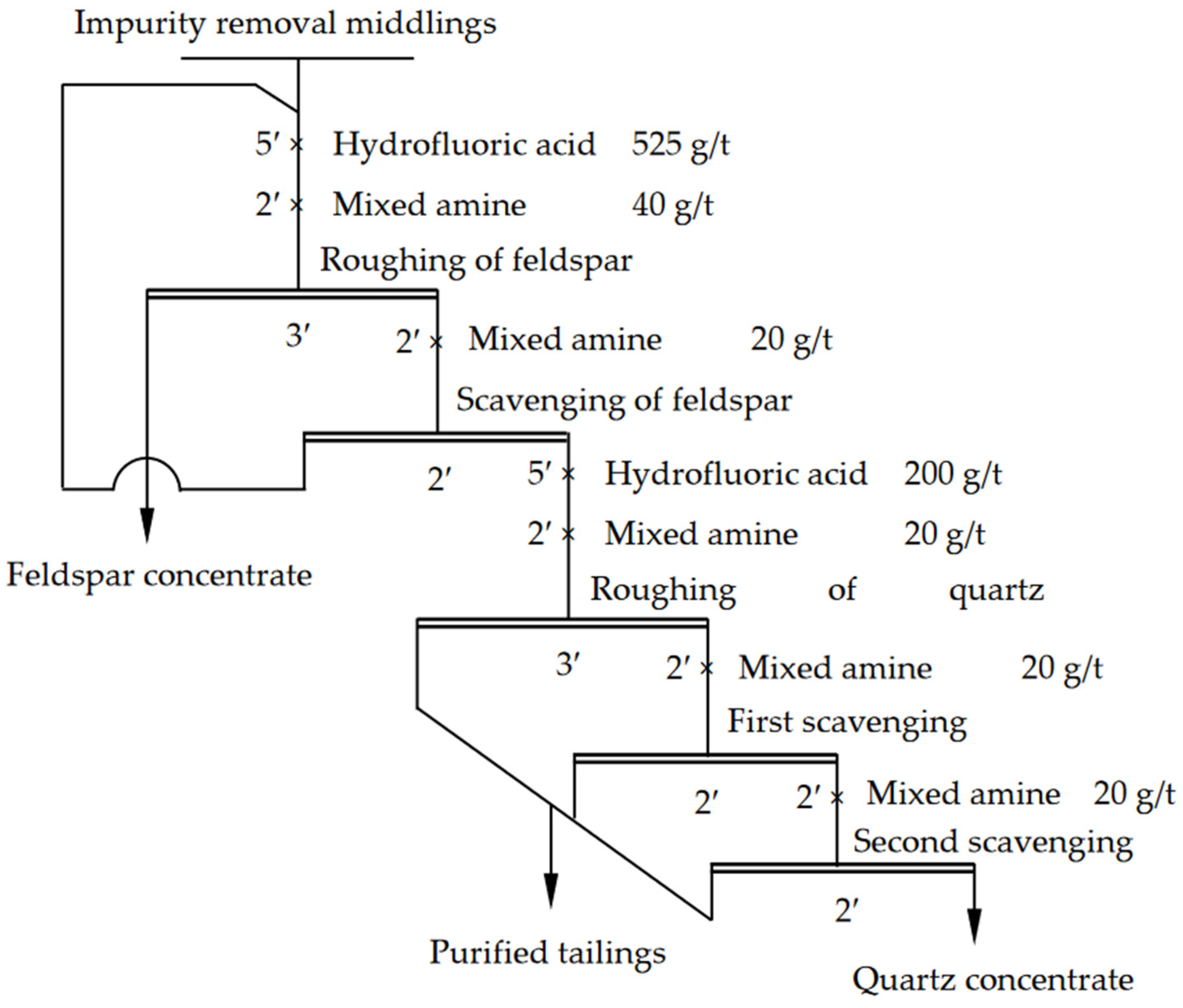
3.4.2. Recovery of Quartz and Feldspar through Flotation Separation
3.5. Characteristics Analysis of Products
3.5.1. Analysis of Mineral Contents in Samples
3.5.2. Evaluation of Quartz Properties
3.6. Economic Benefit and Environmental Impact Assessment
4. Conclusions
- (1)
- The process mineralogy of the tailings samples obtained from the quartz vein-hosted gold deposits showed that the content of non-metallic minerals in the tailings was approximately 98%, comprising quartz, potassium feldspar, plagioclase, calcite, and sericite. Quartz, feldspar, and sericite, and that these were worth recovering because of their high concentration in the tailings. Moreover, the gold distribution in the tailings was 0.20 g/t, which also had a certain recovery value. The particle size distribution analysis indicated that the particles whose size fraction was +0.074 mm achieved 37.68%, in which the distribution of gold and quartz reached 49% and 55%, respectively. The occurrence characteristics analysis showed that the quartz, K-feldspar, and sericite were partly metasomatized and associated.
- (2)
- According to the process mineralogical properties of the tailings samples and the experimental results, an entire recovery process for valuable minerals in the tailings was established. Gold coarse ore, feldspar concentrates, and quartz concentrates were recycled following this process. A 1.74% yield of the sulfur rough concentrate was obtained, and this had a 3.24 g/t Au grade. The yield of the feldspar concentrates was 10.08%, and the K2O content was up to 7.56%. Additionally, a 14.25% yield of the quartz concentrate and a 99.87% grade of SiO2 were also achieved.
- (3)
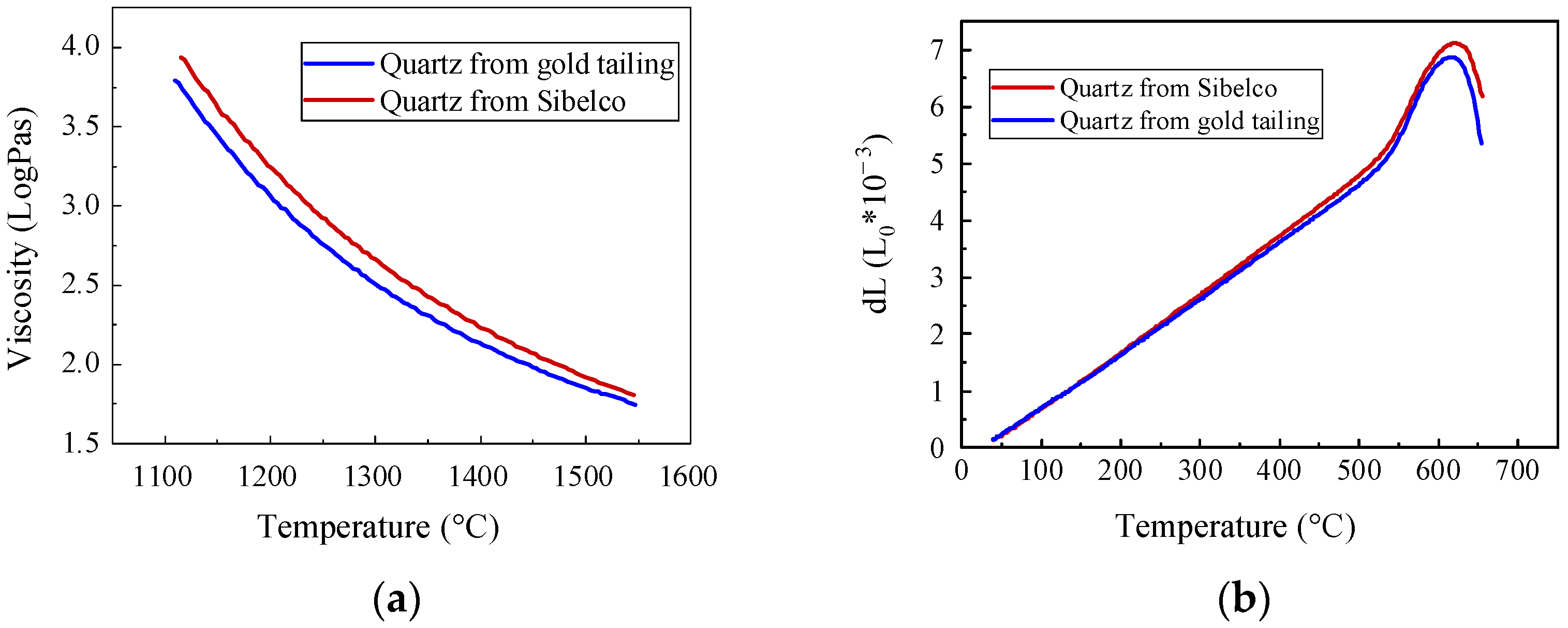
- By analyzing the comparative experimental results of the Sibelco and gold tailings quartz, we found that the high-temperature viscosity of the gold tailings quartz was slightly lower than that of the Sibelco quartz, and that the properties of molten glass products were largely the same for both quartz types.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ince, C. Reusing gold-mine tailings in cement mortars: Mechanical properties and social-economic developments for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2019, 238, 117871. [Google Scholar] [CrossRef]
- World Gold Council. Available online: https://www.gold.org/industry-standards/responsible-gold-mining (accessed on 4 July 2022).
- Norgate, T.; Haque, N. Using life cycle assessment to evaluate some environmental impacts of gold production. J. Clean. Prod. 2012, 29–30, 53–63. [Google Scholar] [CrossRef]
- Lu, Y.; Li, G.; Liu, W.; Yuan, H.; Xiao, D. The application of microwave digestion in decomposing some refractory ore samples with solid fusion agent. Talanta 2018, 186, 538–544. [Google Scholar] [CrossRef]
- Deng, K.; Yin, P.; Liu, X.; Tang, Q.; Qu, R. Modeling, analysis and optimization of adsorption parameters of Au (III) using low-cost agricultural residuals bucwheat hulls. J. Ind. Eng. Chem. 2014, 20, 2428–2438. [Google Scholar] [CrossRef]
- Zheng, W. Analysis of the proportion of aerated concrete block materials produced by using gold tailings. Sci. Technol. Vis. 2013, 8, 24–25. (In Chinese) [Google Scholar]
- China Gold Association. Available online: http://cngold.org.cn/news/show-1574.html (accessed on 6 July 2022).
- Roy, S.; Adhikari, G.R.; Gupta, R.N. Use of gold mill tailings in making bricks: A feasibility study. Waste Manag. Res. 2007, 25, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.P.; Shekdar, A.V. Sustainable waste management in the Indian mining industry. Waste Manag. Res. 2005, 23, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Reddy, B.V.V. Characterization of Kolar gold field mine tailings for cyanide and acid drainage. Geotech. Geol. Eng. 2006, 24, 1545–1559. [Google Scholar] [CrossRef]
- Barcelos, D.A.; Pontes, F.; Silva, F.; Castro, D.C.; dos Anjos, N.O.A.; Castilhos, Z.C. Gold mining tailing: Environmental availability of metals and human health risk assessment. J. Hazard. Mater. 2020, 397, 122721. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Fu, D. Handing and Comprehensive Utilization of Gold Tailings. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2005. [Google Scholar]
- Andrews, J.W.; Moreno, G.J.; Nairn, W.R. Potentialrecovery of aluminum, titanium, lead, and zinc from tailings in the abandoned Picher mining district of Oklahoma. Miner. Econ. 2013, 26, 61–69. [Google Scholar] [CrossRef]
- Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Falagan, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Wei, Z.; Yin, G.Z.; Wang, J.G.; Wan, L.; Li, G. Design, construction and management of tailings storage facilities for surface disposal in China: Case studies of failures. Waste Manag. Res. 2012, 31, 106–112. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W. Comprehensive Utilization and Resource Recovery of Metal Mine Tailings; Metallurgical Industry Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Gaustad, G.; Krystofik, M.; Bustamante, M.; Badami, K. Circular economy strategies for mitigating critical material supply issues. Resour. Conserv. Recycl. 2018, 135, 24–33. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Gerven, T.V.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial processresidues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Altinkaya, P.; Liipo, J.; Kolehmainen, E.; Haapalainen, M.; Leikola, M.; Lundström, M. Leaching of trace amounts of metals from flotation tailings in cupric chloride solutions. Min. Metall. Explor. 2019, 36, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, C.; Li, L.; Wang, Y. Recovery of gold and iron from the cyanide tailings by magnetic roasting. Rare Met. Mater Eng. 2013, 42, 1805–1809. [Google Scholar] [CrossRef]
- Zhijie, C.; Zijie, R.; Huimin, G.; Renji, Z.; Yulin, J.; Chunge, N. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. The flotation behavior and adsorption mechanism of a new cationic collector on the separation of spodumene from feldspar and quartz. Sep. Purif. Technol. 2021, 264, 118445. [Google Scholar] [CrossRef]
- Barros Galvão, J.L.; Andrade, H.D.; Brigolini, G.J.; Fiorotti Peixoto, R.A.; Mendes, J.C. Reuse of iron ore tailings from tailings dams as pigment for sustainable paints. J. Clean. Prod. 2018, 200, 412–422. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. Reuse of base-metal tailings as aggregates for rendering mortars: Assessment of immobilization performances and environmental behavior. Constr. Build. Mater. 2015, 96, 296–306. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Reusing copper tailings in concrete: Corrosion performance and socioeconomic implications for the lefke-xeros area of cyprus. J. Clean. Prod. 2016, 112, 420–429. [Google Scholar] [CrossRef]
- Uribe, L.; Moraga, C.; Rivas, F. Using gold –silver tailings on the elaboration of ceramic foams. J. Sustain. Metall. 2021, 7, 364–376. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Wang, W.; Yang, Y.; Zhuang, S.; Lu, T.; Hou, Z. Utilizing gold mine tailings to produce sintered bricks. Constr. Build. Mater. 2021, 282, 122655. [Google Scholar] [CrossRef]
- Çelik, Ö.; Elbeyli, I.Y.; Piskin, S. Utilization of gold tailings as an additive in portland cement. Waste Manag. Res. 2006, 24, 215. [Google Scholar] [CrossRef]
- Saunders, J.A.; Hofstra, A.H.; Goldfarb, R.J.; Reed, M.H. 13.15—Geochemistry of hydrothermal gold deposits. Treatise Geochem. 2014, 13, 383–424. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Zhong, Q.; Li, Q.; Yang, Y.; Jiang, T. Study on the oxygen pressure alkali leaching of gold with generated thiosulfate from sulfur oxidation. Hydrometallurgy 2018, 177, 178–186. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Li, Q.; Wei, S. Feasibility study on desliming of flotation feed with cyclone classifier. Coal Prep. Technol. 2020, 4, 52–55. (In Chinese) [Google Scholar]
- Chen, W.; Cao, J.; Luo, L. Flotation separation of quartz from feldspar under hydrofluoric acid-free and less sulfuric acid conditions. Min. Metall. Eng. 2003, 3, 35–37. (In Chinese) [Google Scholar]
- QB/T 1636-1992; Feldspar for Ceramics. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2007. (In Chinese)
- DB43T 1167-2016; High Purity (SiO2 ≥ 99.997%) Quartz Sand. Local Standards of Hunan Province: Changsha, China, 2016. (In Chinese)




| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | CaO | MgO | S | TiO2 | CuO | NiO | MnO | WO3 | Pb | Zn | Au *(g/t) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 72.91 | 11.50 | 4.16 | 4.17 | 1.21 | 4.04 | 1.11 | 0.12 | 0.24 | 0.014 | 0.018 | 0.010 | 0.025 | 0.005 | 0.007 | 0.20 |
| Phase | Elemental Gold | Gold in Sulfide | Gold in Hematite, Limonite | Gold in Others | Total Gold |
|---|---|---|---|---|---|
| Content | 0.03 | 0.12 | 0.04 | 0.02 | 0.21 |
| Distribution | 14.29 | 57.14 | 19.05 | 9.52 | 100.00 |
| Particle Size (mm) | Distribution (%) | Cumulative Distribution (%) |
|---|---|---|
| +0.154 | 16.75 | 16.75 |
| −0.154 + 0.074 | 20.93 | 37.68 |
| −0.074 + 0.045 | 28.61 | 66.29 |
| −0.045 + 0.038 | 13.47 | 79.76 |
| −0.038 + 0.019 | 6.35 | 86.11 |
| −0.019 | 13.89 | 100.00 |
| Total | 100.00 | / |
| Minerals | Quartz | K-Feldspar | Plagioclase | Sericite | Calcite | Magnetite, Hematite | Mica | Chlorite | Limonite, Lmenite | Magnetite, Pyrite | Amphiboles | Rutile | Chalcopyrite | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 40.3 | 32.8 | 9.6 | 7.2 | 5.1 | 1.0 | 1.1 | 0.7 | 0.5 | 0.4 | 0.4 | 0.3 | 0.1 | 0.5 |
| Item | Yield | Grade | Distribution | ||||
|---|---|---|---|---|---|---|---|
| Au */(g/t) | SiO2 | K2O | Au | SiO2 | K2O | ||
| Underflow | 75.07 | 0.23 | 75.36 | 4.31 | 86.29 | 77.43 | 75.73 |
| Overflow | 24.93 | 0.11 | 66.13 | 4.16 | 13.71 | 22.57 | 24.27 |
| Feeding | 100.00 | 0.20 | 73.05 | 4.27 | 100.00 | 100.00 | 100.00 |
| Item | Yield | Grade | Recovery | ||||
|---|---|---|---|---|---|---|---|
| Au */(g/t) | SiO2 | Fe2O3 | Au | SiO2 | Fe2O3 | ||
| Flow-off I | 24.33 | 0.11 | 66.38 | 3.49 | 12.78 | 22.23 | 20.76 |
| Flow-off II | 26.19 | 0.13 | 67.27 | 2.88 | 16.27 | 24.25 | 18.44 |
| Magnetic concentrate | 14.96 | 0.41 | 67.07 | 14.17 | 29.30 | 13.81 | 51.84 |
| Sulfur rough concentrate | 1.71 | 3.18 | 61.23 | 11.43 | 25.98 | 1.44 | 4.78 |
| Desulphurized tailings | 32.81 | 0.10 | 84.71 | 0.52 | 15.67 | 38.27 | 4.18 |
| Feeding | 100.00 | 0.21 | 72.64 | 4.09 | 100.00 | 100.00 | 100.00 |
| Minerals | Quartz | K-Feldspar | Plagioclase | Calcite | Chlorite | Sericite | Mica | Hematite, Limonite | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content | 59.6 | 30.5 | 7.3 | 0.8 | 0.3 | 0.8 | 0.2 | 0.3 | 0.2 |
| Chemical Composition | Al2O3 | SiO2 | Na2O | K2O | Fe2O3 | MgO | CaO | S | Au * (g/t) |
|---|---|---|---|---|---|---|---|---|---|
| Content | 6.77 | 84.71 | 1.33 | 3.46 | 0.52 | 0.61 | 1.76 | 0.03 | 0.10 |
| Item | Yield | Grade of Fe2O3 | Recovery Rate of Fe2O3 | ||
|---|---|---|---|---|---|
| Feed * | Run-of-Mine ** | Feed | Run-of-Mine | ||
| Iron-containing impurities | 21.53 | 7.06 | 0.52 | 95.55 | 3.99 |
| Impurity removal middlings | 78.47 | 25.75 | 0.03 | 4.45 | 0.19 |
| Feeding | 100.00 | 32.81 | 0.53 | 100.00 | 4.18 |
| Item | Yield | Grade of K2O | Recovery of K2O | ||
|---|---|---|---|---|---|
| Feed | Run-of-Mine | Feed | Run-of-Mine | ||
| Feldspar concentrate | 39.16 | 10.08 | 7.56 | 90.51 | 18.36 |
| Feldspar middlings | 60.84 | 15.67 | 0.51 | 9.49 | 1.93 |
| Feeding | 100.00 | 25.75 | 3.46 | 100.00 | 20.29 |
| Item | Yield | Grade of SiO2 | Recovery of SiO2 | ||
|---|---|---|---|---|---|
| Feed | Run-of-Mine | Feed | Run-of-Mine | ||
| Quartz concentrate | 90.93 | 14.25 | 99.87 | 92.15 | 19.50 |
| Purified tailings | 9.07 | 1.42 | 85.32 | 7.85 | 1.66 |
| Feeding | 100.00 | 15.67 | 98.55 | 100.00 | 21.16 |
| Chemical Composition | Au */(g/t) | Na2O | K2O | Al2O3 | SiO2 | Fe2O3 | CaO | MgO |
|---|---|---|---|---|---|---|---|---|
| Magnetic concentrate | 0.35 | 0.62 | 3.71 | 9.73 | 68.19 | 14.05 | 0.98 | 0.15 |
| Sulfur rough concentrate | 3.26 | 1.15 | 3.24 | 10.76 | 61.75 | 11.57 | 0.31 | 0.11 |
| Chemical Composition | Na2O | K2O | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Feldspar concentrate | 3.27 | 7.48 | 18.25 | 70.51 | 0.043 | 0.26 | 0.08 | 0.011 | / |
| Quartz concentrate | 0.01 | 0.02 | 0.03 | 99.87 | 0.014 | 0.01 | 0.01 | 0.01 | 0.019 |
| Intrinsic Viscosity (PaS) | Temperature (°C) | Fitted Calculation (°C) | ||
|---|---|---|---|---|
| Gold Tailings | Sibelco | Gold Tailings | Sibelco | |
| 102 | 1509.6 | 1535.2 | 1512.8 | 1536.4 |
| 102.5 | 1375.5 | 1402.2 | 1372.3 | 1402.8 |
| 103 | 1263.6 | 1289.8 | 1260.5 | 1289.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, Q.; Jiang, T. Comprehensive Utilization of Tailings in Quartz Vein-Hosted Gold Deposits. Minerals 2022, 12, 1481. https://doi.org/10.3390/min12121481
Chen L, Li Q, Jiang T. Comprehensive Utilization of Tailings in Quartz Vein-Hosted Gold Deposits. Minerals. 2022; 12(12):1481. https://doi.org/10.3390/min12121481
Chicago/Turabian StyleChen, Liuhui, Qian Li, and Tao Jiang. 2022. "Comprehensive Utilization of Tailings in Quartz Vein-Hosted Gold Deposits" Minerals 12, no. 12: 1481. https://doi.org/10.3390/min12121481




