A Combined EMPA and LA-ICP-MS Study of Muscovite from Pegmatites in the Chinese Altai, NW China: Implications for Tracing Rare-Element Mineralization Type and Ore-Forming Process
Abstract
:1. Introduction
2. Regional Geological Setting
3. Geology of Pegmatites
4. Samples and Analytical Methods
4.1. Samples
4.2. Analytical Methods
4.2.1. EMPA
4.2.2. LA-ICP-MS
5. Results
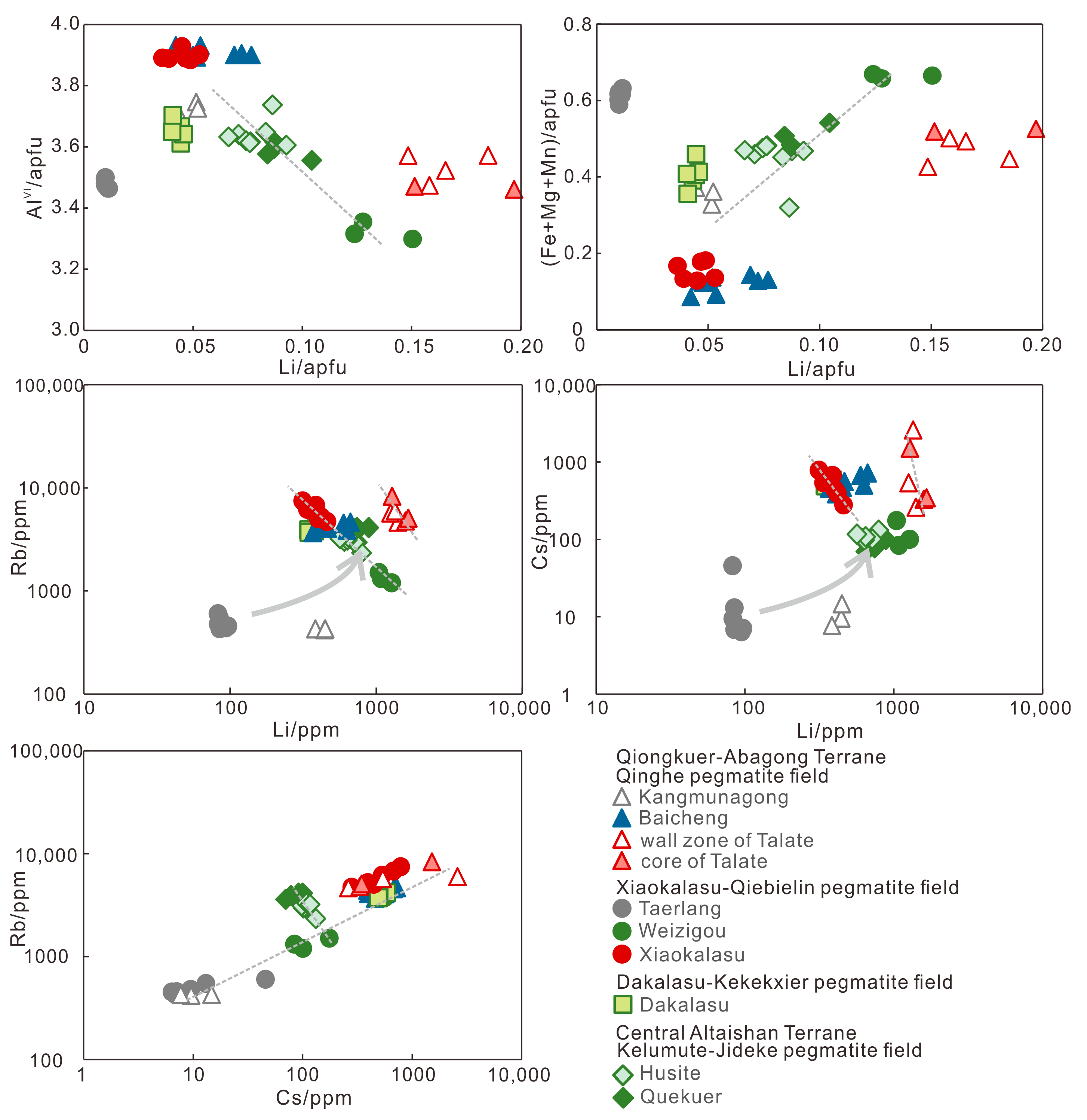
5.1. Major Elements
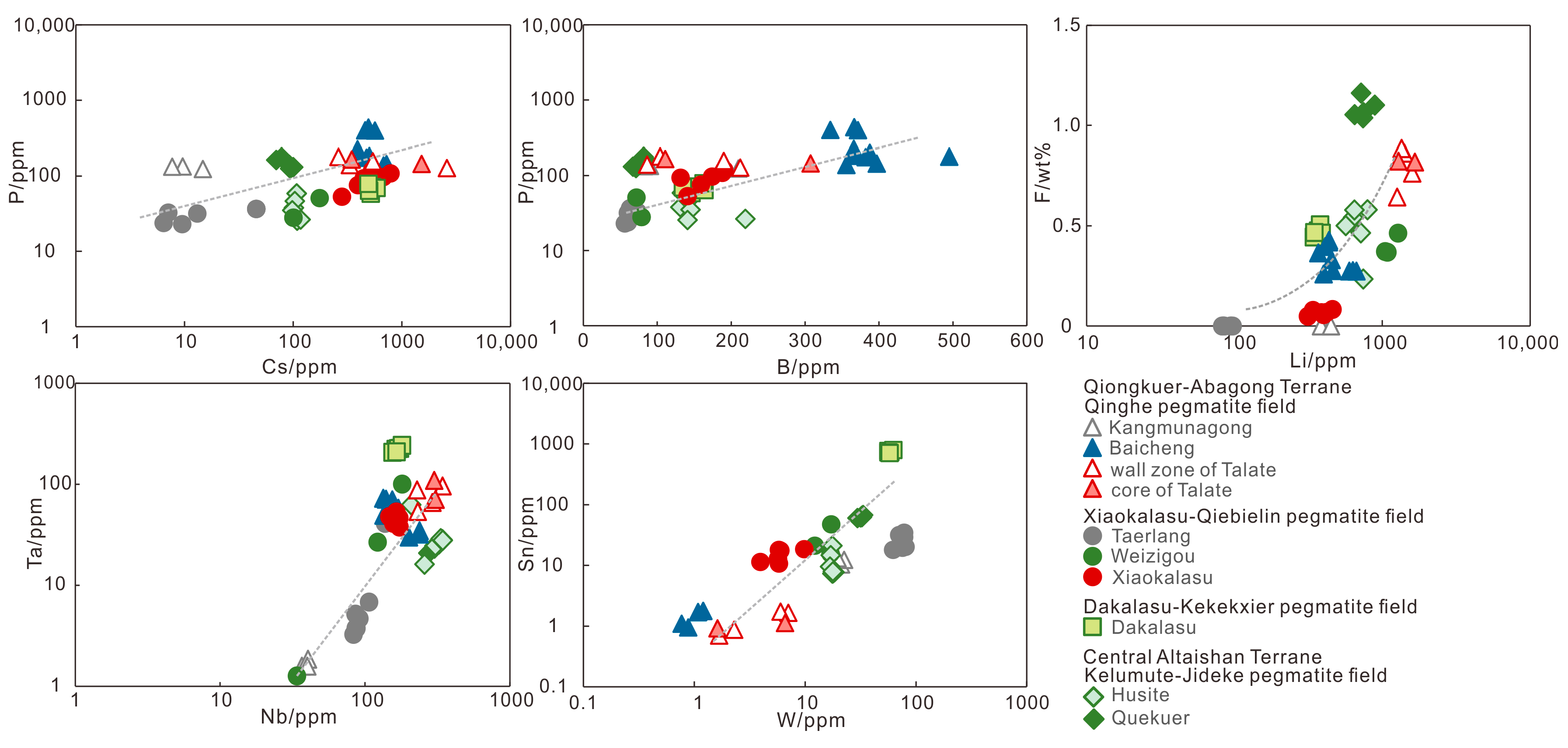
5.2. Trace Components
6. Discussion
6.1. Substitutions among Alkali Elements
6.2. Implications of Fluxes and HFSE
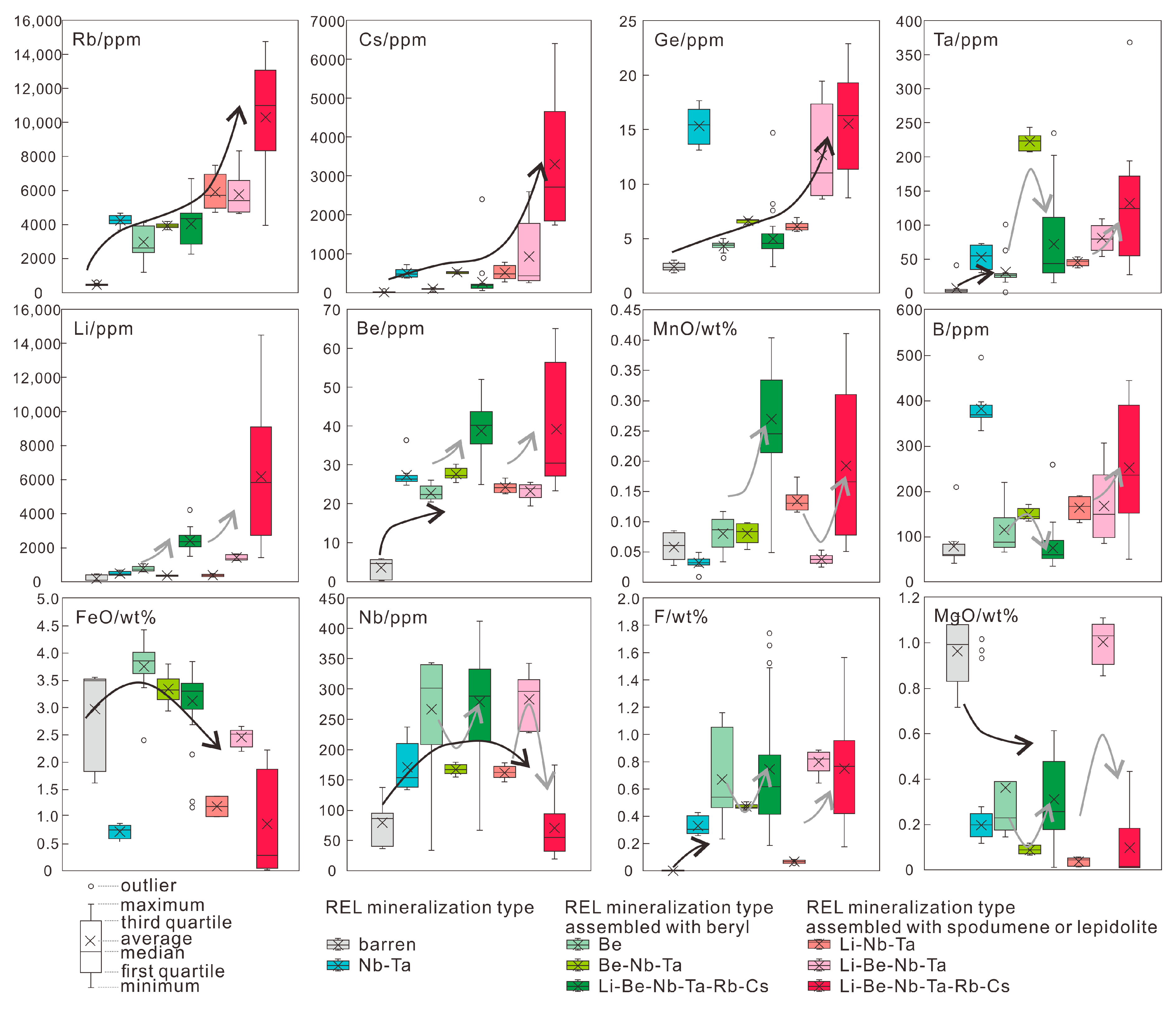
6.3. The Evolution Degree of Different REL Pegmatites
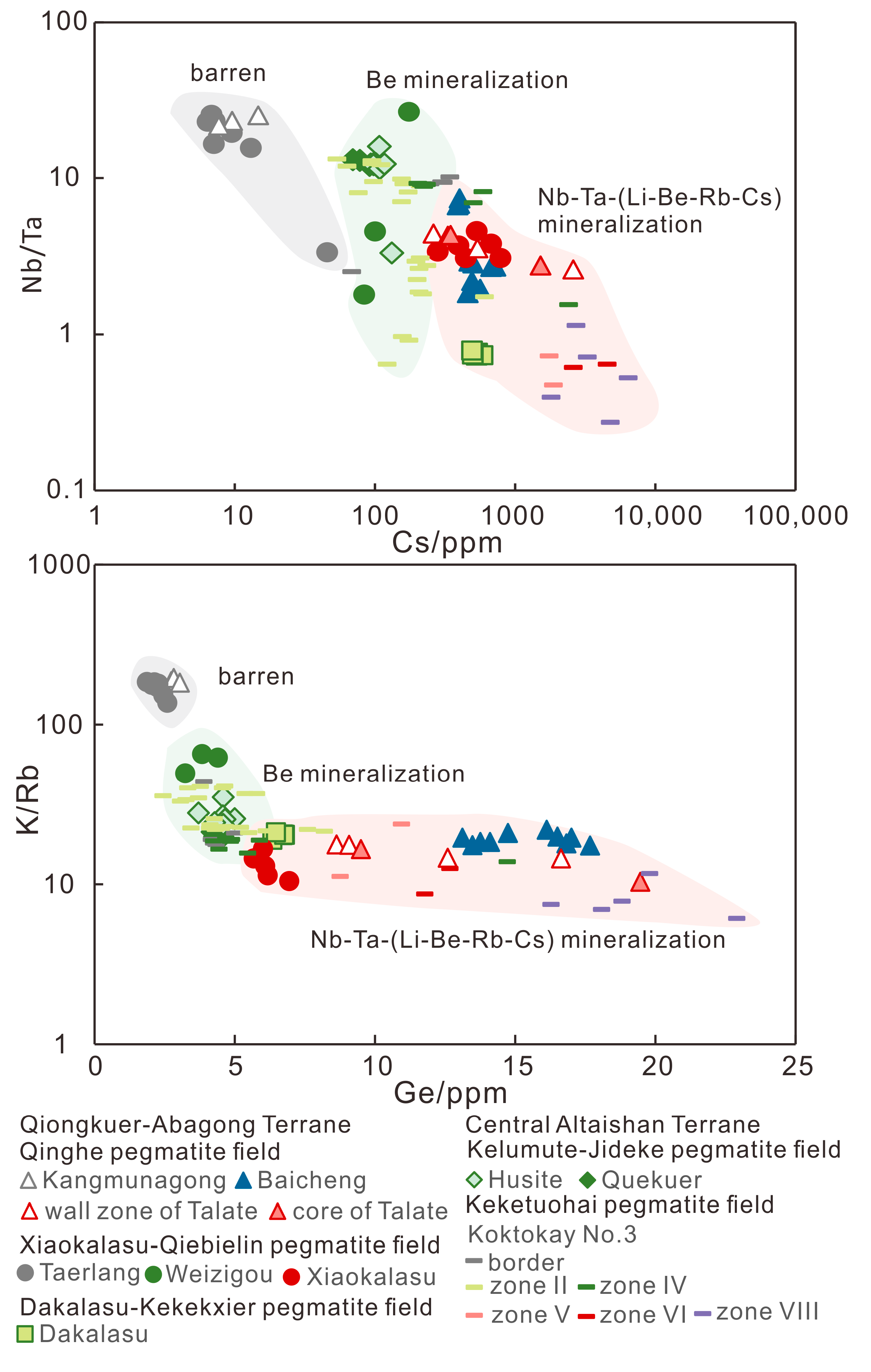
6.4. Possible Indicators of Different REL Mineralization
- (1)
- Barren, Be-mineralized and Li-mineralized pegmatites
- (2)
- Nb-Ta-, beryl- and spodumene-(lepidolite)-bearing pegmatites
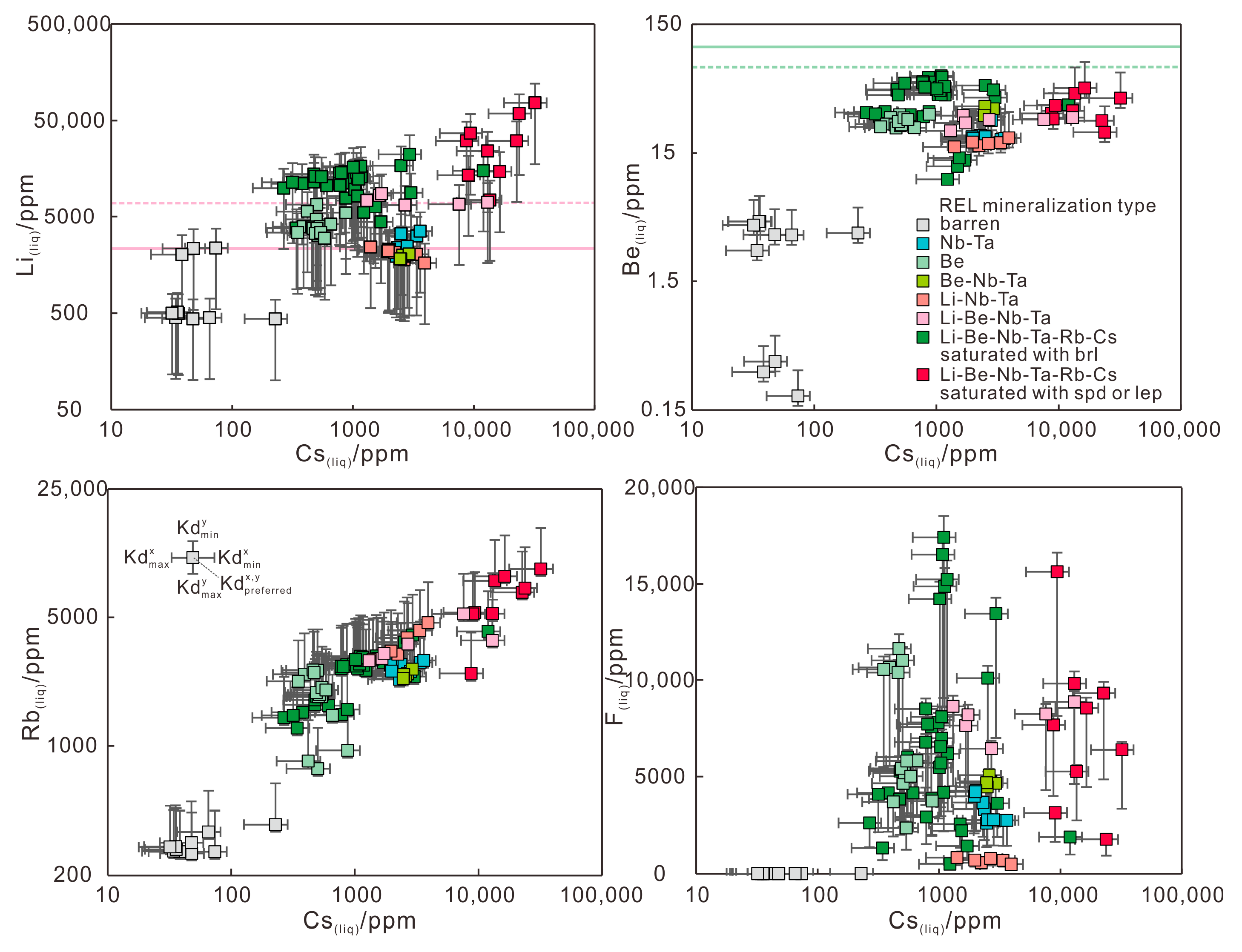
6.5. Evaluation of the REL Concentrations of Liquids
6.5.1. Be Content of Beryl-Forming Magma for Be-Mineralized Pegmatite
6.5.2. Li Content of Spodumene-Forming Magma for Li-Mineralized Pegmatite
7. Conclusions
- Li+ accompanied with Fe, Mg and Mn substitute for Al3+ at the octahedral site in muscovite from the REL pegmatite in the Chinese Altai. The muscovite from some of the Be pegmatites shows a substitution of Rb by Cs at interlayer space.
- The lenses of the Baicheng Nb-Ta pegmatite are produced at late fluid-rich stage with high fluxes including P and B. The enrichment of HFSE in muscovite indicates a Nb-Ta-Sn-W rich pegmatite magma for the Dakalasu Be-Nb-Ta pegmatite.
- From barren pegmatite, beryl-bearing zone, to spodumene-bearing zone, the pegmatite-forming magma basically display an increasing evolution degree.
- The possible indicators of muscovite for barren, Be- and Li-mineralized pegmatites mainly controlled by evolution degree are summarized. In the Chinese Altai, the muscovites from barren pegmatites contain ca. 400–600 ppm Rb, ca. 5–50 ppm, and Cs <3 ppm Ge with <10 ppm Ta and <10 ppm Be; those intergrown with beryl have ca. 1200–4000 ppm Rb, ca. 100–500 ppm Cs, ca. 4–6 ppm Ge and ca. 3–4 wt% FeO; those assembled with spodumene host >4500 ppm Rb, ca. 6–12 ppm Ge and ca. 1–2.5 wt% FeO with large variations of Cs (>300 ppm). The plots of Nb/Ta vs. Cs and K/Rb vs. Ge are proposed to discriminate barren, Be- and Nb-Ta-(Li-Be-Rb-Cs) pegmatites.
- The Li, Be, Rb, Cs and F concentrations of liquid were evaluated using the trace element compositions of muscovites from barren and REL pegmatites in the Chinese Altai. The high Rb and Cs contents of liquid and lower Be contents than beryl saturation value indicate that both highly evolved pegmatite magma and low temperature at emplacement contribute to beryl formation. The liquids saturated with spodumene have large variations of Li, possibly related to metastable state at Li unsaturation–supersaturation or heterogeneous distribution of lithium in the system.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jahns, R.H.; Burnham, C.W. Experimental studies of pegmatite genesis: I. A model for the derivation and crystallization of granitic pegmatites. Econ. Geol. 1969, 64, 843–864. [Google Scholar] [CrossRef]
- London, D. Pegmatites; Special Publication 10; The Canadian Mineralogist: Quebec, QC, Canada, 2008; pp. 1–347. [Google Scholar]
- Černý, P. Geochemical and petrogenitic features of mineralization in rare-element granite pegmatites in the light of current research. Appl. Geochem. 1992, 7, 393–416. [Google Scholar]
- London, D. Granitic pegmatites: An assessment of current concepts and directions for the future. Lithos 2005, 81, 281–303. [Google Scholar] [CrossRef]
- Canosa, F.; Matin-Izard, A.; Fuertes-Fuente, M. Evolved granitic systems as a source of rare-element deposits: The Ponte Segade case (Galicia, NW Spain). Lithos 2012, 153, 165–176. [Google Scholar] [CrossRef]
- Černý, P. (Ed.) Petrogenesis of granitic pegmatites. In Granitic Pegmatites in Science and Industry; Short Course Handbook 8; Mineralogical Association of Canada: Quebec, QC, Canada, 1982; pp. 405–462. [Google Scholar]
- Černý, P.; Meintzer, R.E.; Anderson, A.J. Extreme fractionation in rare-element granitic pegmatites: Selected examples of data and mechanisms. Can. Mineral. 1985, 23, 382–421. [Google Scholar]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Smeds, S.A. Trace elements in potassium-feldspar and muscovite as a guide in the prospecting for lithium- and tin-bearing pegmatites in Sweden. J. Geochem. Explor. 1992, 42, 351–369. [Google Scholar] [CrossRef]
- Pichavant, M.; Villaros, A.; Deveaud, S.; Scaillet, B.; Lahlafi, M. Influence of redox state on mica crystallization in leucogranitic and pegmatitic liquids. Can. Mineral. 2016, 54, 559–581. [Google Scholar] [CrossRef]
- Jolliff, B.L.; Papike, J.J.; Shearer, C.K. Fractionation trends in mica and tourmaline as indicators of pegmatite internal evolution: Bob Ingersoll pegmatite, Black Hills, South Dakota, USA. Geochim. Cosmochim. Acta 1987, 51, 519–534. [Google Scholar] [CrossRef]
- Kile, D.E.; Foord, E.E. Micas from the Pikes Peak batholith and its cogenetic granitic pegmatites, Colorado: Optical properties, composition, and correlation with pegmatite evolution. Can. Mineral. 1998, 36, 463–482. [Google Scholar]
- Roda, E.; Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, J.; de Parseval, P. Mineralogy and geochemistry of micas from the Pinilla de Fermoselle pegmatite (Zamora, Spain). Eur. J. Mineral. 2006, 18, 369–377. [Google Scholar] [CrossRef]
- Roda, E.; Keller, P.; Pesquera, A.; Fontan, F. Micas of the muscovite-lepidolite series from Karibib pegmatites, Namibia. Mineral. Mag. 2007, 71, 41–62. [Google Scholar] [CrossRef]
- Černý, P.; Burt, D.M. Paragenesis, crystallochemical characteristics, and geochemical evolution of micas in granite pegmatites. In Micas; Bailey, S.W., Ed.; Reviews in mineralogy 13; Mineralogical Society of America: Washington, DC, USA, 1984; pp. 257–297. [Google Scholar]
- Henderson, C.M.B.; Martin, J.S.; Mason, R.A. Compositional relations in Li-micas from S.W. England and France: An ion- and electron-microprobe study. Mineral. Mag. 1989, 53, 427–449. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Lugli, C.; Poppi, L.; Foord, E.E.; Kile, D.E. Crystal chemical variations in Li- and Fe-rich micas from Pikes Peak batholith (central Colorado). Am. Mineral. 2000, 85, 1275–1286. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegmatites. Part I: Anatomy and internal evolution pegmatite deposits. Geosci. Can. 1991, 18, 49–67. [Google Scholar]
- Černý, P. The Tanco rare-element pegmatite deposit, Manitoba: Regional context, internal anatomy, and global comparisons. In Rare Element Geochemistry and Mineral Deposits; Linnen, R.L., Samson, I.M., Eds.; Short Course Notes 17; Geological Association of Canada: Quebec, QC, Canada, 2004; pp. 184–231. [Google Scholar]
- Foord, E.E.; Černý, P.; Jackson, L.L.; Sherman, D.M.; Eby, R.K. Mineralogical and geochemical evolution of micas from miarolitic pegmatites of the anorogenic Pikes Peak batholith, Colorado. Mineral. Petrol. 1995, 55, 1–26. [Google Scholar] [CrossRef]
- Wise, M.A. Trace element chemistry of lithium—Rich micas from rare—Element granitic pegmatites. Mineral. Petrol. 1995, 55, 203–215. [Google Scholar] [CrossRef]
- Pesquera, A.; Torres-Ruiz, J.; Gel-Crespo, P.; Velilla, N. Chemistry and genetic implications of tourmaline and Li-F-Cs micas from the Valdeflores area (Cáceres, Spain). Am. Mineral. 1999, 84, 55–69. [Google Scholar] [CrossRef]
- Clarke, D.B.; Bogutyn, P.A. Oscillatory epitactic-growth zoning in biotite and muscovite from the Lake Lewis leucogranite, South Mountain batholith, Nova Scotia, Canada. Can. Mineral. 2003, 41, 1027–1047. [Google Scholar] [CrossRef]
- Viana, R.R.; Jordt-Evengelista, H.; Stern, W.B. Geochemistry of muscovite from pegmatites of the Eastern Brazilian pegmatite province: A clue to petrogenesis and mineralization potential. Eur. J. Mineral. 2007, 19, 745–755. [Google Scholar] [CrossRef]
- Vieira, R.R.; Roda-Robles, E.; Pesquera, A.; Lima, A. Chemical variation and significance of micas from the Fregeneda-Almendra pegmatitic field (Central-Iberian zone, Spain and Portugal). Am. Mineral. 2011, 96, 637–645. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.L.; He, P.L.; Li, W.X.; Yu, Y.; Chen, L.L. In situ analyses of micas in the Yashan granite, South China: Constraints on magmatic and hydrothermal evolutions of W and Ta-Nb bearing granites. Ore Geol. Rev. 2015, 65, 793–810. [Google Scholar] [CrossRef]
- Villaros, A.; Pichavant, M. Mica-liquid trace elements partitioning and the granite-pegmatite connection: The St-Sylvestre complex (Western French Massif Central). Chem. Geol. 2019, 528, 119265. [Google Scholar] [CrossRef]
- Zou, T.R.; Li, Q.C. Rare and Rare Earth Metallic Deposits in Xinjiang, China; Geological Publishing House: Beijing, China, 2006; pp. 1–284, (In Chinese with English Abstract). [Google Scholar]
- Chen, F.W.; Li, H.Q.; Wang, D.H.; Cai, H.; Chen, W. New chronological evidence for Yanshanian diagenetic mineralization in China’s Altay orogenic belt. Chin. Sci. Bull. 2000, 45, 108–114. [Google Scholar] [CrossRef]
- Wang, D.H.; Chen, Y.C.; Zou, T.R.; Xu, Z.G.; Li, H.Q.; Chen, W.; Chen, F.W.; Tian, F. 40Ar/39Ar dating for the Azubai rare metal-gem deposit in Altay, Xinjiang-New evidence for Yanshanian mineralization of rare metals. Geol. Rev. 2000, 46, 307–311, (In Chinese with English Abstract). [Google Scholar]
- Wang, D.H.; Chen, Y.C.; Xu, Z.G. Chronological study of Caledonian metamorphic pegmatite muscovite deposits in the Altay Mountains, northwestern China, and its significance. Acta Geol. Sin. 2001, 75, 419–425, (In Chinese with English Abstract). [Google Scholar]
- Wang, D.H.; Chen, Y.C.; Xu, Z.G. 40Ar/39Ar isotope dating on muscovite from Indosinian rare metal deposits in Central Altay, Northwestern China. Bull. Mineral. Petrol. Geochem. 2003, 22, 14–17, (In Chinese with English Abstract). [Google Scholar]
- Wang, D.H.; Zou, T.R.; Xu, Z.G.; Yu, J.J.; Fu, X.F. Advance in the study of using pegmatite deposits as the tracer of orogenic process. Adv. Earth Sci. 2004, 19, 614–620, (In Chinese with English Abstract). [Google Scholar]
- Ren, B.Q.; Zhang, H.; Tang, Y.; Lv, Z.H. LA-ICP-MS U-Pb zircon geochronology of the Altai pegmatites and its geological significance. Acta Mineral. Sin. 2011, 31, 587–596, (In Chinese with English Abstract). [Google Scholar]
- Lv, Z.H.; Zhang, H.; Tang, Y.; Guan, S.J. Petrogenesis and magmatic-hydrothermal evolution time limitation of Kelumute No.112 pegmatite in Altay, Northwestern China: Evidence from zircon U-Pb and Hf isotopes. Lithos 2012, 154, 374–391. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Qin, K.Z.; Tang, D.M.; Tian, Y.; Cao, M.J.; Wang, C.L. Formation age and evolution time span of the Koktokay No.3 pegmatite, Altai, NW China: Evidence from U-Pb zircon and 40Ar/39Ar muscovite ages. Resour. Geol. 2015, 65, 210–231. [Google Scholar] [CrossRef]
- Lv, Z.H.; Zhang, H.; Tang, Y.; Liu, Y.L.; Zhang, X. Petrogenesis of syn-orogenic rare metal pegmatites in the Chinese Altai: Evidences from geology, mineralogy, zircon U-Pb age and Hf isotope. Ore Geol. Rev. 2018, 95, 161–181. [Google Scholar] [CrossRef]
- Lv, Z.H.; Zhang, H.; Tang, Y. Anatexis origin of rare metal/earth pegmatites: Evidences from the Permian pegmatites in the Chinese Altai. Lithos 2021, 380–381, 105865. [Google Scholar] [CrossRef]
- Zhang, A.C.; Wang, R.C.; Hu, H.; Zhang, H.; Zhu, J.C.; Chen, X.M. Chemical evolution of Nb-Ta oxides and zircon from the Koktokay No.3 granitic pegmatite, Altai, northwestern China. Mineral. Mag. 2004, 68, 739–756. [Google Scholar] [CrossRef]
- Zhang, A.C.; Wang, R.C.; Jiang, S.Y.; Hu, H.; Zhang, H. Chemical and textural features of tourmaline from the spodumene-subtype Koktokay No. 3 pegmatite, Altai, northwestern China: A record of magmatic to hydrothermal evolution. Can. Mineral. 2008, 46, 41–58. [Google Scholar] [CrossRef]
- Zhang, A.C.; Wang, R.C.; Li, Y.; Hu, H.; Lu, X.; Ji, J.; Zhang, H. Tourmalines from the Koktokay No. 3 pegmatite, Altai, NW China: Spectroscopic characterization and relationships with the pegmatite evolution. Eur. J. Mineral. 2008, 20, 143–154. [Google Scholar] [CrossRef]
- Liu, C.Q.; Zhang, H. The lanthanide tetrad effect in apatite from the Altay No.3 pegmatite, Xingjiang, China: An intrinsic feature of the pegmatite magma. Chem. Geol. 2005, 214, 61–77. [Google Scholar] [CrossRef]
- Wang, R.C.; Hu, H.; Zhang, A.C.; Fontan, F.; Parseval, P.D.; Jiang, S.Y. Cs-dominant polylithionite in the Koktokay #3 pegmatite, Altai, NW China: In situ micro-characterization and implication for the storage of radioactive cesium. Contrib. Mineral. Petrol. 2007, 153, 355–367. [Google Scholar]
- Cao, M.J.; Zhou, Q.F.; Qin, K.Z.; Tang, D.M.; Evans, N.J. The tetrad effect and geochemistry of apatite from the Altay Koktokay No.3 pegmatite, Xinjiang, China: Implications for pegmatite petrogenesis. Mineral. Petrol. 2013, 107, 985–1005. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Qin, K.Z.; Tang, D.M.; Ding, J.G.; Guo, Z.L. Mineralogy and significance of micas and feldspars from the Koktokay No.3 pegmatitic rare-element deposit, Altai. Acta Petrol. Sin. 2013, 29, 3004–3022, (In Chinese with English Abstract). [Google Scholar]
- Zhou, Q.F.; Qin, K.Z.; Tang, D.M.; Wang, C.L.; Tian, Y.; Sakyi, P.A. Mineralogy of the Koktokay No.3 pegmatite, Altai, NW China: Implications for evolution and melt-fluid processes of rare-metal pegmatites. Eur. J. Mineral. 2015, 27, 433–457. [Google Scholar] [CrossRef]
- Xiao, X.C.; Tang, Y.Q.; Feng, Y.; Zhu, B.; Li, J.; Zhou, M. Tectonics in Northern Xinjiang and Its Neighbouring Areas; Geological Publishing: Beijing, China, 1992; pp. 1–169, (In Chinese with English Abstract). [Google Scholar]
- Windley, B.F.; Krӧner, A.; Guo, J.; Qu, G.; Li, Y.; Zhang, C. Neoproterozoic to Paleozoic geology of the Altai orogen, NW China: New zircon age data and tectonic evolution. J. Geol. 2002, 110, 719–737. [Google Scholar] [CrossRef]
- Wang, T.; Hong, D.W.; Jahn, B.M.; Tong, Y.; Wang, Y.B.; Han, B.F.; Wang, X.X. Timing, petrogenesis and setting of Paleozoic syn-orogenic intrusions from the Altai Mountains, NW China: Implications for tectonic evolution of an accretionary orogen. J. Geol. 2006, 114, 735–751. [Google Scholar] [CrossRef]
- Chen, B.; Jahn, B.M. Geochemical and isotopic studies of the sedimentary and granitic rocks of the Altai orogen of northwest China and their tectonic implications. Geol. Mag. 2002, 139, 1–13. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Yuan, C.; Sun, M.; Han, C.M.; Lin, S.F.; Chen, H.L.; Yan, Q.R.; Liu, D.Y.; Qin, K.Z.; et al. Paleozoic multiple subduction-accretion processes of the southern Altaids. Am. J. Sci. 2009, 309, 221–270. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, T.; Hong, D.W.; Dai, Y.J. TIMS U-Pb zircon ages of Fuyun post-orogenic linear granite plutons on the southern margin of Altay orogenic belt and their implications. Acta Petrol. Mineral. 2006, 29, 619–641, (in Chinese with English Abstract). [Google Scholar]
- Yuan, C.; Sun, M.; Long, X.P.; Xia, X.P.; Xiao, W.J.; Li, X.H.; Lin, S.F.; Cai, K.D. Constraining the deposition time and tectonic background of the Habahe Group of the Altai. Acta Petrol. Sin. 2007, 23, 1635–1644, (In Chinese with English Abstract). [Google Scholar]
- Cai, K.D.; Sun, M.; Yuan, C.; Zhao, G.C.; Xiao, W.J.; Long, X.P.; Wu, F.Y. Prolonged magmatism, juvenile nature and tectonic evolution of the Chinese Altai, NW China: Evidence from zircon U-Pb and Hf isotopic study of Paleozoic granitoids. J. Asian Earth Sci. 2011, 42, 949–968. [Google Scholar] [CrossRef]
- Cai, K.D.; Sun, M.; Yuan, C.; Long, X.P.; Xiao, W.J. Geological framework and Paleozoic tectonic history of the Chinese Altai, NW China: A review. Russ. Geol. Geophys. 2011, 52, 1619–1633. [Google Scholar] [CrossRef]
- Shen, X.M.; Zhang, H.X.; Wang, Q.; Wyman, D.A.; Yang, Y.H. Late Devonian-Early Permian A-type granites in the southern Altay Range, Northwest China: Petrogenesis and implications for tectonic setting of “A2-type” granites. J. Asian Earth Sci. 2011, 42, 986–1007. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Badarch, G.; Sun, S.; Li, J.L.; Qin, K.Z.; Wang, Z. Palaeozoic accretionary and convergent tectonics of the southern Altaids: Implications for the growth of central Asia. J. Geol. Soc. Lond. 2004, 161, 339–342. [Google Scholar] [CrossRef]
- Sengӧr, A.M.C.; Natalín, B.A.; Burtman, V.S. Evolution of the Altaid tentonic collage and Paleozoic crustal growth in Eurasia. Nature 1993, 364, 299–307. [Google Scholar] [CrossRef]
- He, G.Q.; Li, M.S.; Liu, D.Q.; Zhou, N.H. Palaeozoic Crustal Evolution and Mineralization in Xinjiang of China; Xinjiang People’s Publishing House: Urumqi, China, 1994; pp. 1–437. (In Chinese) [Google Scholar]
- Qin, K.Z. Metallogeneses in Relation to Central-Asia Style Orogeny of Northern Xinjiang. Post-Doctoral Research Report; Institute of Geology and Geophysics, Chinese Academy of Sciences: Beijing, China, 2000; (In Chinese with English Abstract). [Google Scholar]
- Sun, M.; Long, X.P.; Cai, K.D.; Jiang, Y.D.; Wang, B.Y.; Yuan, C.; Zhao, G.C.; Xiao, W.J.; Wu, F.Y. Early Paleozoic ridge subduction in the Chinese Altai: Insight from the abrupt change in zircon Hf isotopic composition. Sci. China 2009, 52, 1345–1358. [Google Scholar] [CrossRef]
- Li, J.Y.; Xiao, W.J.; Sun, G.H.; Gao, L.M. Neoproterozoic-Paleozoic tectonostratigraphy, magmatic activities and tectonic evolution of eastern Xinjiang, NW China. In Tectonic Evolutioin and Metallogeny of the Chinese Altay and Tianshan; Mao, J.W., Goldfarb, R.J., Seltmann, R., Wang, D.H., Xiao, W.J., Hart, C., Eds.; IAGOD Guidebook Series; CERCAMS/NHM 10: London, UK, 2003; pp. 31–74. [Google Scholar]
- Wang, T.; Hong, D.W.; Tong, Y.; Han, B.F.; Shi, Y.R. Zircon U-Pb SHRIMP age and origin of post-orogenic Lamazhao granitic pluton from Altai orogen: Its implications for vertical continental growth. Acta Petrol. Sin. 2005, 21, 640–650, (In Chinese with English Abstract). [Google Scholar]
- Li, P.F.; Yuan, C.; Sun, M.; Long, X.P.; Cai, K.D. Thermochronological constraints on the late Paleozoic tectonic evolution of the southern Chinese Altai. J. Asian. Earth. Sci. 2015, 113, 51–60. [Google Scholar] [CrossRef]
- Broussolle, A.; Aguilar, C.; Sun, M.; Schulmann, K.; Štípská, P.; Jiang, Y.D.; Yu, Y.; Xiao, W.J.; Wang, S.; Míková, J. Polycyclic Paleozoic evolution of accretionary orogenic wedge in the southern Chinese Altai: Evidence from structural relationships and U-Pb geochronology. Lithos 2018, 314, 400424. [Google Scholar]
- Xiao, W.J.; Windley, B.F.; Han, C.M.; Liu, W.; Wan, B.; Zhang, J.E.; Ao, S.J.; Zhang, Z.Y.; Song, D.F. Late Paleozoic to early Triassic multiple roll-back and oroclinal bending of the Mongolia collage in Central Asia. Earth Sci. Rev. 2018, 186, 94–128. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, T.; Kovach, V.P.; Hong, D.W.; Han, B.F. Age and origin of Takeshiken postorogenic alkali-rich intrusive rocks in southern Altai, near the Mongolian border in China and its implicaitons for continental growth. Acta Petrol. Mineral. 2006, 22, 1267–1278, (In Chinese with English Abstract). [Google Scholar]
- Wang, T.; Tong, Y.; Jahn, B.M.; Zou, T.R.; Wang, Y.B.; Hong, D.W.; Han, B.F. SHRIMP U-Pb Zircon geochronology of the Altai No.3 Pegmatite, NW China, and its implications for the origin and tectonic setting of the pegmatite. Ore Geol. Rev. 2007, 32, 325–336. [Google Scholar] [CrossRef]
- Wang, T.; Tong, Y.; Li, S.; Zhang, J.J.; Shi, X.J.; Li, J.Y.; Han, B.F.; Hong, D.W. Spatial and temporal variations of granitoids in the Altay orogen and their implications for tectonic setting and crustal growth: Perspectives from Chinese Altay. Acta Petrol. Mineral. 2010, 29, 595–618, (In Chinese with English Abstract). [Google Scholar]
- Liu, F.; Zhang, Z.X.; Li, Q.; Zhang, C.; Li, C. New precise timing constraint for the Keketuohai No.3 pegmatite in Xinjiang, China and identification of its parental pluton. Ore Geol. Rev. 2014, 56, 209–219. [Google Scholar] [CrossRef]
- Wang, C.L.; Qin, K.Z.; Tang, D.M.; Zhou, Q.F.; Shen, M.D.; Guo, Z.L.; Guo, X.J. Geochronology and Hf isotope of zircon for the Arskartor Be-Nb-Mo deposit in Altay and its geological implications. Acta Petrol. Sin. 2015, 31, 2337–2352, (In Chinese with English Abstract). [Google Scholar]
- Zhou, Q.F.; Qin, K.Z.; Tang, D.M.; Wang, C.L.; Sakyi, P.A. LA-ICP-MS U-Pb zircon, columbite-tantalite and 40Ar/39Ar muscovite age constraints for the rare-element pegmatite dikes in the Altai orogenic belt, NW China. Geol. Mag. 2018, 155, 707–728. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Sun, S.; Li, J.L.; Huang, B.C.; Han, C.M.; Yuan, C.; Sun, M.; Chen, H.L. A tale of amalgamation of three Permo-Triassic collage systems in Central Asia: Oroclines, sutures, and terminal accretion. Annu. Rev. Earth Planet. Sci. 2015, 43, 477–507. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.J.; Cawood, P.A.; Wang, Y.J.; Chen, H.Y.; Zhang, L.; Li, D.F. Late Permian-Triassic metallogeny in the Chinese Altay Orogen: Constraints from mica 40Ar/39Ar dating on ore deposits. Gondwana Res. 2017, 43, 4–16. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, H.; Tang, Y.; Zhang, X.; Lv, Z.H.; Zhao, J.Y. Petrogenesis and tectonic setting of the Middle Permian A-type granites in Altay, Northwestern China: Evidences from geochronological, geochemical, and Hf isotopic studies. Geol. J. 2018, 53, 527–546. [Google Scholar] [CrossRef]
- Yuan, C.; Sun, M.; Xiao, W.J.; Li, X.H.; Chen, H.L.; Lin, S.F.; Xia, X.P.; Long, X.P. Accretionary orogenesis of the Chinese Altai: Insights from Paleozoic granitoids. Chem. Geol. 2007, 242, 22–339. [Google Scholar] [CrossRef]
- Geng, X.X.; Chai, F.M.; Yang, F.Q.; Zuo, W.Z.; Guo, Z.L.; Liu, F.; Zhang, Z.X. Geochronology and genesis of the bimodal volcanic rocks in Dalawuzi from the southern margin of Altay, Xinjiang. Acta Petrol. Sin. 2010, 26, 2967–2980, (In Chinese with English Abstract). [Google Scholar]
- Armstrong, J.T. CITZAF: Combined ZAF and phirho(Z) Electron Beam Correction Programs; California Institute of Technology: Pasadena, CA, USA, 1989. [Google Scholar]
- Xie, L.W.; Zhang, Y.B.; Zhang, H.H.; Sun, J.F.; Wu, F.Y. In situ simultaneous determination of trace elements, U-Pb and Lu-Hf isotopes in zircon and baddeleyite. Chin. Sci. Bull. 2008, 53, 1565–1573. [Google Scholar] [CrossRef]
- Griffin, W.L.; Powell, W.J.; Pearson, N.J.; O’Reilly, S.Y. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation-ICP-MS in the Earth Sciences; Sylvester, P., Ed.; Short Course Series 40, Appendix 2; Mineralogical Association of Canada: Quebec, QC, Canada, 2008; pp. 204–207. [Google Scholar]
- Tischendorf, G.; Gottesmann, B.; Förster, H.-J.; Trumbull, R.B. On Li-bearing micas: Estimating Li from electron microprobe analyses and an improved diagram for graphical representation. Mineral. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Maneta, V.; Baker, D.R. The potential of lithium in alkali feldspars, quartz, and muscovite as a geochemical indicator in the exploration for lithium-rich granitic pegmatites: A case study from the spodumene-rich Moblan pegmatite, Quebec, Canada. J. Geochem. Explor. 2019, 205, 106336. [Google Scholar] [CrossRef]
- Legros, H.; Marignac, C.; Mercadier, J.; Cuney, M.; Richard, A.; Wang, R.C.; Charles, N.; Lespinasse, M.Y. Detailed paragenesis and Li-mica compositions as recorders of the magmatic-hydrothermal evolution of the Maoping W-Sn deposit (Jiangxi, China). Lithos 2016, 264, 108–124. [Google Scholar] [CrossRef]
- Li, P.; Li, J.K.; Chen, Z.Y.; Liu, X.; Huang, Z.B.; Zhou, F.C. Compositional evolution of the muscovite of Renli pegmatite-type rare-metal deposit, northeast Hunan, China: Implications for its petrogenesis and mineralization potential. Ore Geol. Rev. 2021, 138, 104830. [Google Scholar] [CrossRef]
- London, D. A petrologic assessment of internal zonation in granitic pegmatites. Lithos 2014, 184–187, 74–104. [Google Scholar] [CrossRef]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- López-Moro, F.J.; Polonio, F.G.; Gonázlez, T.L.; Contreras, J.L.S.; Fernández, A.G.; Benito, M.C.M. Ta-Sn concentration by muscovite fractionation and degassing in a lens-like granite body: The case study of the Penouta rare-metal albite granite (NW Spain). Ore Geol. Rev. 2017, 82, 10–30. [Google Scholar] [CrossRef]
- Breiter, K.; Hložková, M.; Korbelová, Z.; Galiová, M.V. Diversity of lithium mica compositions in mineralized granite-greisen system: Cnovec Li-Sn-W deposit, Erzgebirge. Ore Geol. Rev. 2019, 106, 12–27. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.C.; Groat, L.A.; Zhu, J.C.; Huang, F.F.; Cempírek, J. A combined EMPA and LA-ICP-MS study of Li-bearing mica and Sn-Ti oxide minerals from the Qiguling topaz rhyolite (Qitianling District, China): The role of fluorine in origin of tin mineralization. Ore Geol. Rev. 2015, 65, 779–792. [Google Scholar] [CrossRef]
- Guimarães, F.S.; de Oliveira, A.L.R.; Amorim, L.E.D.; Rios, F.J.; Lehmann, B.; Hernndez, C.R.; Moraes, R. Lithium-mica composition as pathfinder and recorder of Grenvillian-age greisenization, Rondonia Tin Province, Brazil. Geochemistry 2021, 81, 125737. [Google Scholar] [CrossRef]
- Shi, R.Z.; Zhao, J.X.; Evans, N.J.; Qin, K.Z.; Wang, F.Y.; Li, Z.Z.; Han, R.; Li, X.F. Temporal-spatial variations in Li-Fe mica compositions from the Weilasituo Sn-polymetallic deposit (NE China): Implications for deposit-scale fluid evolution. Ore Geol. Rev. 2021, 134, 104132. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Grégoire, M.; Linnen, R.L.; Béziat, D.; Salvi, S. Trace element geochemistry by laser ablation ICP-MS of micas associated with Ta mineralization in the Tanco pegmatite, Manitoba, Canada. Contrib. Mineral. Petrol. 2008, 155, 791–806. [Google Scholar] [CrossRef]
- Oyarzábal, J.; Galliski, M.A.; Perino, E. Geochemistry of K-feldspar and Muscovite in rare-element pegmatites and granites from the Totoral pegmatite field, San Luis, Argentina. Resour. Geol. 2009, 59, 315–329. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegamatites. Part II: Regional to global environments and petrogenesis. Geosci. Can. 1991, 18, 68–81. [Google Scholar]
- Hulsbosch, N.; Hertogen, J.; Dewaele, S.; André, L.; Muchez, P. Alkali metal and rare earth element evolution of rock-forming minerals from the Gatumba area pegmatites (Rwanda): Quantitative assessment of crystal-melt fractionation in the regional zonation of pegmatite groups. Geochim. Cosmochim. Acta 2014, 132, 349–374. [Google Scholar] [CrossRef]
- Černý, P.; Trueman, D.L.; Ziehlke, D.V.; Goad, B.E.; Paul, B.J. The Cat Lake-Winnipeg River and Wekusku Lake Pegmatite Fields, Manitoba; ER80-1; Department of Energy and Mines, Mineral Resources Division: Winnipeg, MB, Canada, 1981; 234p. [Google Scholar]
- Lu, H.Z.; Wang, Z.G.; Li, Y.S. Magma-fluid transition and the genesis of pegmatite dike No.3, Altay, Xinjiang, Northwest China. Chin. J. Geochem. 1997, 16, 43–52. [Google Scholar]
- Wang, R.C.; Hu, H.; Zhang, A.C.; Fontan, F.; Zhang, H.; Parseval, P.D. Occurrence and late re-equilibration of pollucite from the Koktokay No. 3 pegmatite, Altai, northwestern China. Am. Mineral. 2006, 91, 729–739. [Google Scholar] [CrossRef]
- Wang, R.C.; Che, X.D.; Zhang, W.L.; Zhang, A.C.; Zhang, H. Geochemical evolution and late re-equilibration of Na-Cs-rich beryl from the Koktokay #3 pegmatite (Altai, NW China). Eur. J. Mineral. 2009, 21, 795–809. [Google Scholar]
- Zhou, Q.F. The Geochronology, Mineralogy, Melt-Fluid Evolution and Metallogenesis of the Koktokay No.3 Pegmatitic Rare-Element Deposit, Altai, China. Ph.D. Thesis, Institue of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Van Lichtervelde, M.; Salvi, S.; Beziat, D.; Linnen, R.L. Textural features and chemical evolution in tantalum oxides: Magmatic versus hydroterhmal origins for Ta mineralization in the Tanco Lower Pegmatite, Manitoba, Canada. Econ. Geol. 2007, 102, 257–276. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P.; Beurlen, H. Tantalite-(Mn) from the Borborema Pegmatite Province, northeastern Brazil: Conditions of formation and melt and fluid-inclusion constraints on experimental studies. Mineral. Depos. 2011, 46, 749–759. [Google Scholar] [CrossRef]
- Beurlen, H.; Thomas, R.; da Silva, M.R.R.; Müller, A.; Rhede, D.; Soares, D.R. Perspectives for Li- and Ta-mineralization in the Borborema pegmatite province, NE-Brazil: A review. J. S. Am. Earth Sci. 2014, 56, 110–127. [Google Scholar] [CrossRef]
- Stepanov, A.; Mavrogenes, J.A.; Meffre, S.; Davidson, P. The key role of mica during igneous concentration of tantalum. Contrib. Mineral. Petrol. 2014, 167, 1009. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strngth trace elmenets in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Holtz, F.; Melcher, F. The effect of disequilibrium crystallization on Nb-Ta fractionation in egmatites: Constraints from crystallization experiments of tantalite-tapiolite. Am. Mineral. 2018, 103, 1401–1416. [Google Scholar] [CrossRef]
- Pollard, P.; Pichavant, M.; Charoy, B. Contrasting evolution of fluorine-rich and boron-rich tin systems. Miner. Dep. 1987, 22, 315–321. [Google Scholar] [CrossRef]
- Breiter, K.; Ackerman, L.; Ďurišová, J.; Svojtika, M.; Novák, M. Trace element composition of quartz from different types of pegmatites: A case study from the Moldanubian Zone of the Bohemian Massif (Czech Republic). Mineral. Mag. 2014, 78, 703–722. [Google Scholar] [CrossRef]
- Icenhower, J.P.; London, D. An experimental study of element partitioning between biotite, muscovite and coexisting peraluminous granitic melt at 200 MPa (H2O). Am. Mineral. 1995, 80, 1229–1251. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D. Experimental silicate mineral/melt partition coefficients for beryllium and the crustal Be cycle from migmatite to pegmatite. Geochim. Cosmochim. Acta 2002, 66, 2239–2265. [Google Scholar] [CrossRef]
- Stewart, D.B. Petrogenesis of lithium-rich pegmatites. Am. Mineral. 1978, 63, 970–980. [Google Scholar]
- Pichavant, M.; Herrera, J.V.; Boulmier, S.; Briqueu, L.; Loron, J.L.; Juteau, M.; Marin, L.; Michard, A.; Sheppard, S.M.F.; Treuil, M.; et al. The Macusani glasses SE Peru: Evidence of chemical fractionation in peraluminous magmas. Geochem. Soc. Sp. Pub. 1987, 1, 359–374. [Google Scholar]
- London, D. Geochemistry of alkali and alkaline earth elements in ore-forming granites, pegmatites and rhyolites. In Rare Eelement Geochemistry and Mineral Deposits; Linnen, R.L., Samson, I.M., Eds.; Geological Association of Canada Short Course Notes 17; Geological Association of Canada: Quebec, QC, Canada, 2005; pp. 17–43. [Google Scholar]
- Linnen, R.L.; Van Lichtervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Gallagher, M.J. Compositioin of some Rhodesian lithium-beryllium pegmatites. Trans. Geol. Soc. S. Afr. 1975, 78, 35–41. [Google Scholar]
- Kretz, R.; Loop, J.; Hartree, R. Petrology and Li-Be-B geochemistry of muscovite-biotite granite and associated pegmatite near Yellowknife, Canada. Contrib. Mineral. Petr. 1989, 102, 174–190. [Google Scholar] [CrossRef]
- Stilling, A. Bulk composition of the Tanco pegmatite at Bernic Lake, Manitoba, Canada. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 1998. [Google Scholar]
- Evensen, J.M.; London, D.; Wendlandt, R.F. Solubility and stability of beryl in granitic melts. Am. Mineral. 1999, 84, 733–745. [Google Scholar] [CrossRef]
- London, D.; Evensen, J.M. Beryllium in silicic magmas and the origin of beryl-bearing pegmatites. In Beryllium: Mineralogy, Petrology and Geochemistry; Grew, E.S., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 2002; p. 48. [Google Scholar]
- Wedephol, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Breaks, F.W.; Moore, J.M., Jr. The Ghost Lake batholith, Superior Province of Northwestern Ontario: A fertile, S-type peraluminous granite-rare element pegmatite system. Can. Mineral. 1992, 30, 835–875. [Google Scholar]
- Kaeter, D.; Barros, R.; Menuge, J.F.; Chew, D.M. The magmatic-hydrothermal transition in rare-element pegmatites from southeast Ireland: LA-ICP-MS chemical mapping of muscovite and columbite-tantalite. Geochim. Cosmochim. Acta 2018, 240, 98–130. [Google Scholar] [CrossRef]
- Müller, A.; Romer, R.L.; Pedersen, R.-B. The Sveconorwegian pegmatite province-thousands of pegmatites without parental granites. Can. Mineral. 2017, 55, 283–315. [Google Scholar] [CrossRef]
- Fan, J.J.; Tang, G.J.; Wei, G.J.; Wang, H.; Xu, Y.G.; Wang, Q.; Zhou, J.S.; Zhang, Z.Y.; Huang, T.Y.; Wang, Z.L. Lithium isotope fractionation during fluid exsolution: Implications for Li mineralization of the Bailongshan pegmatites in the West Kunlun, NW Tibet. Lithos 2020, 352–353, 105236. [Google Scholar] [CrossRef]
- Maneta, V.; Baker, D.R.; Minarik, W. Evidence for lithium-aluminosilicate supersaturation of pegmatite-forming melts. Contrib. Mineral. Petrol. 2015, 170, 4. [Google Scholar] [CrossRef]
- Maneta, V.; Baker, D.R. Exploring the effect of lithium on pegmatitic textures: An experimental study. Am. Mineral. 2014, 99, 1383–1403. [Google Scholar] [CrossRef]
- Morgan, G.B.; London, D. Crystallization of the Little Three layered pegmatite-aplite dike, Ramona District, California. Contrib. Mineral. Petrol. 1999, 136, 310–330. [Google Scholar] [CrossRef]




| Pegmatite | Pegmatite Field | Pegmatite Type | Pegmatite Subtype | Mineralization Type | Internal Zonation and Mineralogy Components | Ages | Country Rock |
|---|---|---|---|---|---|---|---|
| Talate | Qinghe | complex | spodumene | Li-Be-Nb-Ta | WZ: fine Ab-Qz-Ms-Grt-Brl; OIZ: graphic intergrowth; IIZ: Spd-Qz-Ab; CZ: blocky Mc and Qz block. | 286 Ma * [72], 386 Ma [37] | Mica schist |
| Baicheng | Qinghe | beryl | beryl-columbite | Nb-Ta | Kf-Ab-Qz-Ms; lenses: Mc block and Qz-Ms-CGM-Tur block; MU: saccharoidal Ab-Qz-Ms. | 297 Ma * [72] | Mica schist |
| Kangmunagong | Qinghe | Barren | Homogeneous body: Kf-Ab-Qz-Ms-Tur-Grt. | 265 Ma * [72] | Mica schist | ||
| Qunkuer | Kelumute- Jideke | beryl | beryl-columbite | Be | saccharoidal Ab-Qz-Ms; coarse Grt-Ab intergrowth; Mc block; smoky Qz-Brl-Ms | 194 Ma [72] | Mica schist |
| Husite | Kelumute- Jideke | beryl | beryl-columbite | Be | BZ: fine Ab-Qz-Ms; WZ: coarse Ms-Qz-Brl-Grt; IZ: blocky Mc and graphic intergrowth with Brl; MU: saccharoidal Ab. | 199 Ma [72] | Mica schist |
| Dakalsu | Dakalasu- Kekexier | beryl | beryl-columbite | Be-Nb-Ta | BZ: fine Ab-Qz-Ms-Grt-Tur; WZ: graphic intergrowth-CGM; IZ: Mc block-Qz-Ms-Brl columns-CGM; MU: Ab; CZ: Qz. | 240 Ma [72]; 258 Ma [38] | Biotite granite (270 Ma) [75] |
| Xiaokalasu | Xiaokalasu- Qiebielin | complex | spodumene | Li-Nb-Ta | WZ: Mc block and fine Ab-Qz-Ms-Grt-CGM; OIZ: Qz-Ms-Kf-Grt; IIZ: Spd-Qz-Ms-CGM and blocky Mc. | 258–262 Ma [72] | Mica schist |
| Weizigou | Xiaokalasu- Qiebielin | beryl | beryl-columbite | Be | BZ: Qz-Ms-Tur; WZ: fine Ab-Qz-Ms-Grt-Brl; IZ: blocky Mc-Brl and coarse Qz-Ms-Brl-Tur block. | 237 Ma [72] | Two mica granite (398–412 Ma) [76] |
| Taerlang | Xiaokalasu-Qiebielin | Barren | BZ: Kf-Qz-Ms-Bi-Grt-Tur;WZ: graphic intergrowth and Qz-Ms block | 248 Ma [72] | Mica schist |
| Pegmatite Field | Pegmatite | Sample | Zone | Mineralogy Components |
|---|---|---|---|---|
| Qinghe | Talate (Li-Be-Nb-Ta) | 12TLT-4 | WZ | Ab (50)—Qz (25)—Ms (15)—Grt (2–5)—Brl (2–5)—CGM (2–5) |
| 12TLT-7 | CZ | Mc (45)—Ab (10)—Qz (30)—Ms (10)—CGM (5) | ||
| Baicheng (Nb-Ta) | BC-2 | Lens | Qz (70)—Ms (10)—Kf (10)—Ab (5)—Col-Tan (2–5) | |
| Kangmunagong (Barren) | KMNG-1 | Kf (55–60)—Qz (25–30)—Ms (5–8)—Tur (5)—Grt (2) | ||
| Kelumute- Jideke | Qunkuer (Be) | QKE-2-1 | Qz (70)—Ms (15)—Ab (10)—Brl (5) | |
| Husite (Be) | HST-5 | IZ | Kf (45)—Qz (35)—Ms (10–15)—Brl (2–5) | |
| HST-7 | IZ | Kf (40)—Qz (45)—Ms (10)—Grt (2–5) | ||
| Dakalasu- Kekexier | Dakalsu (Be-Nb-Ta) | DKLS-3 | IZ | Kf (50–55)—Qz (10–15)—Ms(20)—CGM(5)—Brl(2–5) |
| Xiaokalasu- Qiebielin | Xiaokalasu (Li-Nb-Ta) | XKLS-2(2) | IZ | Ab (50–55)—Qz (15–20)—Spd (25)—Ms (5–10) |
| Weizigou (Be) | WZG-4 | IZ | Qz (60)—Mc (20)—Ms (10)—Brl (5)—Tur (2–5) | |
| Taerlang (Barren) | TEL-1(1) | WZ | Kf (40)—Qz (35–40)—Ms (15)—Bi (5) |
| Pegmatite Dyke | Kangmunagong | Taerlang | Baicheng | Weizigou | Qunkuer | Husite | Dakalasu | Xiaokalasu | Talate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineralization Type | Barren | Barren | Nb-Ta | Be | Be | Be | Be-Nb-Ta | Li-Nb-Ta | Li-Be-Nb-Ta | ||
| Rock Type | Mc-Qz-Ms-Tur-Grt | WZ | Lens | IZ | Qz-Ms-Ab-Brl | IZ | IZ | IIZ | WZ | CZ | |
| Sample No. | KMNG-1 | TEL-1(1) | BC-2 | WZG-4 | QKE-2-1 | HST-5 | HST-7 | DKLS-3 | XKLS-2(2) | 12TLT-4 | 12TLT-7 |
| /wt% | |||||||||||
| SiO2 | 46.95 | 46.70 | 46.70 | 46.36 | 45.98 | 46.78 | 46.60 | 46.46 | 46.26 | 46.16 | 46.15 |
| TiO2 | 0.12 | 0.51 | 0.03 | 1.29 | 0.07 | bdl | 0.09 | 0.23 | 0.03 | 0.34 | 0.41 |
| Al2O3 | 34.65 | 32.41 | 36.90 | 31.22 | 33.45 | 34.64 | 33.95 | 33.38 | 36.81 | 33.90 | 32.45 |
| Cr2O3 | bdl | bdl | 0.03 | 0.02 | bdl | bdl | 0.03 | 0.02 | bdl | 0.03 | 0.02 |
| FeO | 1.86 | 3.48 | 0.83 | 3.94 | 4.12 | 2.40 | 3.50 | 3.79 | 1.02 | 2.30 | 2.55 |
| MnO | 0.04 | 0.06 | 0.03 | 0.11 | 0.06 | 0.04 | 0.09 | 0.10 | 0.13 | 0.04 | 0.04 |
| MgO | 0.81 | 1.06 | 0.21 | 1.02 | 0.17 | 0.24 | 0.24 | 0.10 | 0.04 | 0.92 | 1.11 |
| ZnO | bdl | 0.02 | 0.07 | 0.04 | 0.04 | bdl | bdl | 0.07 | 0.05 | 0.08 | 0.08 |
| CaO | bdl | bdl | 0.02 | bdl | bdl | bdl | 0.02 | 0.02 | bdl | 0.02 | 0.04 |
| Li2O * | 0.08 | 0.02 | 0.10 | 0.28 | 0.15 | 0.16 | 0.15 | 0.08 | 0.10 | 0.34 | 0.28 |
| Na2O | 0.43 | 0.60 | 0.57 | 0.50 | 0.91 | 0.36 | 0.43 | 0.65 | 0.75 | 0.39 | 0.45 |
| K2O | 10.11 | 9.84 | 9.83 | 9.52 | 9.62 | 9.96 | 9.59 | 9.41 | 9.52 | 10.13 | 10.39 |
| F | bdl | bdl | 0.33 | 0.46 | 1.16 | 0.24 | 0.47 | 0.51 | 0.08 | 0.76 | 0.82 |
| O = F | bdl | bdl | 0.14 | 0.20 | 0.49 | 0.10 | 0.20 | 0.21 | 0.03 | 0.32 | 0.35 |
| H2O * | 4.50 | 4.44 | 4.39 | 4.19 | 3.87 | 4.36 | 4.24 | 4.19 | 4.47 | 4.10 | 4.02 |
| Total | 99.54 | 99.13 | 99.90 | 98.73 | 99.12 | 99.07 | 99.19 | 98.77 | 99.23 | 99.17 | 98.47 |
| (O, OH, F) = 24 | |||||||||||
| Si | 6.253 | 6.305 | 6.157 | 6.299 | 6.232 | 6.266 | 6.266 | 6.287 | 6.141 | 6.203 | 6.272 |
| IVAl | 1.747 | 1.695 | 1.843 | 1.701 | 1.768 | 1.734 | 1.734 | 1.713 | 1.859 | 1.797 | 1.728 |
| IVSum | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 |
| VIAl | 3.694 | 3.464 | 3.892 | 3.299 | 3.577 | 3.737 | 3.648 | 3.612 | 3.902 | 3.572 | 3.471 |
| Ti | 0.012 | 0.052 | 0.003 | 0.132 | 0.007 | 0.000 | 0.009 | 0.023 | 0.003 | 0.034 | 0.042 |
| Cr | 0.000 | 0.000 | 0.003 | 0.003 | 0.000 | 0.000 | 0.003 | 0.002 | 0.000 | 0.003 | 0.002 |
| Fe | 0.207 | 0.393 | 0.092 | 0.447 | 0.467 | 0.269 | 0.394 | 0.429 | 0.113 | 0.258 | 0.290 |
| Mn | 0.004 | 0.007 | 0.004 | 0.012 | 0.007 | 0.004 | 0.010 | 0.011 | 0.015 | 0.005 | 0.005 |
| Mg | 0.160 | 0.214 | 0.042 | 0.206 | 0.034 | 0.047 | 0.047 | 0.020 | 0.009 | 0.184 | 0.224 |
| Li | 0.044 | 0.011 | 0.052 | 0.151 | 0.084 | 0.086 | 0.083 | 0.044 | 0.053 | 0.185 | 0.151 |
| Zn | 0.000 | 0.002 | 0.007 | 0.004 | 0.004 | 0.000 | 0.000 | 0.007 | 0.005 | 0.008 | 0.008 |
| VISum | 4.122 | 4.143 | 4.094 | 4.253 | 4.179 | 4.144 | 4.194 | 4.148 | 4.099 | 4.249 | 4.195 |
| Ca | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.003 | 0.002 | 0.000 | 0.002 | 0.006 |
| Na | 0.111 | 0.156 | 0.145 | 0.131 | 0.239 | 0.093 | 0.111 | 0.171 | 0.193 | 0.101 | 0.118 |
| K | 1.717 | 1.695 | 1.654 | 1.650 | 1.664 | 1.702 | 1.645 | 1.625 | 1.612 | 1.736 | 1.802 |
| XIISum | 1.828 | 1.852 | 1.802 | 1.781 | 1.903 | 1.794 | 1.759 | 1.799 | 1.805 | 1.839 | 1.927 |
| F | 0.000 | 0.000 | 0.138 | 0.199 | 0.498 | 0.100 | 0.198 | 0.217 | 0.034 | 0.324 | 0.354 |
| OH | 4.000 | 4.000 | 3.862 | 3.801 | 3.502 | 3.900 | 3.802 | 3.783 | 3.966 | 3.676 | 3.646 |
| /ppm | |||||||||||
| Li | 385 | 94.7 | 453 | 1279 | 717 | 745 | 716 | 380 | 461 | 1591 | 1287 |
| Be | 0.401 | 5.53 | 24.8 | 26.0 | 20.5 | 23.1 | 21.3 | 27.0 | 22.59 | 25.5 | 23.7 |
| B | 82.2 | 60.1 | 382 | 78.9 | 71.3 | 133 | 131 | 146 | 141 | 86 | 307 |
| P | 134 | 23.8 | 175 | 28.1 | 126 | 58.9 | 38.0 | 58.5 | 53.3 | 139 | 145 |
| Sc | 22.9 | 99.5 | 1.7 | 66.7 | 1.86 | 2.11 | 1.36 | 4.48 | 2.19 | 8.79 | 3.94 |
| V | 0.033 | 36.1 | 28.3 | 43.0 | 0.151 | 0.334 | 3.18 | 4.05 | 1.47 | 47.3 | 21.1 |
| Mn | 172 | 536 | 264 | 781 | 320 | 744 | 812 | 617 | 908 | 311 | 246 |
| Co | 0.317 | 1.32 | 0.79 | 1.36 | 0.070 | 0.197 | 0.324 | 0.282 | 0.08 | 6.66 | 5.14 |
| Ga | 60.0 | 145 | 67.3 | 112 | 120 | 114 | 124 | 388 | 122 | 72.1 | 67.7 |
| Ge | 2.82 | 2.27 | 13.8 | 3.84 | 4.28 | 3.71 | 4.58 | 6.79 | 6.01 | 9.08 | 19.5 |
| Rb | 428 | 455 | 4434 | 1205 | 4015 | 2963 | 2964 | 3843 | 4733 | 4774 | 8329 |
| Sr | 3.21 | 16.3 | 6.54 | 9.34 | 1.12 | 3.53 | 0.515 | 0.266 | 3.07 | 3.75 | 4.2 |
| Zr | 1523 | 1.61 | bdl | 1.18 | 0.393 | 0.308 | 1.08 | 1.121 | bdl | 0.73 | 0.237 |
| Nb | 40.3 | 86.3 | 170 | 122 | 301 | 257 | 305 | 169 | 159 | 231 | 300 |
| Mo | 0.112 | 0.324 | bdl | 0.087 | bdl | 0.176 | 0.096 | 0.092 | bdl | 0.093 | bdl |
| In | 0.197 | 0.996 | bdl | 0.31 | 0.256 | 0.123 | 0.045 | 4.86 | 0.079 | 0.069 | 0.021 |
| Sn | 12.4 | 34.7 | bdl | 15.8 | 62.3 | 21.2 | 9.60 | 773 | 18.1 | 1.73 | 0.92 |
| Cs | 7.69 | 6.4 | 472 | 100 | 93.5 | 107 | 101 | 517 | 280 | 332 | 1514 |
| Ba | 6.57 | 315 | 5.08 | 252 | bdl | 1.39 | 1.41 | 1.24 | 2.85 | 82.5 | 28.3 |
| Hf | 0.27 | bdl | bdl | 0.14 | bdl | bdl | 0.195 | 0.826 | bdl | 0.51 | 0.133 |
| Ta | 1.86 | 3.77 | 58.1 | 26.7 | 24.5 | 16.1 | 24.7 | 225 | 46.8 | 53.9 | 109 |
| W | 22.5 | 78.2 | 0.94 | 17.0 | 31.6 | 17.6 | 16.8 | 57.3 | 5.81 | 5.99 | 1.62 |
| Pb | 7.4 | 6.88 | 5.63 | 6.33 | 4.62 | 5.08 | 4.5 | 12.1 | 4.9 | 2.96 | 4.14 |
| Th | bdl | bdl | bdl | 0.032 | bdl | 0.016 | bdl | bdl | bdl | bdl | bdl |
| U | bdl | bdl | 1.53 | 0.082 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| K/Rb | 195.77 | 179.65 | 18.40 | 65.55 | 19.89 | 27.87 | 26.84 | 20.32 | 16.68 | 17.60 | 10.35 |
| K/Cs | 10,904 | 12,761 | 173 | 786 | 854 | 769 | 786 | 151 | 282 | 253 | 56.95 |
| Li/Cs | 50.06 | 14.80 | 0.96 | 12.74 | 7.67 | 6.93 | 7.07 | 0.73 | 1.64 | 4.80 | 0.85 |
| Nb/Ta | 21.69 | 22.90 | 2.92 | 4.56 | 12.28 | 15.93 | 12.37 | 0.75 | 3.40 | 4.28 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Qin, K.; Tang, D.; Wang, C. A Combined EMPA and LA-ICP-MS Study of Muscovite from Pegmatites in the Chinese Altai, NW China: Implications for Tracing Rare-Element Mineralization Type and Ore-Forming Process. Minerals 2022, 12, 377. https://doi.org/10.3390/min12030377
Zhou Q, Qin K, Tang D, Wang C. A Combined EMPA and LA-ICP-MS Study of Muscovite from Pegmatites in the Chinese Altai, NW China: Implications for Tracing Rare-Element Mineralization Type and Ore-Forming Process. Minerals. 2022; 12(3):377. https://doi.org/10.3390/min12030377
Chicago/Turabian StyleZhou, Qifeng, Kezhang Qin, Dongmei Tang, and Chunlong Wang. 2022. "A Combined EMPA and LA-ICP-MS Study of Muscovite from Pegmatites in the Chinese Altai, NW China: Implications for Tracing Rare-Element Mineralization Type and Ore-Forming Process" Minerals 12, no. 3: 377. https://doi.org/10.3390/min12030377





