Influence of Urbanization on Groundwater Chemistry at Lanzhou Valley Basin in China
Abstract
:1. Introduction
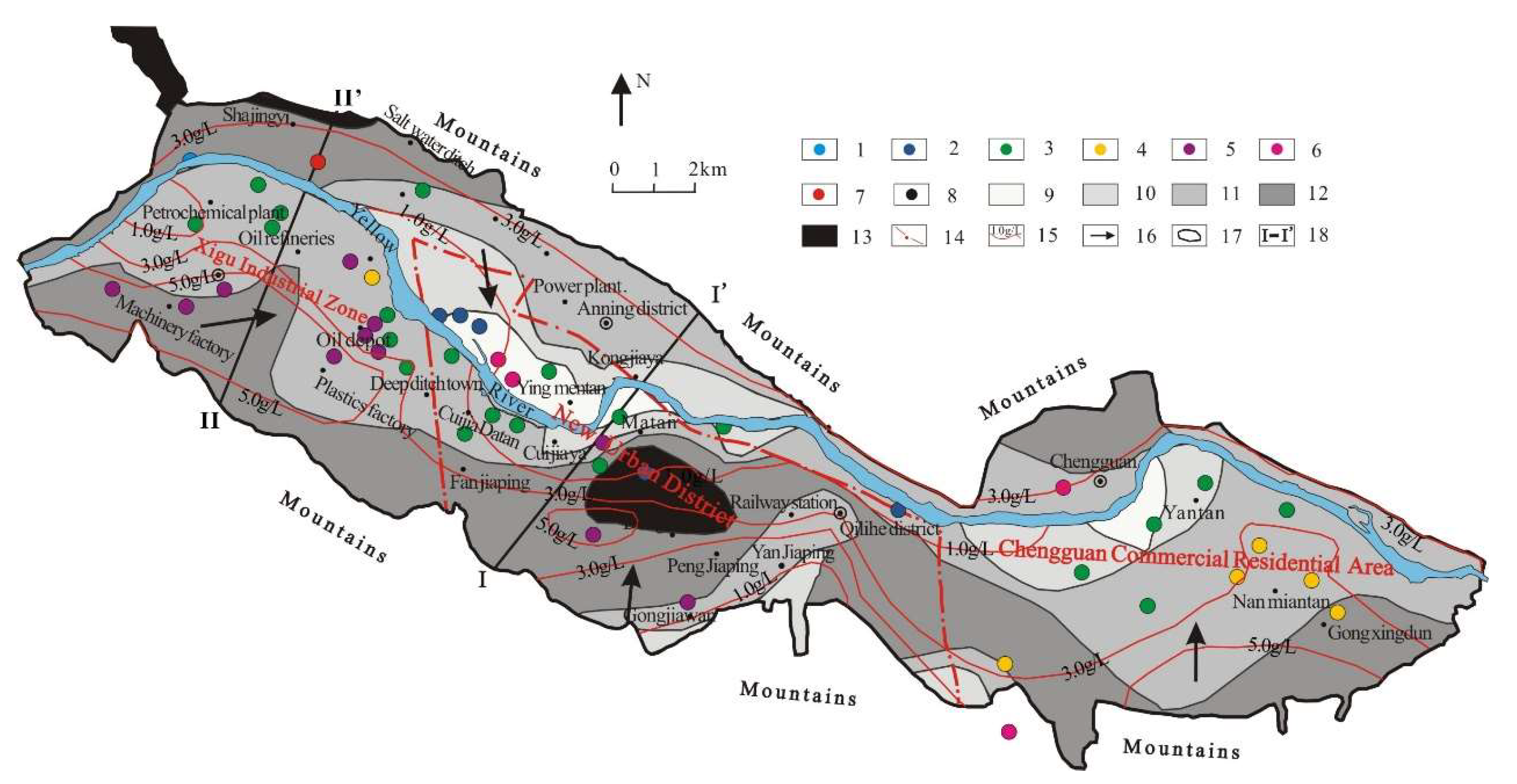
2. Study Area
2.1. Hydrogeological Condition
2.2. Urbanization
2.3. Sample Collection and Analysis
3. Results and Discussion
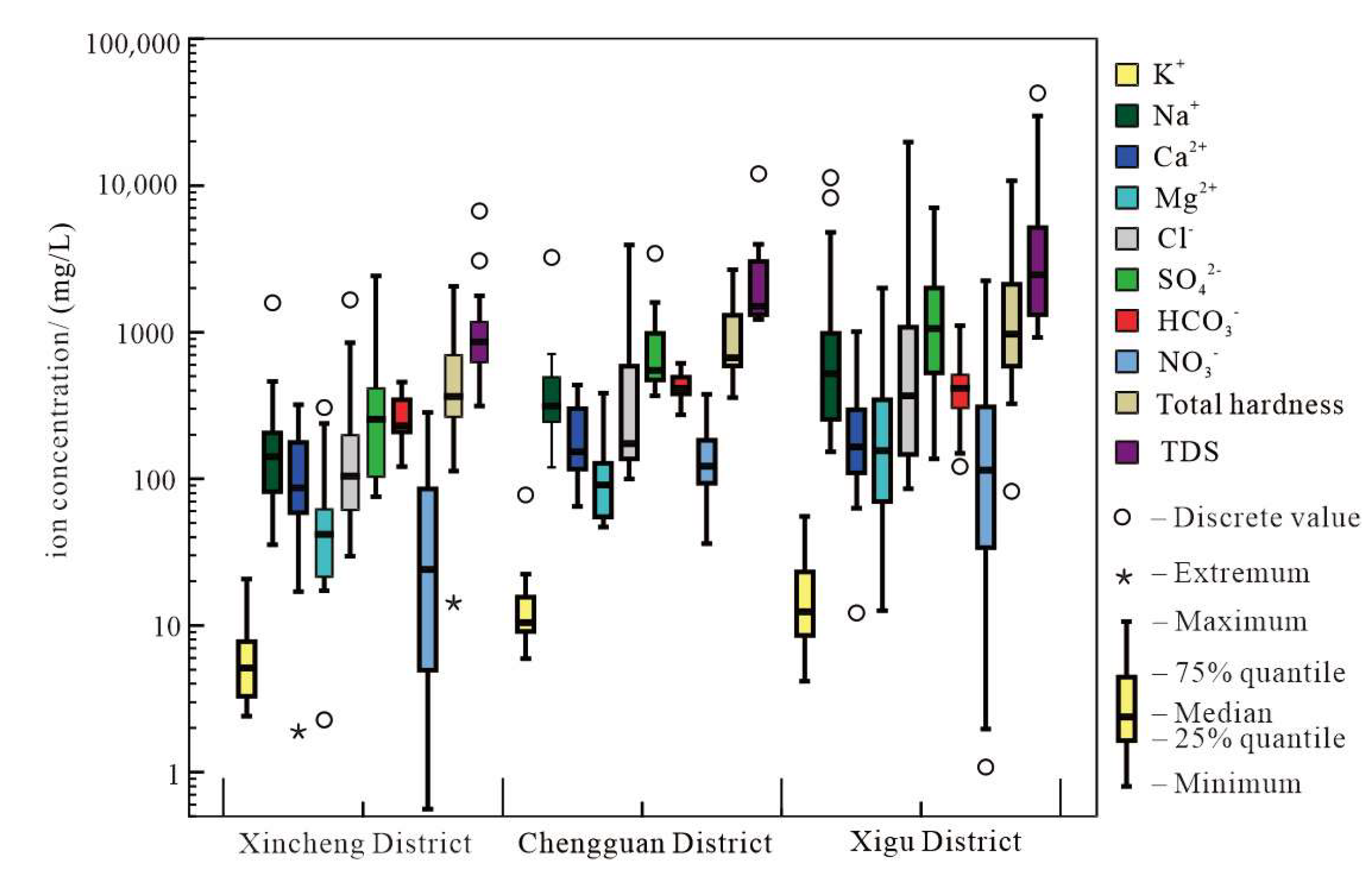
3.1. General Groundwater Chemistry in 2012
3.2. Evolution of Groundwater Hydrochemistry
3.2.1. Groundwater Hydrochemistry in 2012
3.2.2. Characteristics of Groundwater Hydrochemical Evolution with Time
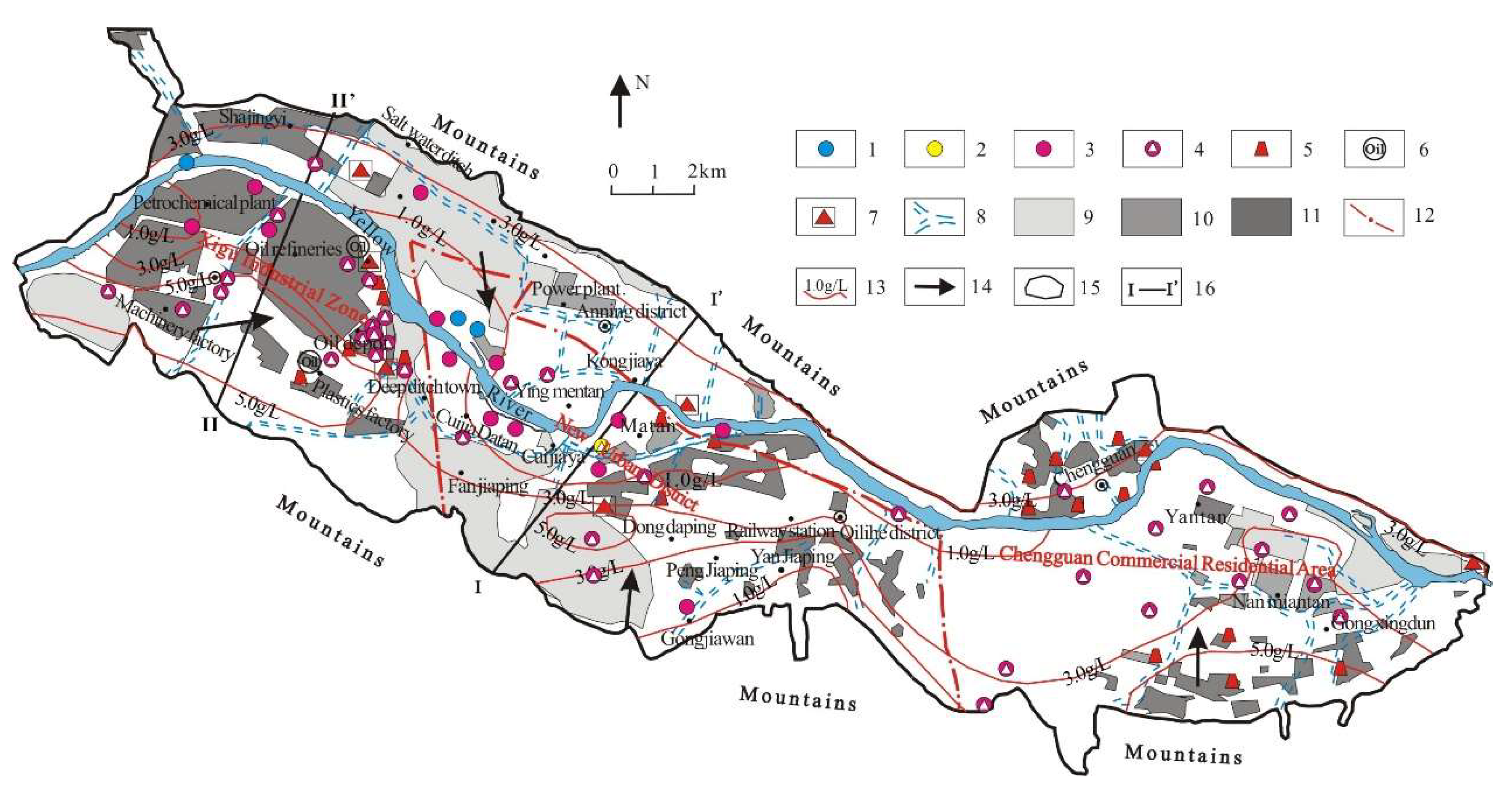
3.3. Identification of Groundwater Chemical Anomalies in Different Urban Functional Areas
3.3.1. Identification of Abnormal Data Based on ‘Hydrochemical’ Method
3.3.2. Groundwater Quality in Different Urban Functional Areas
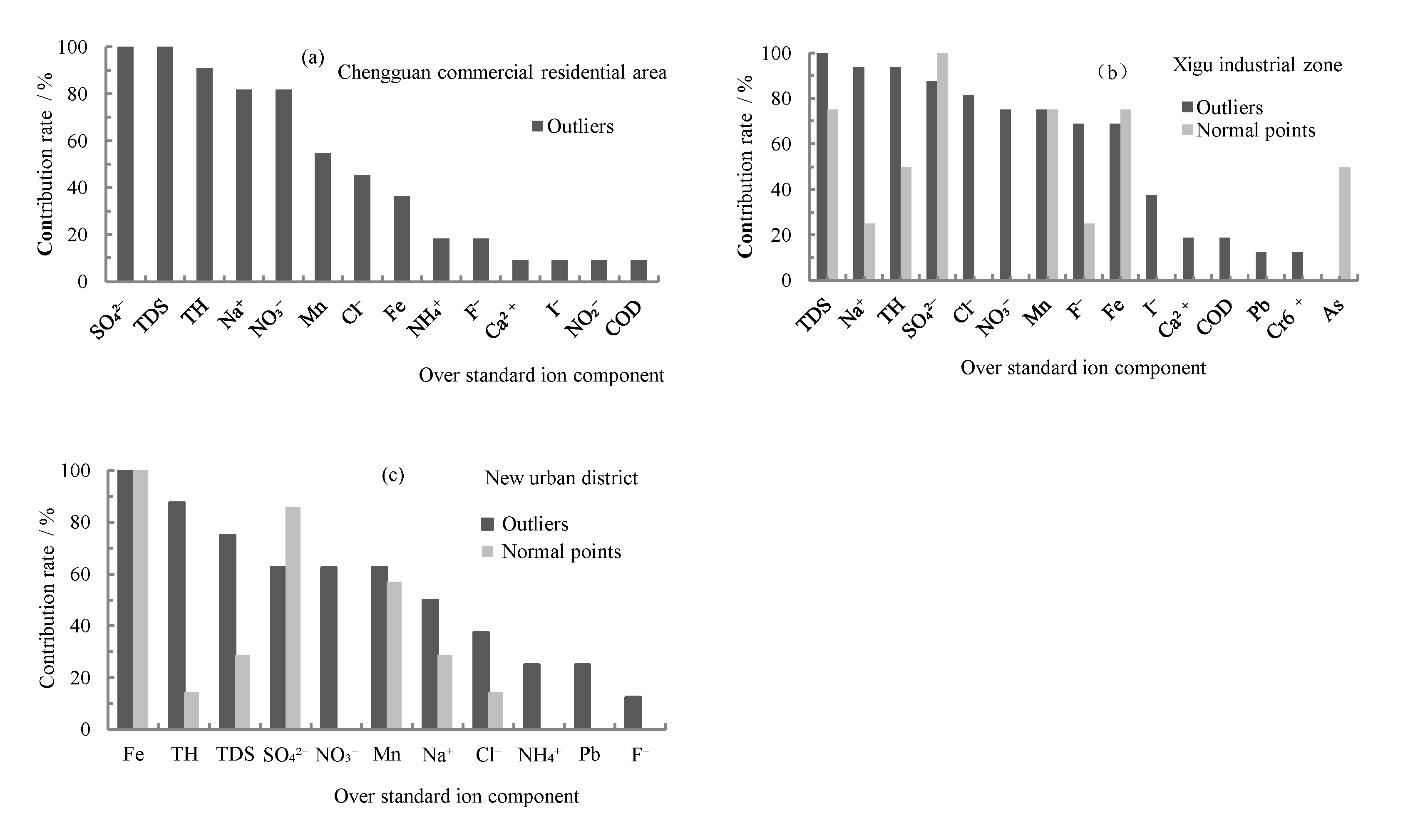
3.4. Main Human Impact Indicators of Poor-Quality Groundwater in Various Urban Functional Areas
3.4.1. Influencing Factors of Poor-Quality Groundwater in Chengguan Commercial Residential Area
3.4.2. Influencing Factors of Poor-Quality Groundwater in the Xigu Industrial Zone
3.4.3. Influencing Factors of Poor-Quality Groundwater in New Urban District
3.5. Water–Rock Interaction and the Cause of Chemical Evolution of Groundwater
3.5.1. Analysis of Chemical Evolution Processes of Groundwater
3.5.2. Driving Factors for Poor-Quality Groundwater in Different Urban Functional Areas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, A.M.; Jing, J.H.; Song, B. Water safety issues of China and ensuring roles of groundwater. Geol. Sin. 2016, 90, 2939–2947. [Google Scholar]
- Zhang, L.Y.; Zhang, Z.; Chen, Y.; Tao, F.L. Spatial pattern of surface water quality in China and its driving factors—Implication for the environment sustainability. Hum. Ecol. Risk Assess. 2019, 25, 1789–1801. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, W.H.; Deng, H.J.; Fang, G.H.; Li, Z. Changes in central Asia’s water tower: Past, present and future. Sci. Rep. 2016, 6, 35458. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.L.; Liu, J.T.; Han, Z.T.; Zhou, B.; Zhu, L.; Chen, X. Chemical Evolution of Groundwater in the Tacheng Basin of Xinjiang in the Process of Urbanization. Chin. J. Environ. Sci. 2020, 41, 1197–1205. [Google Scholar]
- Wang, Y.X.; Zheng, C.M.; Ma, R. Review: Safe and sustainable groundwater supply in China. Appl. Hydrogeol. 2018, 26, 1301–1324. [Google Scholar] [CrossRef]
- Gao, X.B.; Luo, W.T.; Luo, X.S.; Li, C.C.; Zhang, X.; Wang, Y.X. Indigenous microbes induced fluoride release from aquifer sediments. Environ. Pollut. 2019, 252, 580–590. [Google Scholar] [CrossRef]
- Huang, G.X.; Liu, C.Y.; Sun, J.C.; Zhang, M.; Jing, J.H.; Li, L.P. A regional scale investigation on factors controlling the groundwater chemistry of various aquifers in a rapidly urbanized area: A case study of the Pearl River Delta. J. Sci. Total Environ. 2018, 625, 510–518. [Google Scholar] [CrossRef]
- Li, P.Y.; Qian, H.; Howard, K.W.F.; Wu, J.H. Building a new and sustainable ‘Silk Road economic belt’. Environ. Earth Sci. 2015, 74, 7267–7270. [Google Scholar] [CrossRef]
- Peng, C.; He, J.T.; Liao, L.; Zhang, Z.G. Research on the influence degree of human activities on groundwater quality by the method of geochemistry: A case study from Liujiang Basin. Earth Sci. Front. 2017, 24, 321–331. [Google Scholar]
- Zhang, Q.Q.; Sun, J.C.; Liu, J.T.; Huang, G.X.; Lu, C.; Zhang, Y.X. Driving mechanism and sources of groundwater nitrate contamination in the rapidly urbanized region of south China. J. Contam. Hydrol. 2015, 182, 221–230. [Google Scholar] [CrossRef]
- He, S.; Li, P.Y.; Wu, J.H.; Elumalai, V.; Adimalla, N. Groundwater quality under land use/land cover changes: A temporal study from 2005 to 2015 in xi’an, northwest china. Hum. Ecol. Risk Assess. 2020, 26, 2771–2797. [Google Scholar] [CrossRef]
- Gabor, R.S.; Hall, S.J.; Eiriksson, D.P.; Jameel, Y.; Millington, M.; Stout, T.; Barnes, M.L.; Gelderloos, A.; Tennant, H.; Bowen, G.J. Persistent Urban Influence on Surface Water Quality via Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 9477–9487. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N.; Prasad, K.M.; Madhusudhan, B.J.; Krishna, V.S.R.; Anand, A.V.S.S.; Madhnure, P. Impact of Urbanization on Groundwater Quality in Vijayawada Urban Agglomeration, the New Capital Region of Andhra Pradesh, India—A Baseline Study. J. Geol. Soc. India 2016, 87, 539–552. [Google Scholar] [CrossRef]
- Huang, G.X.; Sun, J.C.; Zhan, Y.; Chen, Z.Y.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. J. Sci. Total Environ. 2013, 463–464, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.E.; Huang, G.X.; Hou, Q.X.; Liu, C.Y.; Zhang, Y.; Zhang, Q. Groundwater quality in the Pearl River Delta after the rapid expansion of industrialization and urbanization: Distributions, main impact indicators, and driving forces. J. Hydrol. 2019, 577, 124004. [Google Scholar] [CrossRef]
- Xia, C.C.; Liu, G.D.; Meng, Y.C.; Wang, Z.Y.; Zhang, X.X. Impact of human activities on urban river system and its implication for water-environment risks: An isotope-based investigation in Chengdu, China. Hum. Ecol. Risk Assess. 2021, 27, 1416–1439. [Google Scholar] [CrossRef]
- Li, P.Y.; Tian, R.; Xue, C.Y.; Wu, J.H. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Zhu, G.F.; Su, Y.H.; Huang, C.L.; Feng, Q.; Liu, Z.G. Hydrogeochemical processes in the groundwater environment of Heihe River Basin, northwest China. Environ. Earth Sci. 2010, 60, 139–153. [Google Scholar]
- Gao, X.B.; Su, C.L.; Wang, Y.X.; Hu, Q.H. Mobility of arsenic in aquifer sediments at Datong Basin, northern China: Effect of bicarbonate and phosphate. J. Geochem. Explor. 2013, 135, 93–103. [Google Scholar] [CrossRef]
- Li, C.C.; Gao, X.B.; Liu, Y.S.; Wang, Y.X. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Xue, D.M.; Botte, J.; Baets, B.D.; Accoe, F. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface and groundwater. J. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Porcel, D.; De, H.; Schuth, L.G.C. Urban impacts analysis on hydrochemical and hydrogeological evolution of groundwater in shallow aquifer Linares, Mexico. J. Environ. Earth Sci. 2012, 66, 1871–1880. [Google Scholar]
- Liu, C.Q.; Li, S.L.; Lang, Y.C.; Xiao, H.Y. Using delta 15N-and delta 18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ. Sci. Technol. 2006, 40, 6928. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shi, J.S.; Liu, J.T.; Gui, C.L. Combined Use of Multivariate Statistical Analysis and Hydrochemical Analysis for Groundwater Quality Evolution: A Case Study in North Chain Plain. J. Earth Sci. 2014, 25, 587–597. [Google Scholar] [CrossRef]
- Zhu, D.N.; Zou, S.Z.; Zhou, C.S.; Li, L.J.; Xie, H. Identification of hydeochemical sensitive factors of karst groundwater in different functional urban areas. Chin. J. Carsologica Sin. 2018, 37, 484–492. [Google Scholar]
- Li, J.; Luo, Z.H.; Gu, X.; Ma, Y.H. Effect of urbanization on shallow groundwater in Guiyang area. Chin. J. Saf. Environ. Eng. 2017, 4, 2–9. [Google Scholar]
- Zhang, X.W.; He, J.T.; Peng, C.; Zhang, C.Y.; Ni, Z.H. Comparison of identification methods of main component hydrochemical anomalies in groundwater: A case study of Liujiang basin. Chin. J. Environ. Sci. 2017, 38, 3225–3234. [Google Scholar]
- Lü, X.L.; Liu, J.T.; Zhu, L.; Liu, C.Y.; Liu, J.J. Distribution and source of nitrogen pollution in groundwater of Lanzhou City. J. Arid Land Resour. Environ. 2019, 33, 95–100. [Google Scholar]
- Lü, X.L.; Shao, J.L.; Liu, J.T.; Wen, J.L.; Sun, J.C. Distribution characteristics and origin of total dissolved solids in groundwater under Lanzhou City. J. Arid Land Resour. Environ. 2012, 27, 23–27. [Google Scholar]
- Chin, R. Statistical Yearbook of Lanzhou City; Lanzhou Municipal Bureau of Statistics: Lanzhou, China, 2012. [Google Scholar]
- GB/T8538-1995; Method of Calibration for Natural Mineral Water for Drinking. China Standard Press: Beijing, China, 1996.
- GB/T14848-2017; Environmental Quality Standards for Groundwater. China Standard Press: Beijing, China, 2017.
- Gibbs, R.J. Mechanisms controlling world water chemistry. J. Sci. 1970, 170, 108–1090. [Google Scholar] [CrossRef]
- Bai, F.L.; Shang, R.Y.; Chin. R. Hydrogeology and Engineering Geology Team 1 Bureau of Geology and Mineral Resources of Gansu Province, Lanzhou, Gansu, China. Environmental Geology Exploration Report of Lanzhou City. 1983; Unpublished work. [Google Scholar]
- Lü, X.L.; Shao, J.L.; Liu, J.T.; Huang, G.X.; Zhang, Y.; Cui, H.W.; Wen, J.L.; Liu, J.J.; Sun, J.C. Contamination characteristics and causes of volatile organic compounds in the groundwater at a petrochemical contaminated site. Hydrogeol. Eng. Geol. 2012, 39, 97–102. [Google Scholar]
- Thilagavathi, R.; Chidambaram, S.; Prasanna, M.V.; Thivya, C.; Singaraja, C. A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. J. Appl. Water Sci. 2012, 2, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Karmegam, U.; Chidambaram, S.; Peasanna, M.V.; Manikandan, S.; Johnsonbabu, G.; Dheivanayaki, V.; Paramaguru, P.; Manivannan, R.; Srinivasamoorthy, K.; Anandhan, P. A study on the mixing proportion in groundwater samples by using Piper diagram and Phreeqc model. Chin. J. Geochem. 2011, 30, 490495. [Google Scholar] [CrossRef]
- Dogramci, S.; Skrzypek, G.; Dofson, W.; Grierson, P.F. Stable isotope and hydrochemical evolution of groundwater in the semi-arid Harmersley Basin of subtropical northwest Australia. J. Hydrol. 2012, 475, 281–293. [Google Scholar] [CrossRef]
- Song, Y.H. The Processing and the Application of Stream Sediment Data with Mahalanobis Distance in Sangou Area; Jilin University: Changchun, China, 2019. [Google Scholar]
- Lü, X.L.; Liu, J.T.; Zhu, L.; Liu, J.J.; Liu, C.Y. Distribution and source of Fe and Mn in groundwater of Lanzhou City. J. Arid Land Resour. Environ. 2019, 33, 132–138. [Google Scholar]
- Zhu, L.; Sun, J.C.; Liu, J.T.; Zhang, Y.X.; Liu, C.Y. Distributing regularity and influencing factors of fluorine in Lanzhou City. Environ. Sci. Technol. 2015, 38, 144–148. [Google Scholar]
- Zhang, M.; Huang, G.X.; Liu, C.Y.; Zhang, Y.; Chen, Z.Y.; Wang, J.C. Distributions and origins of nitrate, nitrite, and ammonium in various aquifers in an urbanized coastal area, south China. J. Hydrol. 2020, 582, 124528. [Google Scholar] [CrossRef]
- Huang, G.X.; Liu, C.Y.; Li, L.P.; Zhang, F.E.; Chen, Z.Y. Spatial distribution and origin of shallow groundwater iodide in a rapidly urbanized delta: A case study of the Pearl River Delta. J. Hydrol. 2020, 585, 124860. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, J.T.; Zhang, X.W.; Ni, Z.H. Geochemical Characteristics and Genesis Analyses of High-arsenic groundwater in the Pearl River Delta. Chin. J. Environ. Sci. 2018, 39, 3631–3639. [Google Scholar]







| Items | Standard (mg/L) | Xigu District (n = 20) | New Urban District (n = 21) | Chengguan District (n = 11) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min (mg/L) | Max (mg/L) | Average (mg/L) | Cv (%) | ESR (%) | Min (mg/L) | Max (mg/L) | Average (mg/L) | Cv (%) | ESR (%) | Min (mg/L) | Max (mg/L) | Average (mg/L) | Cv (%) | ESR (%) | ||
| K+ | — | 4.3 | 43.2 | 15.3 | 73 | — | 2.5 | 11.6 | 6.2 | 45.7 | — | 7.9 | 77.8 | 19.1 | 110.3 | — |
| Na+ | 200 | 153.2 | 11,315 | 1663.7 | 180.1 | 80 | 35.4 | 1585 | 223.1 | 148.8 | 28.6 | 119.6 | 3235 | 601.8 | 147.9 | 81.8 |
| Ca2+ | 400 | 12.2 | 1008 | 262.2 | 97.9 | 15 | 1.9 | 321.4 | 112.5 | 71.8 | 0 | 65 | 436.4 | 200.4 | 60.6 | 9.1 |
| Mg2+ | 50 | 12.6 | 1999 | 337.6 | 142.6 | 90 | 2.3 | 305.7 | 61.7 | 120.2 | 38.1 | 46.9 | 383.7 | 119.6 | 83.5 | 81.8 |
| Cl− | 250 | 85.8 | 19,822 | 2266 | 222.9 | 65 | 29.8 | 1663 | 227.9 | 164.4 | 19 | 99.8 | 3939 | 626.2 | 178.9 | 45.5 |
| SO42− | 250 | 136.9 | 7072 | 1868.7 | 112.3 | 90 | 75.3 | 2420 | 405.6 | 128.2 | 52.4 | 368.8 | 3443 | 952.1 | 94.8 | 100.0 |
| HCO3− | — | 120.9 | 1106 | 418.4 | 51 | — | 121.5 | 457.7 | 266.4 | 33.4 | — | 272.9 | 613.1 | 436 | 24.2 | — |
| NO3−-N | 20 | 0.2 | 209.1 | 40.2 | 128.9 | 45 | 0.1 | 64.2 | 15.3 | 128.9 | 19 | 8.2 | 85.4 | 35.6 | 60.6 | 81.8 |
| NO2−-N | 1.0 | ND | 0.396 | 0.049 | 227.9 | 0 | ND | 0.183 | 0.011 | 355.6 | 0 | ND | 3.47 | 0.471 | 225.7 | 9.1 |
| NH4+-N | 0.5 | ND | 0.233 | 0.028 | 235.2 | 0 | ND | 19.4 | 1 | 444.8 | 9.5 | 0 | 1.867 | 0.297 | 191 | 18.2 |
| F− | 1.0 | 0.3 | 3.8 | 1.3 | 68.6 | 60 | 0.1 | 1.2 | 0.3 | 88.3 | 9.5 | 0.3 | 2.3 | 0.7 | 91.9 | 18.2 |
| H2SiO3 | — | 2.8 | 31.8 | 15.6 | 42.2 | — | 2 | 22.9 | 12.4 | 39.7 | — | 15.6 | 24.1 | 18.4 | 12.1 | — |
| Fe | 0.3 | 0 | 39.8 | 7.2 | 145.6 | 70 | ND | 45.2 | 6.2 | 187.8 | 71.4 | ND | 5.7 | 0.8 | 217.4 | 36.4 |
| Mn | 0.1 | 0 | 2.3 | 0.5 | 141.9 | 75 | ND | 1.1 | 0.2 | 163.2 | 42.9 | ND | 0.6 | 0.2 | 105.4 | 54.5 |
| TDS | 1000 | 917.3 | 42,788 | 6927.6 | 158.7 | 95 | 312.4 | 6711 | 1254.4 | 111.2 | 38.1 | 1223 | 12053 | 2903.7 | 109.3 | 100 |
| TH | 450 | 82.2 | 10,755 | 2045.3 | 127.6 | 85 | 14.2 | 2062 | 535 | 91.2 | 38.1 | 357.3 | 2669 | 992.8 | 68.8 | 90.9 |
| pH | 6.5–8.5 | 7.2 | 8.8 | 7.6 | 5.8 | 9.5 | 5.7 | 9.7 | 7.7 | 8.6 | 9.5 | 6.9 | 8 | 7.3 | 4.8 | 0 |
| Hydrochemical Diagram | Abnormal Data Identification (Group) |
|---|---|
| Piper hydrochemistry diagram of piper | 5 |
| Ion exchange diagram | 11 |
| Na+ + K+–Cl− | 9 |
| Ca2+ + Mg2+–HCO3− | 17 |
| Ca2+ + Mg2+–O42− | 7 |
| Total number | 38 |
| Chemical Parameters | PC1 | PC2 | PC3 |
|---|---|---|---|
| TDS | 0.996 | 0.000 | −0.013 |
| Electrical conductivity | 0.992 | −0.026 | −0.022 |
| Total hardness | 0.991 | 0.041 | 0.005 |
| Na+ | 0.989 | −0.018 | −0.021 |
| Mg2+ | 0.986 | 0.043 | 0.010 |
| Cl− | 0.973 | −0.083 | −0.015 |
| Ca2+ | 0.957 | 0.031 | −0.010 |
| NO3− | 0.935 | −0.131 | −0.012 |
| SO42− | 0.910 | 0.201 | −0.005 |
| F− | 0.812 | 0.256 | 0.064 |
| Pb | 0.077 | 0.804 | 0.184 |
| I− | 0.134 | 0.702 | −0.049 |
| Fe | −0.092 | 0.655 | −0.101 |
| Mn | −0.033 | 0.648 | 0.645 |
| As | 0.086 | −0.180 | 0.809 |
| Eh | 0.080 | −0.125 | −0.758 |
| Eigenvalue | 9.180 | 2.171 | 1.697 |
| Explained variance (%) | 57.374 | 13.568 | 10.604 |
| Cumulative % of variance | 57.374 | 70.941 | 81.545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lü, X.; Han, Z.; Li, H.; Zheng, Y.; Liu, J. Influence of Urbanization on Groundwater Chemistry at Lanzhou Valley Basin in China. Minerals 2022, 12, 385. https://doi.org/10.3390/min12030385
Lü X, Han Z, Li H, Zheng Y, Liu J. Influence of Urbanization on Groundwater Chemistry at Lanzhou Valley Basin in China. Minerals. 2022; 12(3):385. https://doi.org/10.3390/min12030385
Chicago/Turabian StyleLü, Xiaoli, Zhantao Han, Haijun Li, Yuejun Zheng, and Jingtao Liu. 2022. "Influence of Urbanization on Groundwater Chemistry at Lanzhou Valley Basin in China" Minerals 12, no. 3: 385. https://doi.org/10.3390/min12030385






