Application of a Superplasticizer in Scheelite Selective Flotation from Calcite
Abstract
:1. Introduction
2. Materials and Method
2.1. Samples
2.2. Chemicals
2.3. Method
2.3.1. Single Mineral Flotation Tests
2.3.2. Contact Angle Tests
2.3.3. Zeta-Potential Tests
2.3.4. Fourier Transform Infrared (FTIR) Measurements
2.3.5. X-ray Photoelectron Spectroscopy Analysis
2.3.6. Microscope Observation Tests
3. Results and Discussion
3.1. Single Mineral Flotation Tests
3.2. Contact Angle Test
3.3. Zeta-Potential Tests
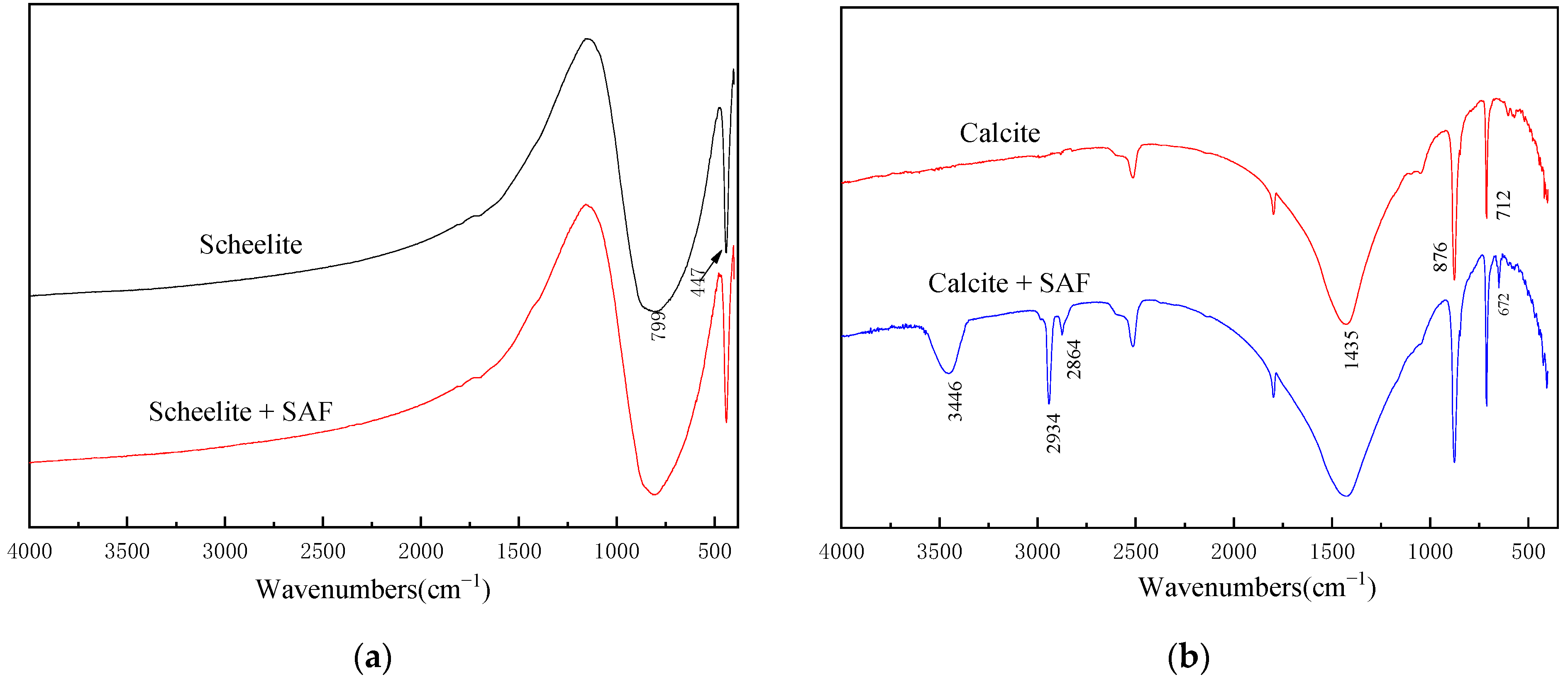
3.4. FTIR Analysis
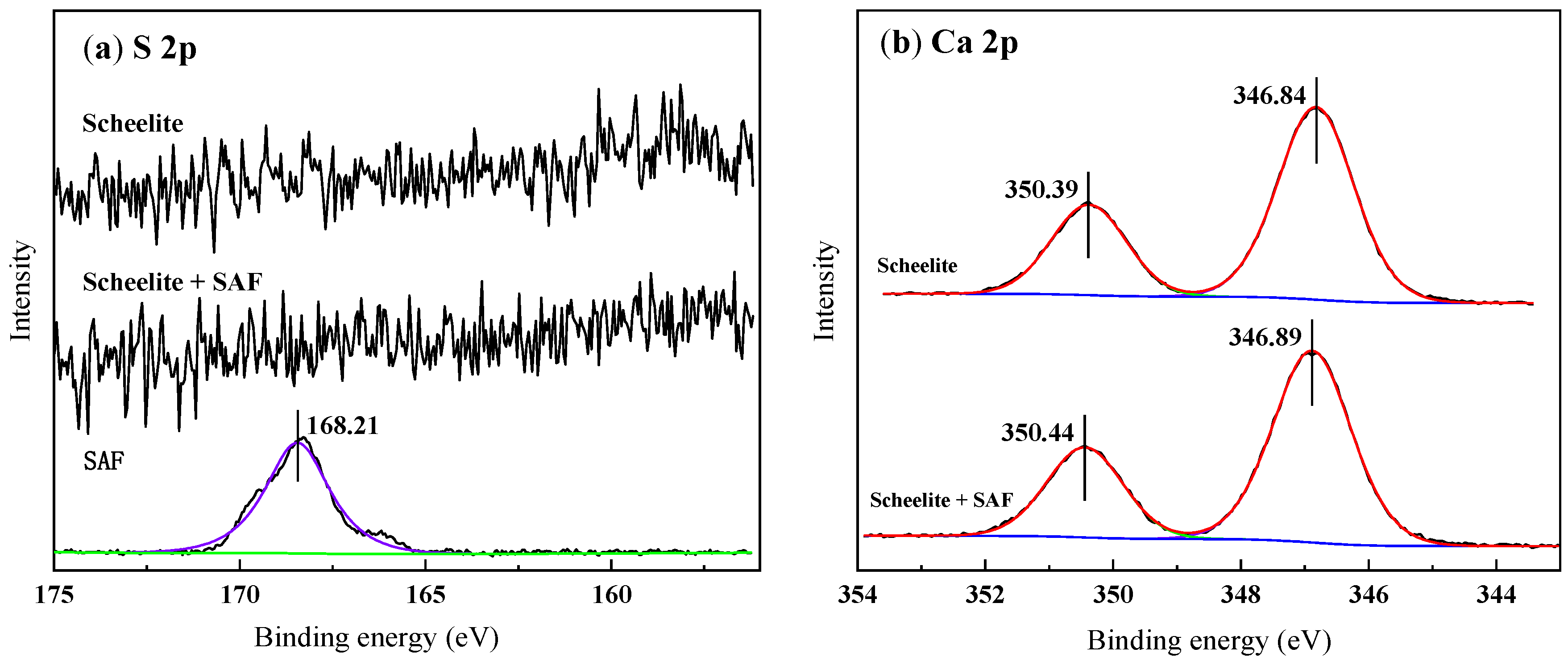
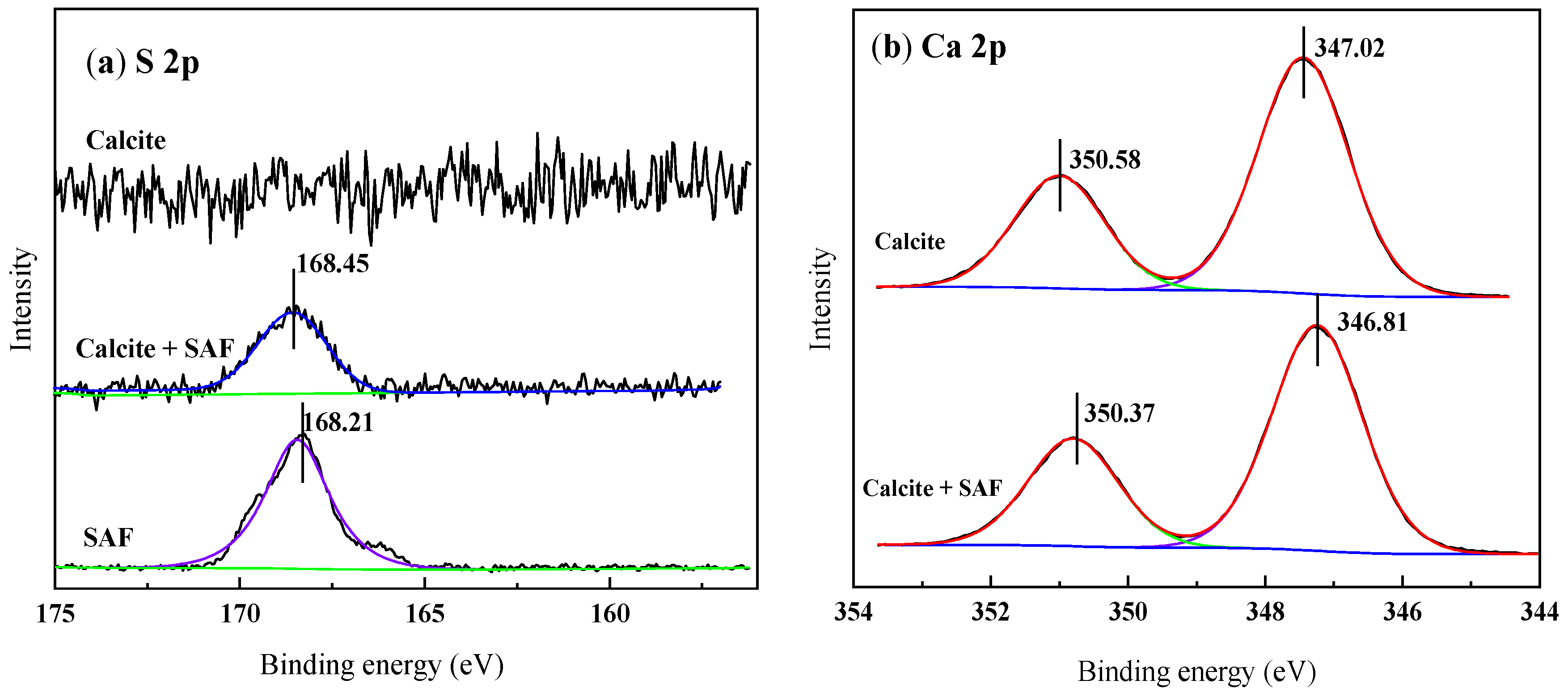
3.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.6. Microscope Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ITIA. Tungsten Properties. 2017. Available online: https://www.itia.info/tungsten-properties.html (accessed on 28 January 2016).
- Jewell, S.; Kimball, S.M. Mineral Commodity Summaries 2016. pp. 180–181. Available online: https://www.usgs.gov/centers/national-minerals-information-center/mineral-commodity-summaries (accessed on 28 January 2016).
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippov, L.; Filippov, I.; Badawi, M. The challenge of tungsten skarn processing by froth flotation: A review. Front. Chem. 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Filippov, L.O.; Shokhin, V.N.; Yenbaeva, L.I.; Ignatkina, V.A. Improvement of engineering data for flotation of scheelite using combination of sodium oleate and Exol-B. Tsvetnye Met. 1993, 1, 60–64. [Google Scholar]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W. Anisotropic surface properties of calcite: A consideration of surfacebroken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Role of sodium carbonate in scheelite flotation-A multi-faceted reagent. Miner. Eng. 2018, 129, 120–128. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodiumsilicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2016, 52, 567–573. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.L.; Sun, W.J.; Wang, J.J.; Gao, Z.Y.; Wang, L.; Zhang, C.Y. Selective Separation of Scheelite from Calcite by Self-Assembly of H2SiO3 Polymer Using Al3+ in Pb-BHA Flotation. Minerals 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, S.; Kalpakli, A.O.; Kahruman, C.; Yusufoglu, I. The investigation of dissolution behavior of gangue materials during the dissolution of scheelite concentrate in oxalic acid solution. Hydrometallurgy 2013, 136, 15–26. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Hu, Y. Investigation of two-stage depressing by using hydrophilic polymer to improve the process of fluorite flotation. J. Clean. Prod. 2018, 193, 228–235. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Santana, R.C.; Ataíde, C.H.; Barrozo, M.A. Recovery of apatite from flotation tailings. Sep. Purif. Technol. 2011, 79, 79–84. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C.; Xu, F. The flotation separation of scheelite from calcite and fluorite using dextran sulfate sodium as depressant. Int. J. Miner. Process. 2017, 169, 53–59. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Yin, W.; Liang, X. Flotation separation of scheelite from calcite using carboxyl methyl cellulose as depressant. Miner. Eng. 2018, 127, 329–333. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, J.; Deng, X.; Liu, Y. Graft copolymerization of black liquor and sulfonated acetone formaldehyde and research on concrete performance. Constr. Build. Mater. 2015, 83, 308–313. [Google Scholar] [CrossRef]
- Xu, Y.; Hua, M.; Chen, D.; Liu, Z.; Yu, Y.; Zhang, H.; Guo, J. Performance and working mechanism of amphoteric polycarboxylate-based dispersant and sulfonated acetone formaldehyde polycondensate-based dispersant in oil well cement. Constr. Build. Mater. 2020, 233, 117147. [Google Scholar] [CrossRef]
- Zhou, M.; Qiu, X.; Yang, D.; Ouyang, X. Physicochemical Behavior of Sulphonated Acetone-Formaldehyde Resin and Naphthalene Sulfonate-Formaldehyde Condensate in Coal-Water Interface. J. Dispers. Sci. Technol. 2009, 30, 353–360. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Zhu, J.; Qu, Q.; Xiong, W. Syntheses and evaluations of three sulfonated polycondensate dispersants for coal–water slurries. Powder Technol. 2014, 254, 572–578. [Google Scholar] [CrossRef]
- Thongtem, T.; Phuruangrat, A.; Thongtem, S. Haracterization of MeWO4 (Me = Ba, Sr and Ca) nanocrystallines prepared by sonochemical method. Appl. Surf. Sci. 2008, 254, 7581–7585. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Kuz’kin, S.F.; Solynshkin, V.I. Infrared spectra of calcite, scheelite and apatite after treatment with flotation regulators. Izv. Vyssh. Uchebn. Zaved. Tsvetn. Metall. 1963, 6, 28–32. [Google Scholar]
- Yoon, R.H.; Mao, L. Application of extended DLVO theory, IV: Derivation of flotation rate equation from first principles. J. Colloid Interface Sci. 1996, 181, 613–626. [Google Scholar] [CrossRef]
- Ninham, B.W. On progress in forces since the DLVO theory. Adv. Colloid Interface Sci. 1999, 83, 1–17. [Google Scholar] [CrossRef]
- Claesson, P.M.; Blom, C.E.; Herder, P.C.; Ninham, B. Interactions between water–stable hydrophobic Langmuir–Blodgett monolayers on mica. J. Colloid Interface Sci. 1986, 114, 234–242. [Google Scholar] [CrossRef]
- Mishchuk, N.A. The model of hydrophobic attraction in the framework of classical DLVO forces. Adv. Colloid Interface Sci. 2011, 168, 149–166. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.; Park, K.; Han, Y.; Kim, H. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
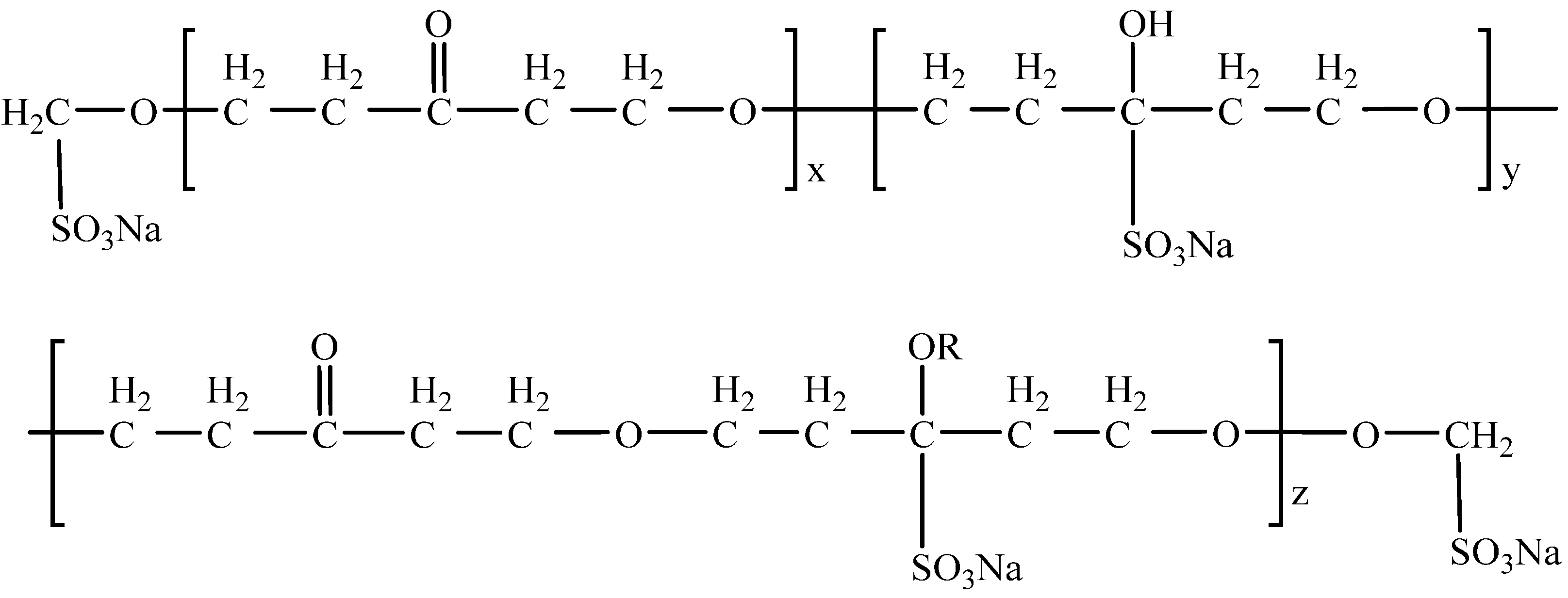


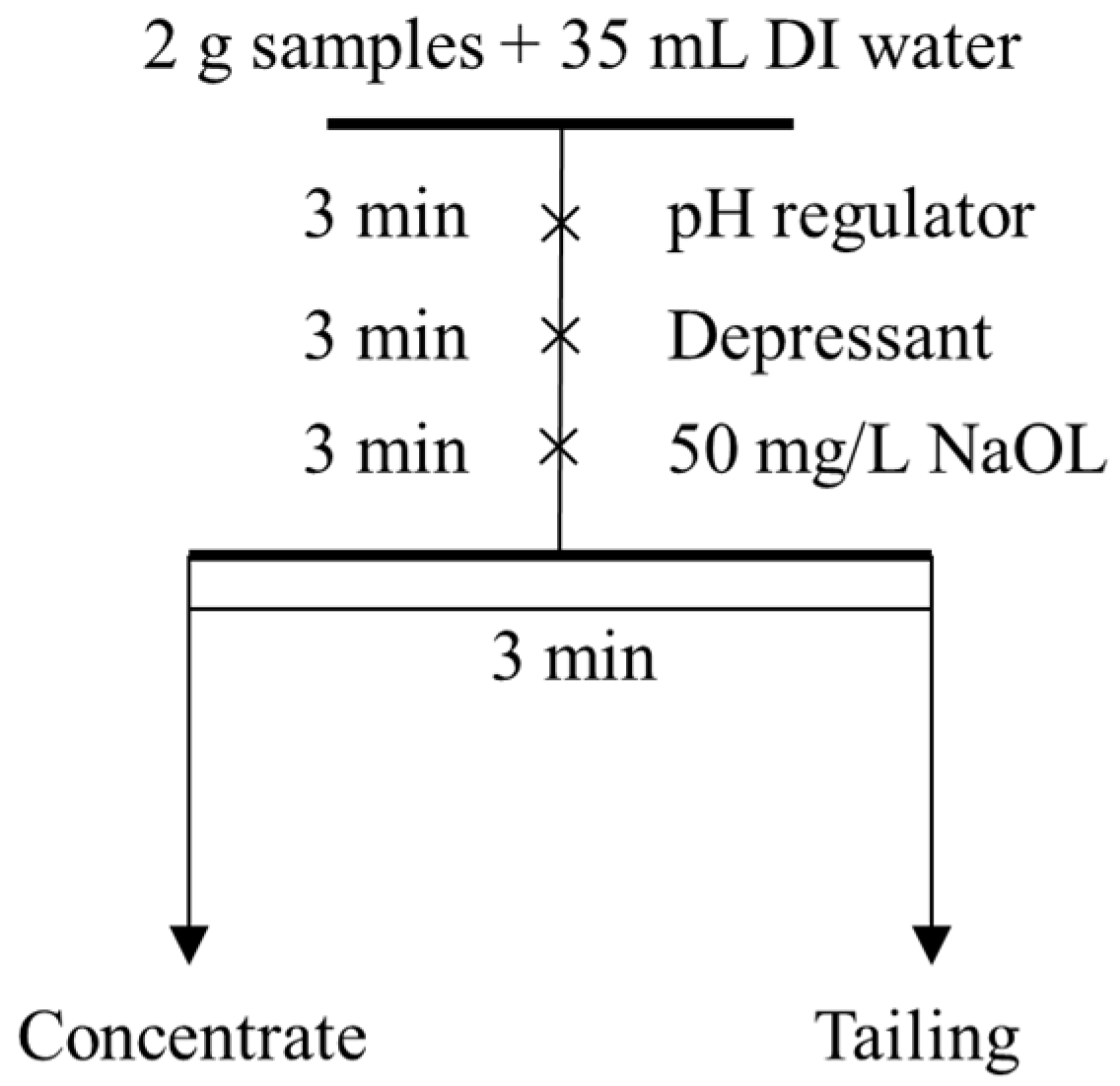

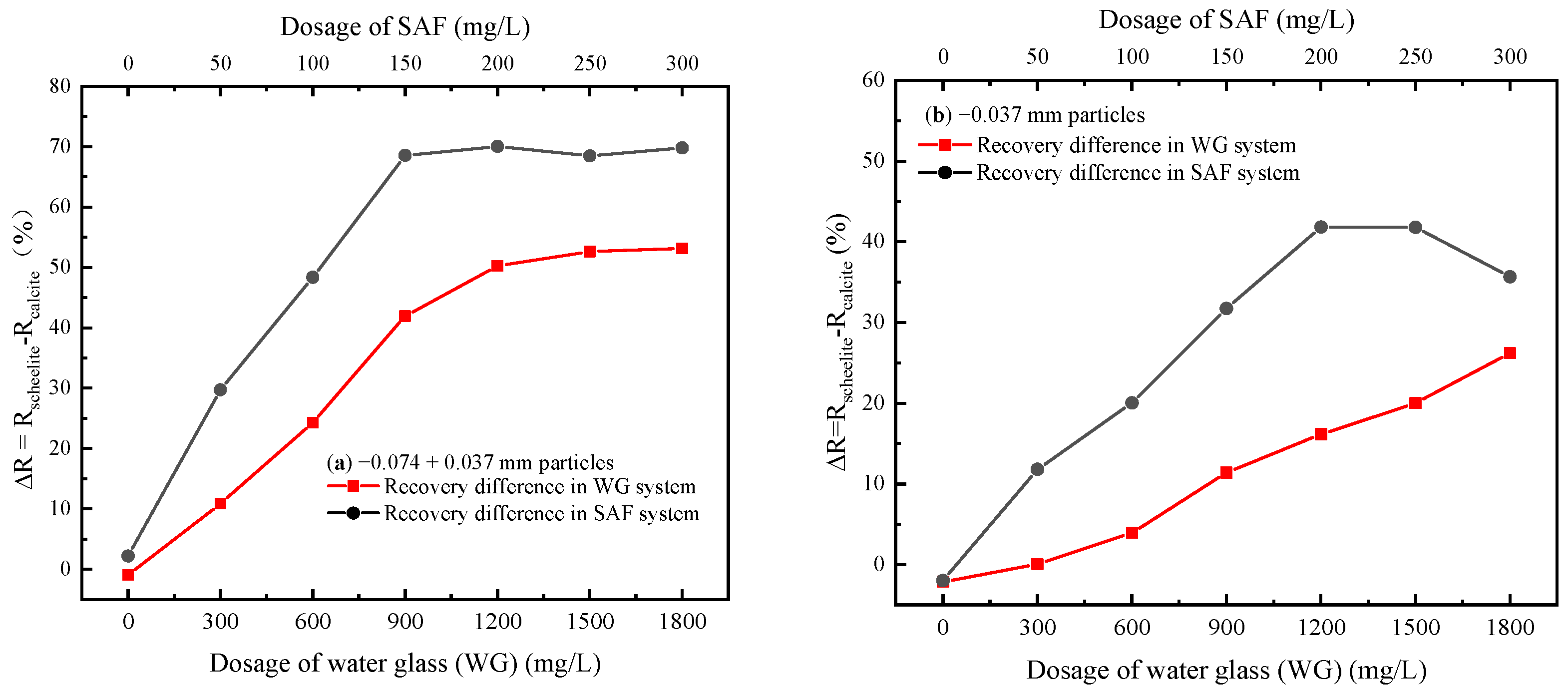

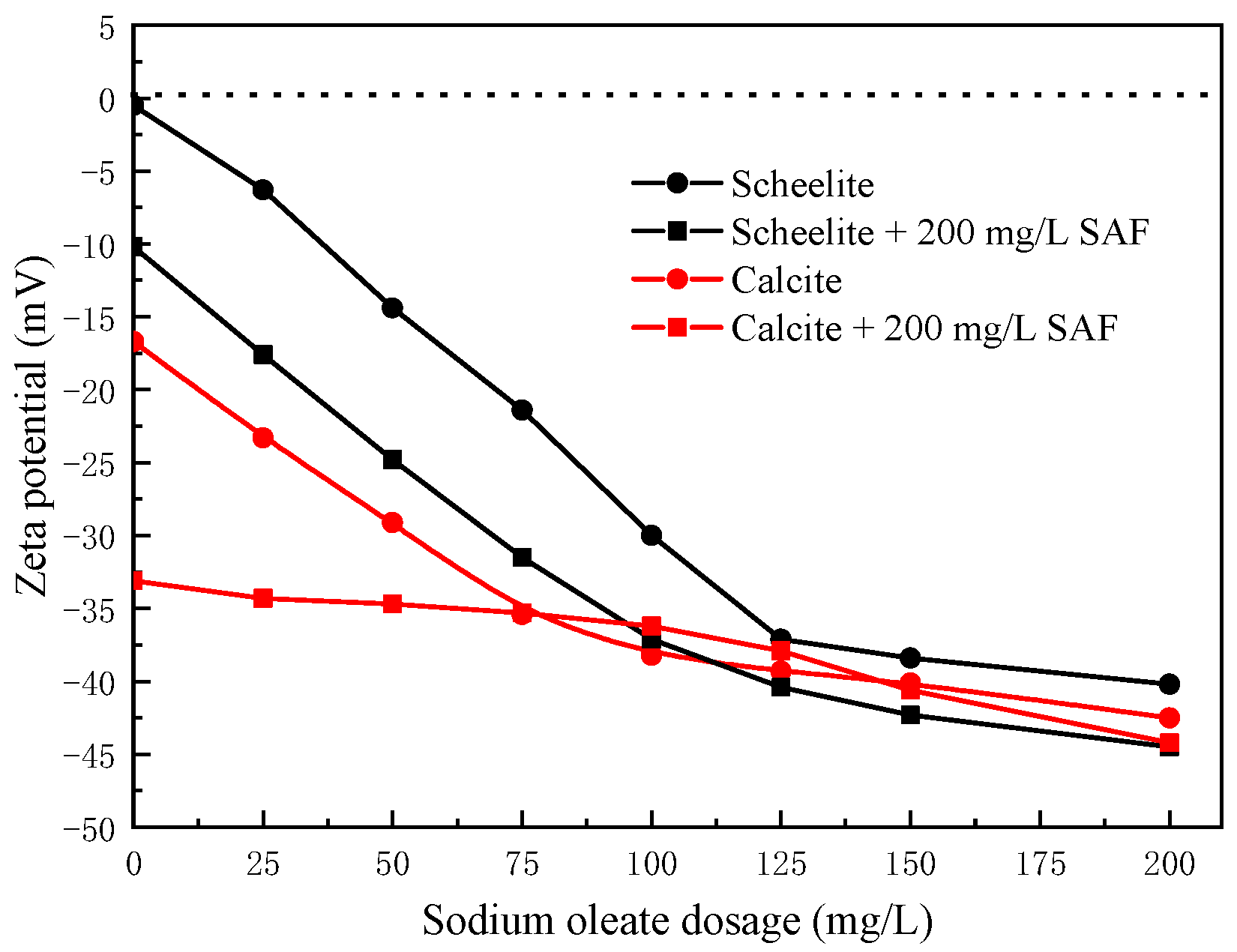


| Minerals | Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | WO3 | CaF2 | CaCO3 | P2O5 | Al2O3 | MgO | Fe | SiO2 | |
| Scheelite | 16.74 | 79.50 | — | — | — | — | — | — | 3.76 |
| Calcite | — | — | — | 97.41 | — | — | — | — | 2.59 |
| Samples | Element Content (%) | ||||
|---|---|---|---|---|---|
| C 1s | O 1s | S 2p | W 4f | Ca 2p | |
| Scheelite | 27.93 | 45.29 | - | 12.54 | 14.25 |
| Scheelite + SAF | 29.51 | 44.21 | - | 12.40 | 13.88 |
| Calcite | 42.41 | 40.70 | - | - | 16.89 |
| Calcite + SAF | 41.98 | 41.07 | 0.40 | - | 16.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Pan, G.; Chu, H.; Lu, D.; Zheng, X. Application of a Superplasticizer in Scheelite Selective Flotation from Calcite. Minerals 2022, 12, 483. https://doi.org/10.3390/min12040483
Wang Y, Pan G, Chu H, Lu D, Zheng X. Application of a Superplasticizer in Scheelite Selective Flotation from Calcite. Minerals. 2022; 12(4):483. https://doi.org/10.3390/min12040483
Chicago/Turabian StyleWang, Yuhua, Gaochan Pan, Haoran Chu, Dongfang Lu, and Xiayu Zheng. 2022. "Application of a Superplasticizer in Scheelite Selective Flotation from Calcite" Minerals 12, no. 4: 483. https://doi.org/10.3390/min12040483






