A New Approach Determining a Phase Transition Boundary Strictly Following a Definition of Phase Equilibrium: An Example of the Post-Spinel Transition in Mg2SiO4 System
Abstract
:1. Introduction
2. Definition of Phase Equilibrium
3. Problems with Previous Experiments
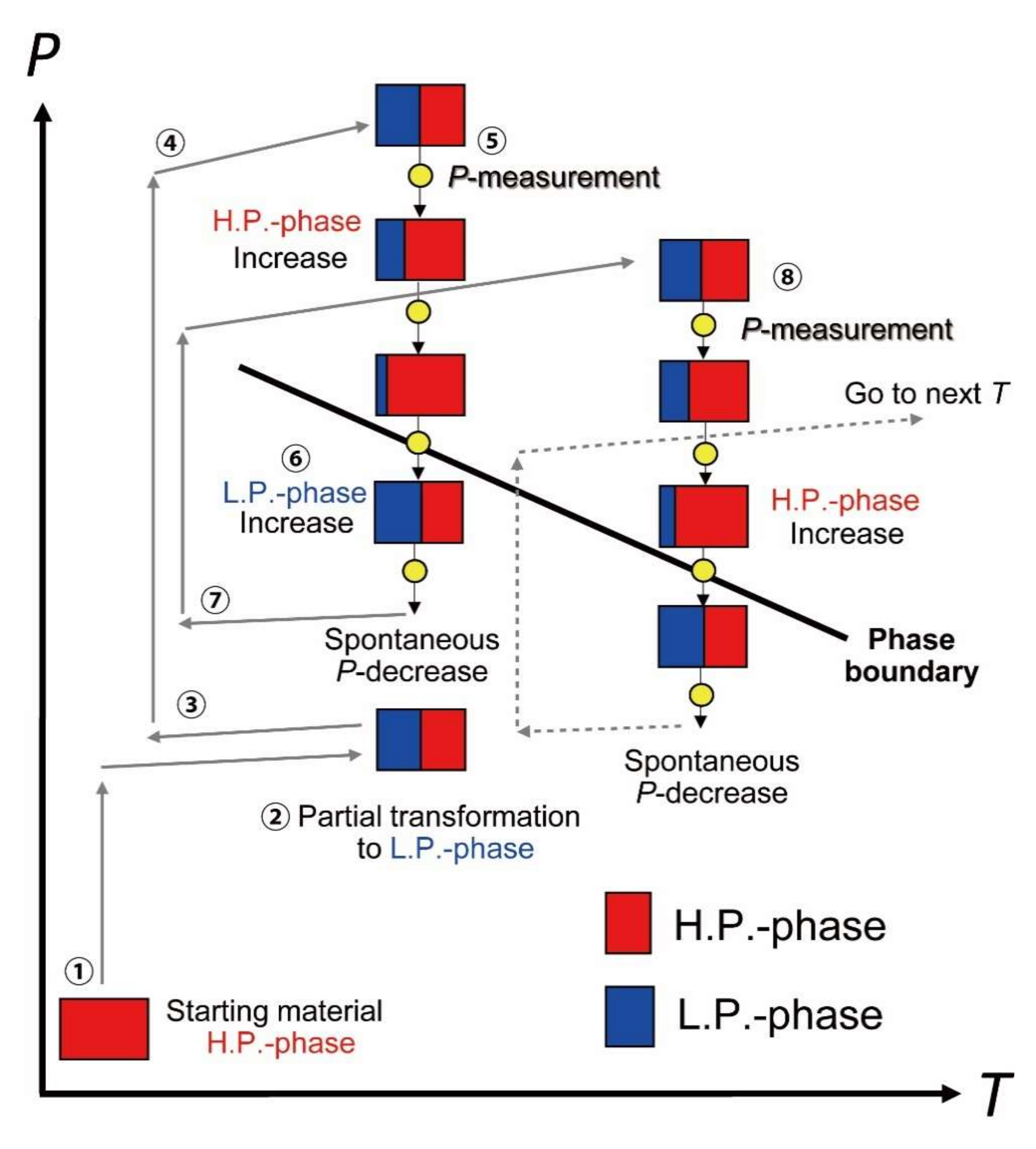
4. A Novel Strategy to Determine a Phase Boundary
- (1)
- The starting material is the H.P.-phase pre-synthesized at the lowest temperature, where the H.P.-phase can be synthesized. Since considerable excess Gibbs energy can drive the phase transition to form the H.P.-phase by overcoming the sluggish kinetics resulting from the low temperature, the synthesis pressure should be as high as possible.
- (2)
- We load the starting material of the H.P.-phase in a multi-anvil cell, compress it, and heat it to the lowest possible temperature where the H.P.-phase can transform to the L.P.-phase to convert the H.P.-phase partially to the L.P.-phase and obtain the coexistence of the H.P.- and L.P.-phases. The pressure should be the lowest in the L.P.-phase stability field to realize the maximum deficient Gibbs energy and enables the partial transition of the H.P.-phase to the L.P.-phase at the lowest temperature. However, since the lower limit of the L.P.-phase stability field is often not well determined, the pressure is higher in practice. This procedure also releases the sample stresses stored during the initial compression, which helps decrease the pressure drop in the later stages. In many cases, the heating in this step reduces the sample pressure by several GPa.
- (3)
- After a sufficient amount of L.P.-phase is obtained to observe using X-ray diffraction and to trace the relative intensity change in the phases, we cool the sample by several hundred K to stop the transition and bring it to higher pressures than the supposed phase boundary.
- (4)
- We heat the sample to the lowest temperature, where the phase transitions can occur kinetically.
- (5)
- We take diffraction patterns of the pressure standard and sample alternatively by keeping the press load and temperature constant. The pressure spontaneously drops gradually. We measure the sample pressure and observe the change in the ratio of the H.P.-phase to the L.P.-phase from the diffraction intensity at constant temperature and monotonically decreasing pressure. L.P.-phase should slowly transform into the H.P.-phase at the beginning because the sample is initially in the stability field of the H.P.-phase, as written in (3). If the pressure drop ceases, we slightly decrease the press load to enhance the pressure drop. We note that the sample pressure is immediately changed by the decrease in press load and kept constant because factors for complicated pressure change shown in Figure 2 have been removed at the initial heating.
- (6)
- At some time, the H.P.-phase/L.P.-phase ratio should start to decrease because the sample enters the L.P.-phase stability field. Therefore, we bracket the phase boundary with the lowest pressure where the H.P.-phase/L.P.-phase ratio increases and the highest pressure where this ratio decreases.
- (7)
- We decrease the sample temperature by several hundred K to stop the progression of the transition, compress the sample to a pressure sufficiently above the phase boundary determined in the previous temperature condition, and heat it to a higher temperature by 50 or 100 K than in the previous stage.
- (8)
- We repeat the procedure from Step (5) to (7) to bracket the phase boundary every 50 or 100 K until the phase transition does not proceed due to the long time annealing.
5. A Practical Example to Determine a Phase Boundary: The Post-Spinel Transition in Mg2SiO4
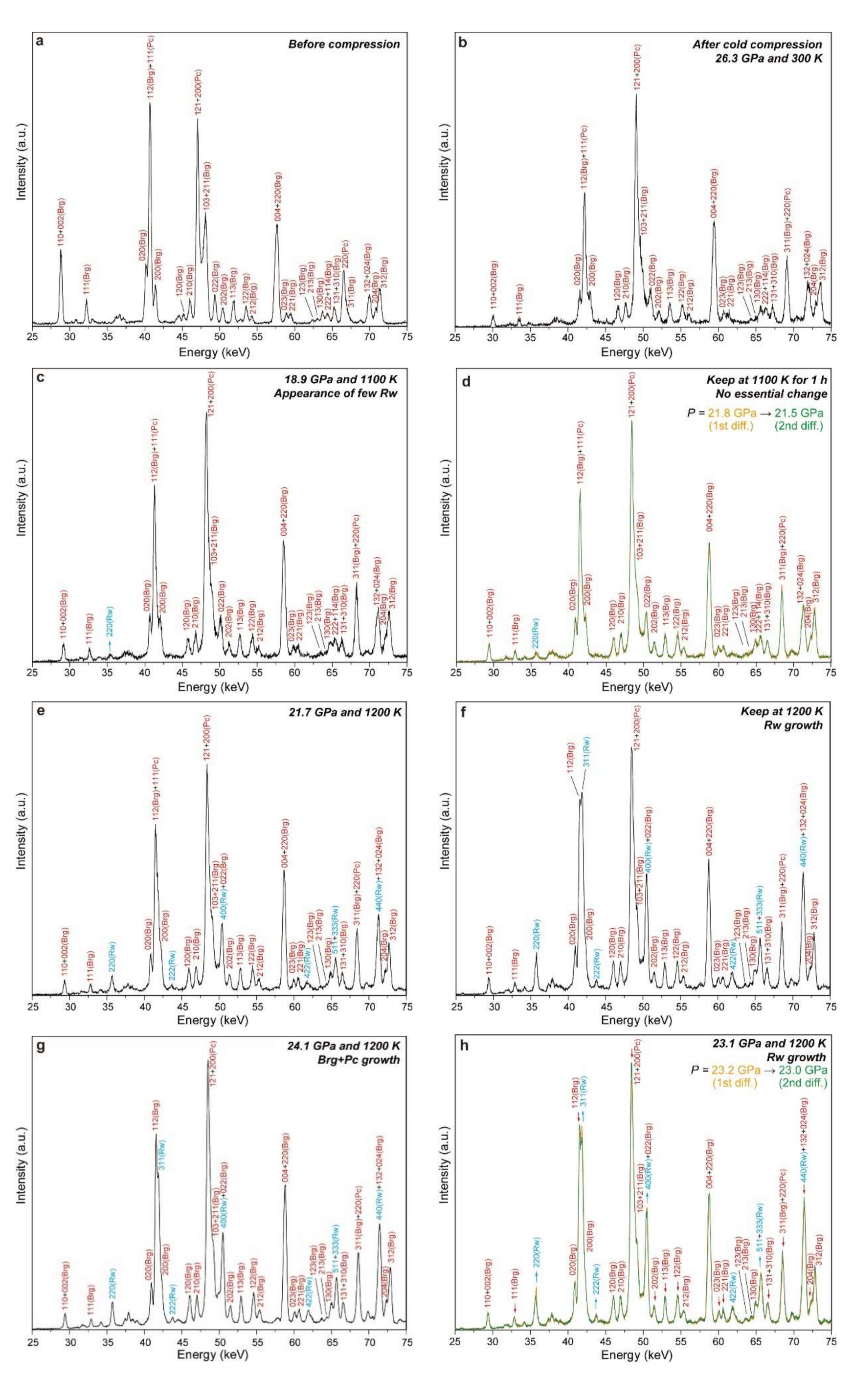
5.1. Preparation of Highly Reactive Starting Material
5.2. Determination of Phase Stability by X-ray Diffraction Profile
6. Discussion
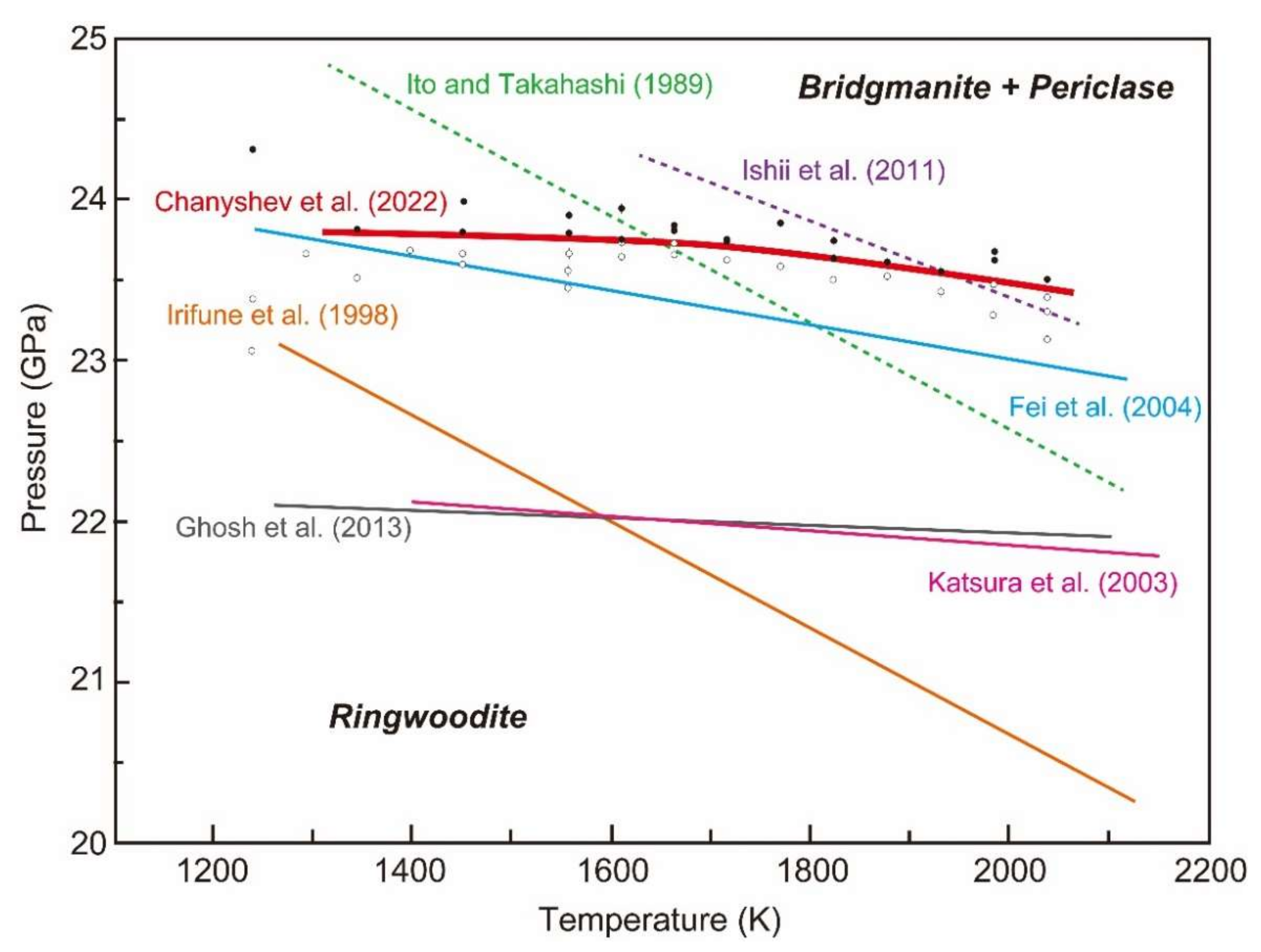
6.1. Comparison with Previously Determined Phase Boundaries
6.2. Mantle Phase Transitions to Be Redetermined Using the New Strategy
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faccenda, M.; Dal Zilio, L. The role of solid–solid phase transitions in mantle convection. Lithos 2017, 268, 198–224. [Google Scholar] [CrossRef]
- Ballmer, M.D.; Schmerr, N.C.; Nakagawa, T.; Ritsema, J. Compositional mantle layering revealed by slab stagnation at ~1000-km depth. Sci. Adv. 2015, 1, e1500815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billen, M.I. Slab dynamics in the transition zone. Phys. Earth Planet. Int. 2010, 183, 296–308. [Google Scholar] [CrossRef]
- Bina, C.R.; Stein, S.; Marton, F.C.; Van Ark, E.M. Implications of slab mineralogy for subduction dynamics. Phys. Earth Planet. Int. 2001, 127, 51–66. [Google Scholar] [CrossRef]
- Bunge, H.P.; Richards, M.A.; Baumgardne, J.R. A sensitivity study of three-dimensional spherical mantle convection at 108 Rayleigh number: Effects of depth-dependent viscosity, heating mode, and an endothermic phase change. J. Geophys. Res. Solid Earth 1997, 102, 11991–12007. [Google Scholar] [CrossRef]
- Čížková, H.; Bina, C.R. Effects of mantle and subduction-interface rheologies on slab stagnation and trench rollback. Earth Planet. Sci. Lett. 2013, 379, 95–103. [Google Scholar] [CrossRef]
- Schubert, G.; Yuen, D.A.; Turcotte, D.L. Role of phase transitions in a dynamic mantle. Geophys. J. Int. 1975, 42, 705–735. [Google Scholar] [CrossRef]
- Tackley, P.J.; Stevenson, D.J.; Glatzmaier, G.A.; Schubert, G. Effects of an endothermic phase transition at 670 km depth in a spherical model of convection in the Earth’s mantle. Nature 1993, 361, 699–704. [Google Scholar] [CrossRef]
- Tackley, P.J.; Stevenson, D.J.; Glatzmaier, G.A.; Schubert, G. Effects of multiple phase transitions in a three-dimensional spherical model of convection in Earth’s mantle. J. Geophys. Res. Solid Earth 1994, 99, 15877–15901. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Yamagishi, Y.; Hamano, Y.; Stegman, D.R. Mechanism for generating stagnant slabs in 3-D spherical mantle convection models at Earth-like conditions. Phys. Earth Planet. Int. 2010, 183, 341–352. [Google Scholar] [CrossRef]
- Ito, E.; Takahashi, E. Postspinel transformations in the system Mg2SiO4-Fe2SiO4 and some geophysical implications. J. Geophys. Res. Solid Earth 1989, 94, 10637–10646. [Google Scholar] [CrossRef]
- Katsura, T.; Ito, E. The system Mg2SiO4-Fe2SiO4 at high pressures and temperatures: Precise determination of stabilities of olivine, modified spinel, and spinel. J. Geophys. Res. Solid Earth 1989, 94, 15663–15670. [Google Scholar] [CrossRef]
- Ishii, T.; Kojitani, H.; Akaogi, M. Post-spinel transitions in pyrolite and Mg2SiO4 and akimotoite–perovskite transition in MgSiO3: Precise comparison by high-pressure high-temperature experiments with multi-sample cell technique. Earth Planet. Sci. Lett. 2011, 309, 185–197. [Google Scholar] [CrossRef]
- Katsura, T. Phase-Relation Studies of Mantle Minerals by In Situ X-ray Diffraction Using Multianvil Apparatus. In Advances in High-Pressure Mineralogy. GSA Spec.; Ohtani, E., Ed.; Geological Society of America: Boulder, CO, USA, 2007; Volume 421, pp. 189–205. [Google Scholar]
- Ishii, T.; Huang, R.; Fei, H.; Koemets, I.; Liu, Z.; Maeda, F.; Yuan, L.; Wang, L.; Druzhbin, D.; Yamamoto, T.; et al. Complete agreement of the post-spinel transition with the 660-km seismic discontinuity. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Huang, R.; Myhill, R.; Fei, H.; Koemets, I.; Liu, Z.; Maeda, F.; Yuan, L.; Wang, L.; Druzhbin, D.; et al. Sharp 660-km discontinuity controlled by extremely narrow binary post-spinel transition. Nat. Geosci. 2019, 12, 869–872. [Google Scholar] [CrossRef]
- Chanyshev, A.; Ishii, T.; Bondar, D.; Bhat, S.; Kim, E.J.; Farla, R.; Nishida, K.; Liu, Z.; Wang, L.; Nakajima, A.; et al. Depressed 660-km discontinuity caused by akimotoite–bridgmanite transition. Nature 2022, 601, 69–73. [Google Scholar] [CrossRef]
- Fei, Y.; Van Orman, J.; Li, J.; Van Westrenen, W.; Sanloup, C.; Minarik, W.; Hirose, K.; Komabayashi, T.; Walter, M.; Funakoshi, K.I. Experimentally determined postspinel transformation boundary in Mg2SiO4 using MgO as an internal pressure standard and its geophysical implications. J. Geophys. Res. Solid Earth 2004, 109, B02305-1. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Ohtani, E.; Litasov, K.D.; Suzuki, A.; Dobson, D.; Funakoshi, K. Effect of water in depleted mantle on post-spinel transition and implication for 660 km seismic discontinuity. Earth Planet. Sci. Lett. 2013, 371, 103–111. [Google Scholar] [CrossRef]
- Hirose, K.; Komabayashi, T.; Murakami, M.; Funakoshi, K.I. In situ measurements of the majorite-akimotoite-perovskite phase transition boundaries in MgSiO3. Geophys. Res. Lett. 2001, 28, 4351–4354. [Google Scholar] [CrossRef] [Green Version]
- Hirose, K.; Fei, Y.; Ono, S.; Yagi, T.; Funakoshi, K.I. In situ measurements of the phase transition boundary in Mg3Al2Si3O12: Implications for the nature of the seismic discontinuities in the Earth’s mantle. Earth Planet. Sci. Lett. 2001, 184, 567–573. [Google Scholar] [CrossRef]
- Irifune, T.; Nishiyama, N.; Kuroda, K.; Inoue, T.; Isshiki, M.; Utsumi, W.; Funakoshi, K.; Urakawa, S.; Uchida, T.; Katsura, T.; et al. The postspinel phase boundary in Mg2SiO4 determined by in situ X-ray diffraction. Science 1998, 279, 1698–1700. [Google Scholar] [CrossRef] [PubMed]
- Katsura, T.; Yamada, H.; Shinmei, T.; Kubo, A.; Ono, S.; Kanzaki, M.; Yoneda, A.; Walter, M.J.; Ito, E.; Urakawa, S.; et al. Post-spinel transition in Mg2SiO4 determined by high P–T in situ X-ray diffractometry. Phys. Earth Planet. Int. 2003, 136, 11–24. [Google Scholar] [CrossRef]
- Katsura, T.; Yamada, H.; Nishikawa, O.; Song, M.; Kubo, A.; Shinmei, T.; Yokoshi, S.; Aizawa, Y.; Yoshino, T.; Walter, M.J.; et al. Olivine-wadsleyite transition in the system (Mg,Fe)2SiO4. J. Geophys. Res. Solid Earth 2004, 109, B02209. [Google Scholar] [CrossRef]
- Litasov, K.; Ohtani, E.; Sano, A.; Suzuki, A.; Funakoshi, K. In situ X-ray diffraction study of post-spinel transformation in a peridotite mantle: Implication for the 660-km discontinuity. Earth Planet. Sci. Lett. 2005, 238, 311–328. [Google Scholar] [CrossRef]
- Ono, S.; Katsura, T.; Ito, E.; Kanzaki, M.; Yoneda, A.; Walter, M.J.; Urakawa, S.; Utsumi, W.; Funakoshi, K. In situ observation of ilmenite-perovskite phase transition in MgSiO3 using synchrotron radiation. Geophys. Res. Lett. 2001, 28, 835–838. [Google Scholar] [CrossRef]
- Yagi, T.; Akaogi, M.; Shimomura, O.; Suzuki, T.; Akimoto, S.I. In situ observation of the olivine-spinel phase transformation in Fe2SiO4 using synchrotron radiation. J. Geophys. Res. Solid Earth 1987, 92, 6207–6213. [Google Scholar] [CrossRef]
- Kuroda, K.; Irifune, T.; Inoue, T.; Nishiyama, N.; Miyashita, M.; Funakoshi, K.; Utsumi, W. Determination of the phase boundary between ilmenite and perovskite in MgSiO3 by in situ X-ray diffraction and quench experiments. Phys. Chem. Miner. 2000, 27, 523–532. [Google Scholar] [CrossRef]
- Hirose, K.; Sinmyo, R.; Sata, N.; Ohishi, Y. Determination of post-perovskite phase transition boundary in MgSiO3 using Au and MgO pressure standards. Geophys. Res. Lett. 2006, 33, L01310. [Google Scholar] [CrossRef]
- Ishii, T.; Yamazaki, D.; Tsujino, N.; Xu, F.; Liu, Z.; Kawazoe, T.; Yamamoto, T.; Druzhbin, D.; Wang, L.; Higo, Y.; et al. Pressure generation to 65 GPa in a Kawai-type multi-anvil apparatus with tungsten carbide anvils. High Press. Res. 2017, 37, 507–515. [Google Scholar] [CrossRef]
- Ishii, T.; Liu, Z.; Katsura, T. A breakthrough in pressure generation by a Kawai-type multi-anvil apparatus with tungsten carbide anvils. Engineering 2019, 5, 434–440. [Google Scholar] [CrossRef]
- Ishii, T.; Miyajima, N.; Criniti, G.; Hu, Q.; Glazyrin, K.; Katsura, T. High pressure-temperature phase relations of basaltic crust up to mid-mantle conditions. Earth Planet. Sci. Lett. 2022, 584, 117472. [Google Scholar] [CrossRef]
- Kubo, T.; Ohtani, E.; Kato, T.; Urakawa, S.; Suzuki, A.; Kanbe, Y.; Funakoshi, K.I.; Utsumi, W.; Kikegawa, T.; Fujino, K. Mechanisms and kinetics of the post-spinel transformation in Mg2SiO4. Phys. Earth Planet. Int. 2002, 129, 153–171. [Google Scholar] [CrossRef]
- Nishihara, Y.; Doi, S.; Kakizawa, S.; Higo, Y.; Tange, Y. Effect of pressure on temperature measurements using WRe thermocouple and its geophysical impact. Phys. Earth Planet. Int. 2020, 298, 106348. [Google Scholar] [CrossRef]
- Kubo, A.; Akaogi, M. Post-garnet transitions in the system Mg4Si4O12–Mg3Al2Si3O12 up to 28 GPa: Phase relations of garnet, ilmenite and perovskite. Phys. Earth Planet. Int. 2000, 121, 85–102. [Google Scholar] [CrossRef]
- Sawamoto, H. Phase diagram of MgSiO3 at pressures up to 24 GPa and temperatures up to 2200 C: Phase stability and properties of tetragonal garnet. High Press. Res. Miner. Phys. 1987, 209, 219. [Google Scholar]
- Suzuki, A.; Ohtani, E.; Morishima, H.; Kubo, T.; Kanbe, Y.; Kondo, T.; Okuda, T.; Terasaki, H.; Kato, T.; Kikegawa, T. In situ determination of the phase boundary between wadsleyite and ringwoodite in Mg2SiO4. Geophys. Res. Lett. 2000, 27, 803–806. [Google Scholar] [CrossRef]
- Inoue, T.; Irifune, T.; Higo, Y.; Sanehira, T.; Sueda, Y.; Yamada, A.; Shinmei, T.; Yamazaki, D.; Ando, J.; Funakoshi, K.; et al. The phase boundary between wadsleyite and ringwoodite in Mg2SiO4 determined by in situ X-ray diffraction. Phys. Chem. Miner. 2006, 33, 106–114. [Google Scholar] [CrossRef]
- Ulmer, P.; Stalder, R. The Mg(Fe)SiO3 orthoenstatite-clinoenstatite transitions at high pressures and temperatures determined by Raman-spectroscopy on quenched samples. Am. Mineral. 2001, 86, 1267–1274. [Google Scholar] [CrossRef]
- Akashi, A.; Nishihara, Y.; Takahashi, E.; Nakajima, Y.; Tange, Y.; Funakoshi, K.I. Orthoenstatite/clinoenstatite phase transformation in MgSiO3 at high-pressure and high-temperature determined by in situ X-ray diffraction: Implications for nature of the X discontinuity. J. Geophys. Res. Solid Earth 2009, 114, B04206. [Google Scholar] [CrossRef]
- Morishima, H.; Kato, T.; Suto, M.; Ohtani, E.; Urakawa, S.; Utsumi, W.; Shimomura, O.; Kikegawa, T. The phase boundary between α-and β-Mg2SiO4 determined by in situ X-ray observation. Science 1994, 265, 1202–1203. [Google Scholar] [CrossRef]
- Ono, S.; Kikegawa, T.; Higo, Y.; Tange, Y. Precise determination of the phase boundary between coesite and stishovite in SiO2. Phys. Earth Planet. Int. 2017, 264, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Utsumi, W.; Liebermann, R.C. In situ X-ray observations of the coesite-stishovite transition: Reversed phase boundary and kinetics. Phys. Chem. Miner. 1996, 23, 1–10. [Google Scholar] [CrossRef]
- Katsura, T.; Ueda, A.; Ito, E.; Morooka, K. Postspinel Transition in Fe2SiO4. In High Pressure Temperature Research: Properties of Earth and Planetary Materials; Manghnani, M.H., Yagi, T., Eds.; American Geophysical Union: Washington, DC, USA, 1998; Volume 101, pp. 435–440. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, T.; Chanyshev, A.; Katsura, T. A New Approach Determining a Phase Transition Boundary Strictly Following a Definition of Phase Equilibrium: An Example of the Post-Spinel Transition in Mg2SiO4 System. Minerals 2022, 12, 820. https://doi.org/10.3390/min12070820
Ishii T, Chanyshev A, Katsura T. A New Approach Determining a Phase Transition Boundary Strictly Following a Definition of Phase Equilibrium: An Example of the Post-Spinel Transition in Mg2SiO4 System. Minerals. 2022; 12(7):820. https://doi.org/10.3390/min12070820
Chicago/Turabian StyleIshii, Takayuki, Artem Chanyshev, and Tomoo Katsura. 2022. "A New Approach Determining a Phase Transition Boundary Strictly Following a Definition of Phase Equilibrium: An Example of the Post-Spinel Transition in Mg2SiO4 System" Minerals 12, no. 7: 820. https://doi.org/10.3390/min12070820




