Flotation Separation of Covellite and Enargite via Oxidation Treatment
Abstract
:1. Introduction
2. Materials and Methods
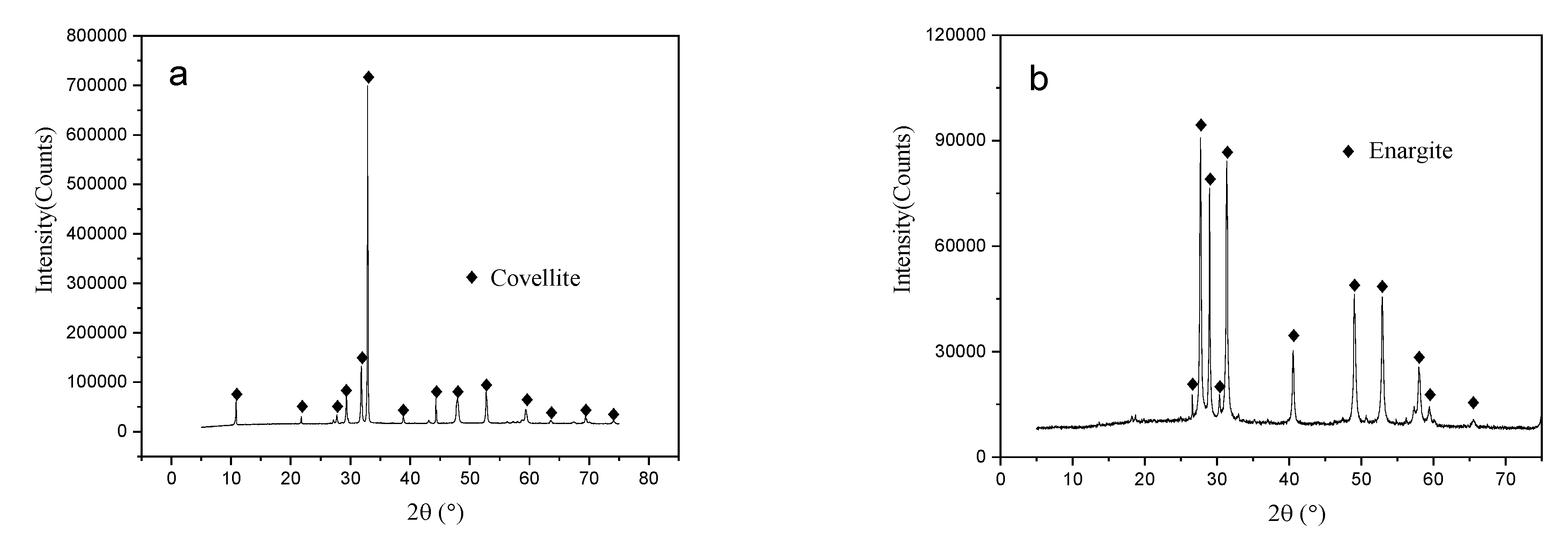
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation Experiments
2.2.2. X-ray Photoelectron Spectroscopy
2.2.3. Scanning Electron Microscopy
2.2.4. Contact Angle Measurements
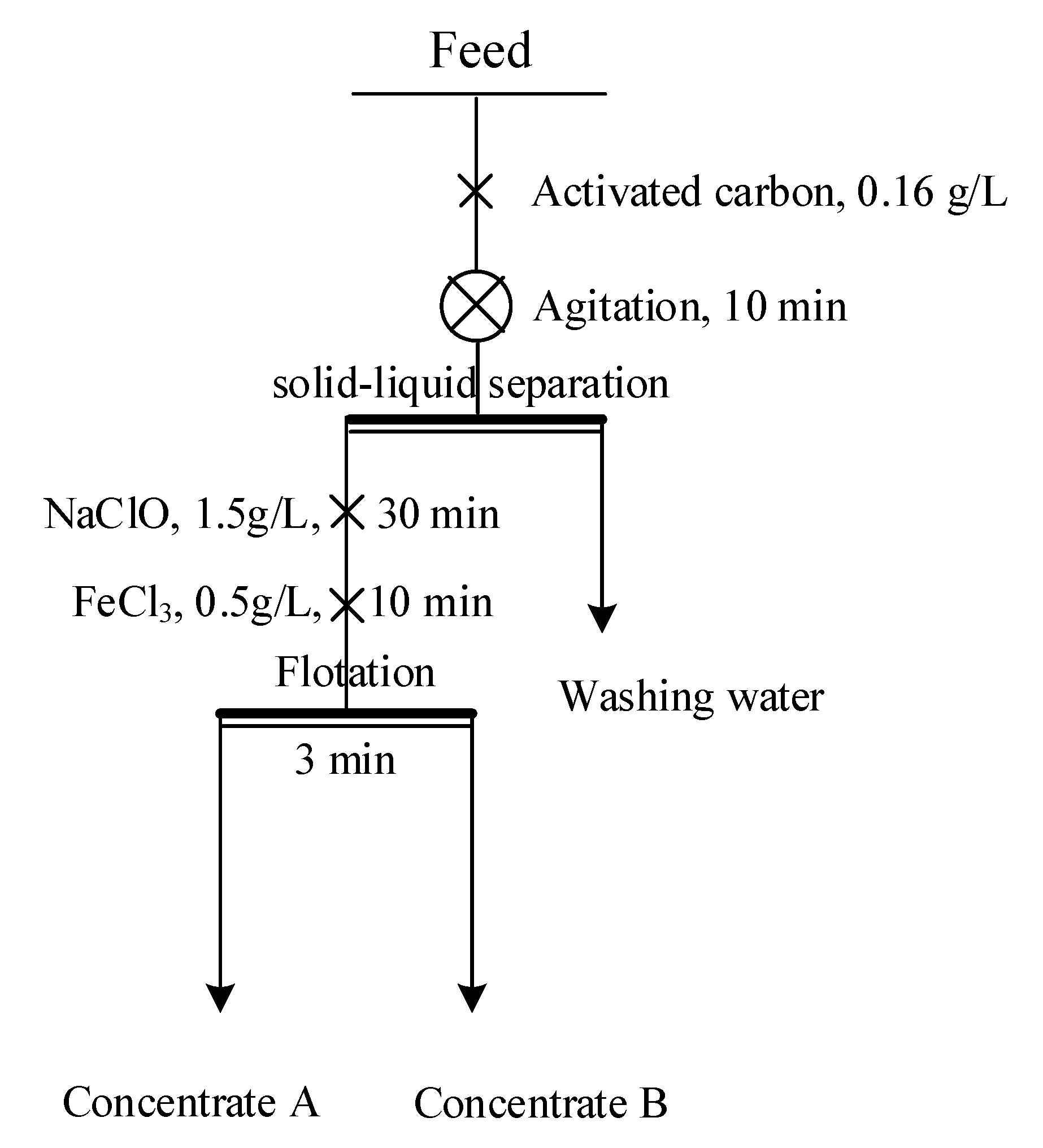
2.2.5. Bench-Scale Flotation
3. Results
3.1. Micro-Flotation of the Pure Mineral Sample
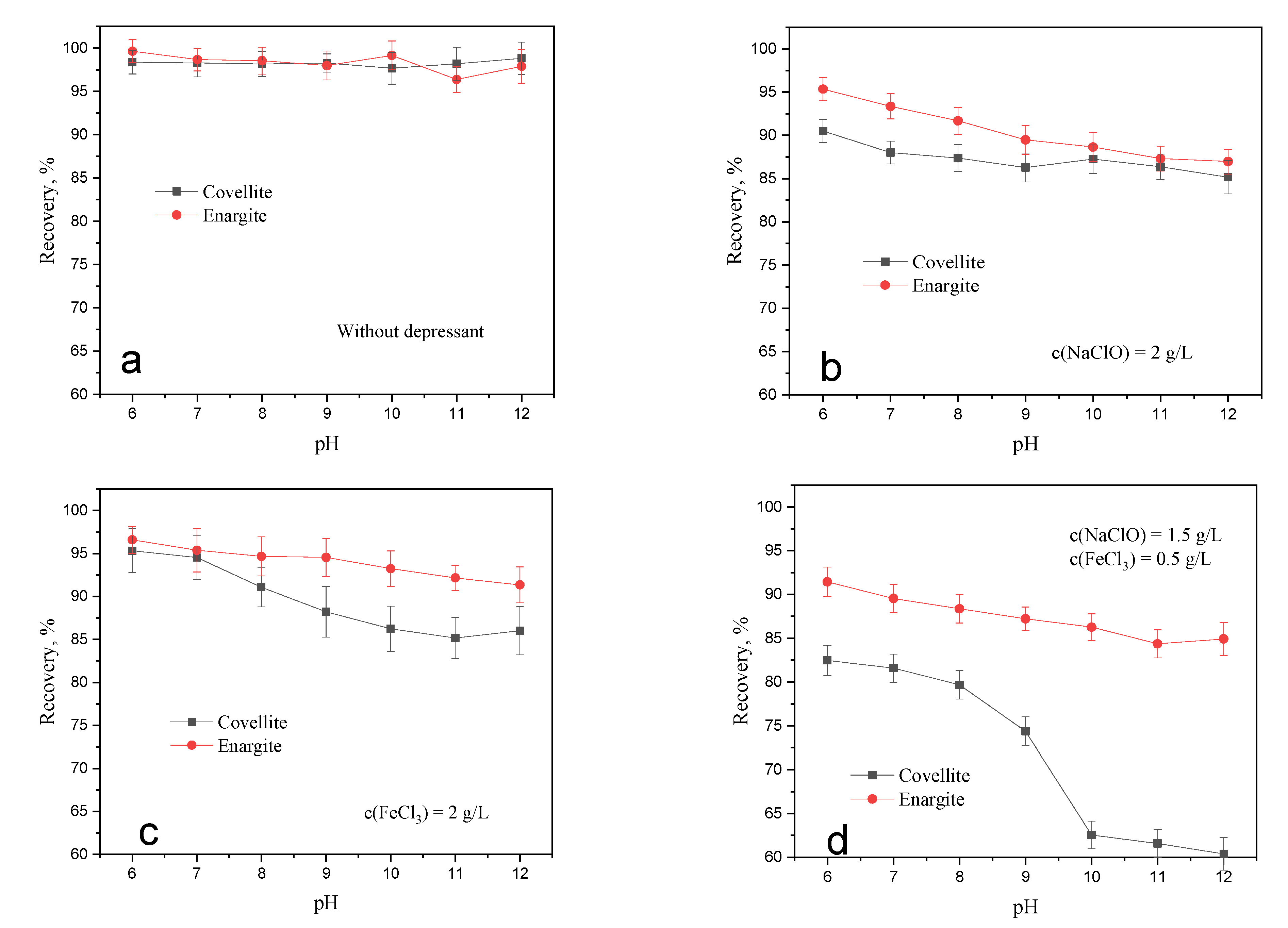
3.1.1. Flotation Performance of Covellite and Enargite
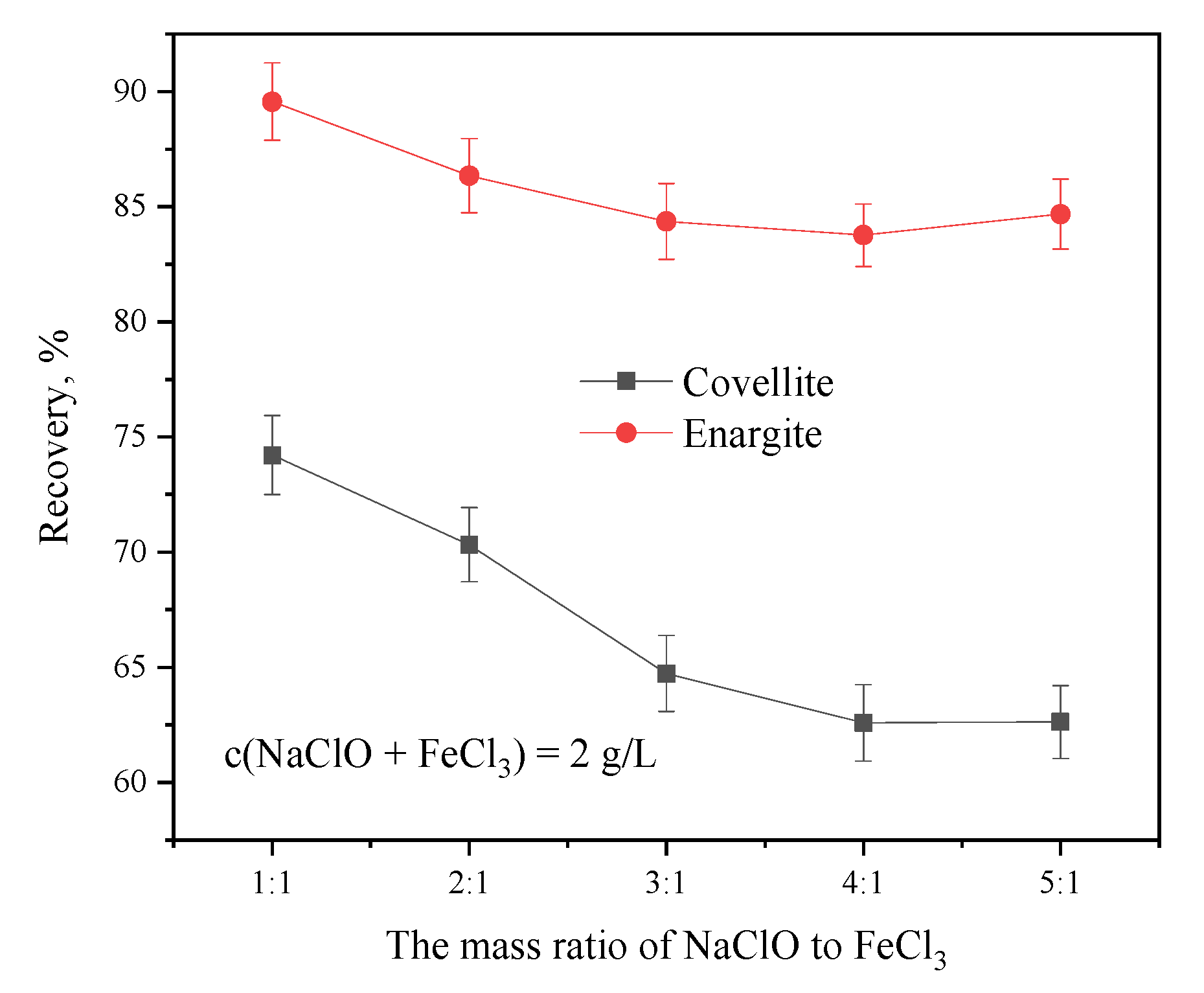
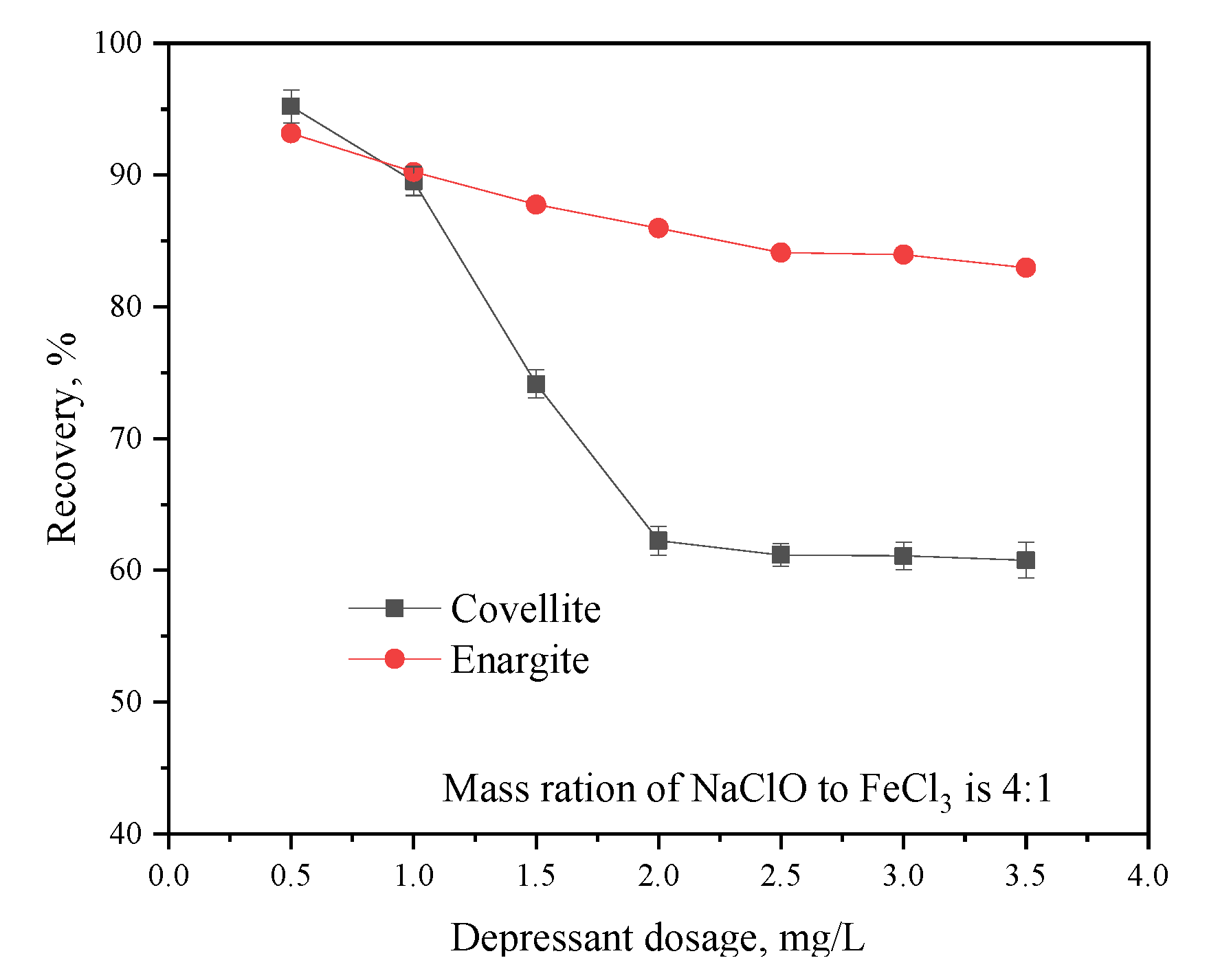
3.1.2. Effect of Depressant Dosage Ratio on the Separation of Covellite and Enargite
3.1.3. Effect of the Depressant Dosage on the Flotation Separation of Covellite and Enargite
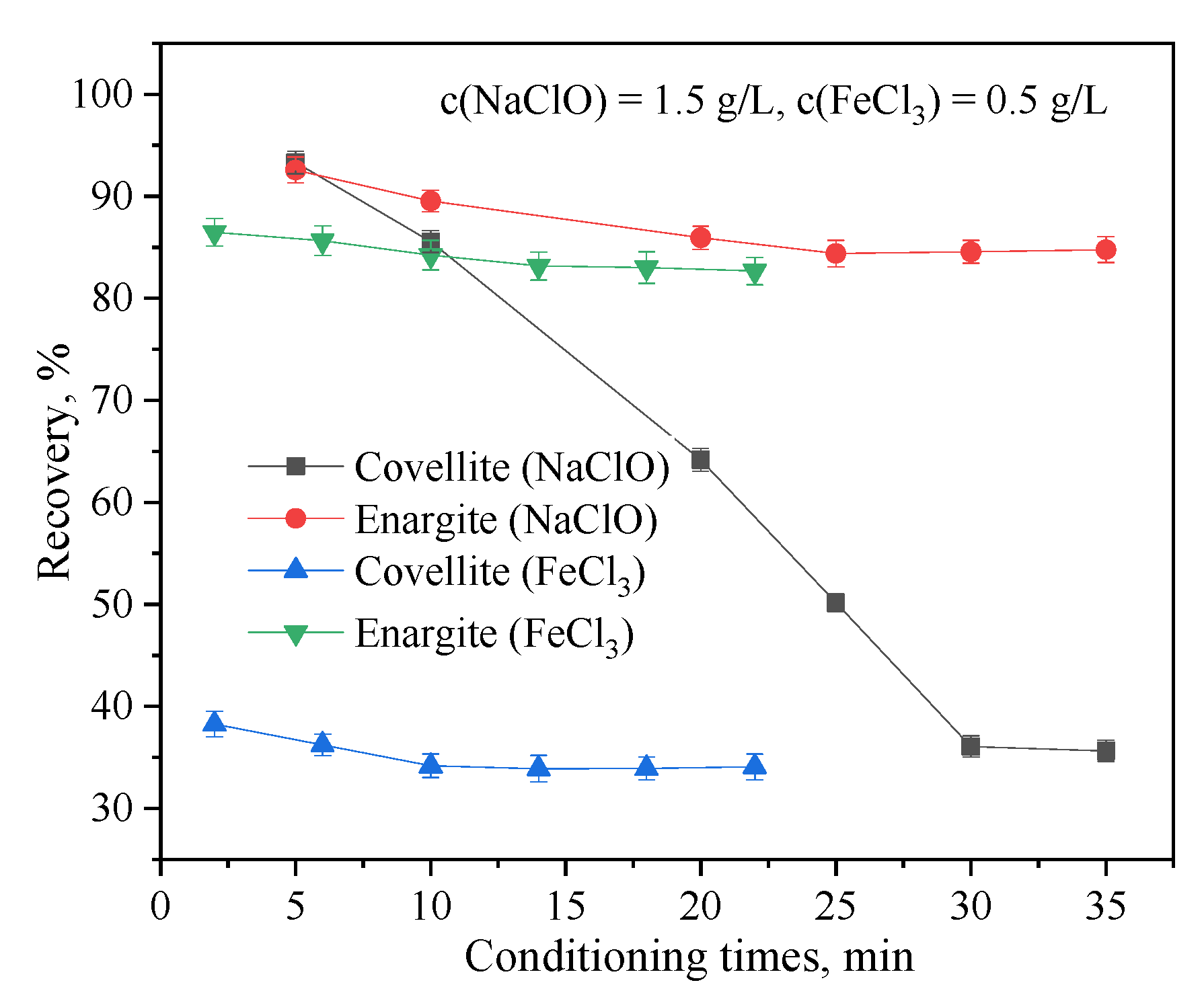
3.1.4. Effects of the Conditioning Times of Depressants on the Flotation Separation of Covellite and Enargite
3.2. Separation Mechanism of Depressants
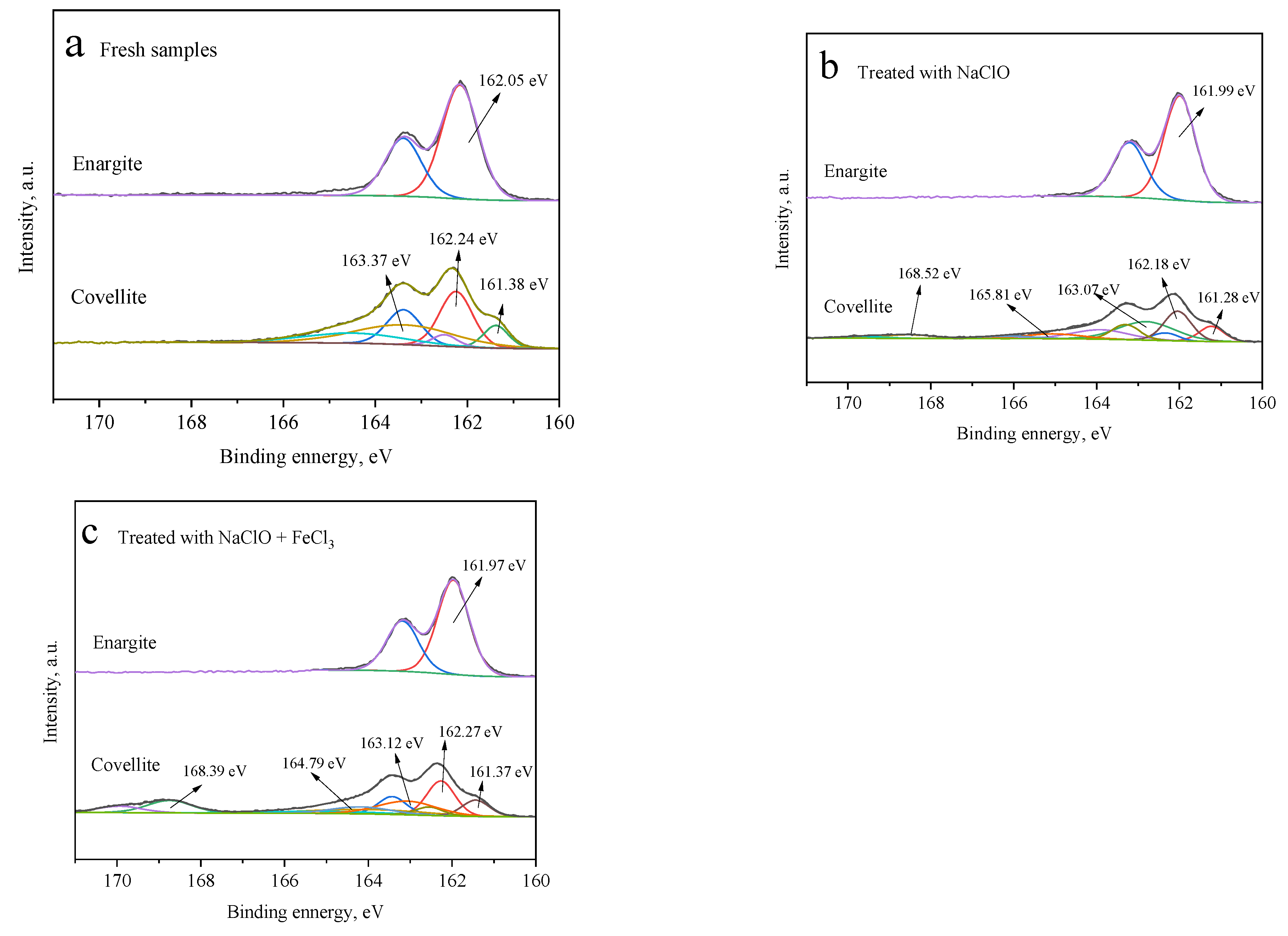
3.2.1. XPS Analysis
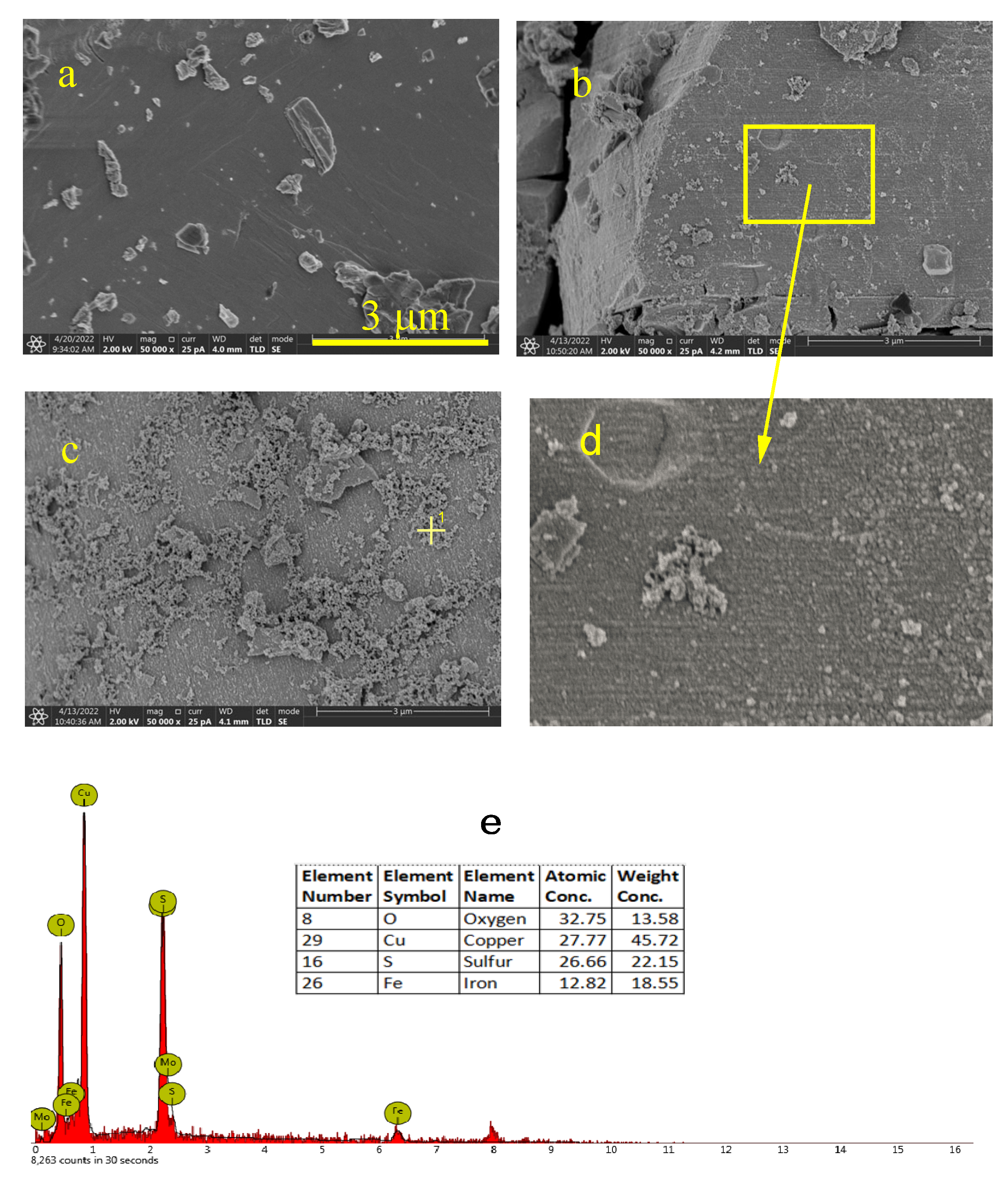
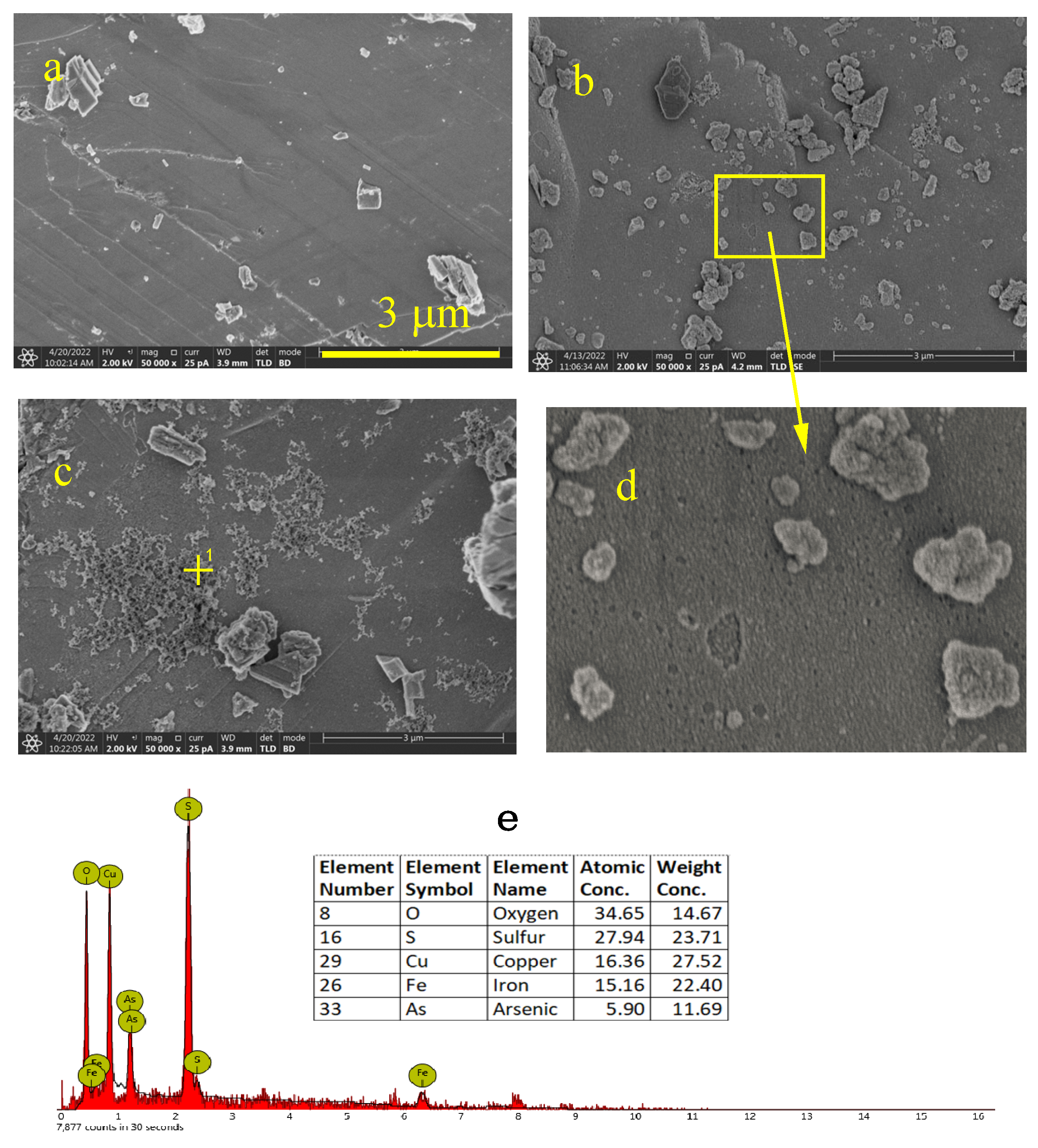
3.2.2. SEM Analysis
3.2.3. Contact Angle Measurements
3.2.4. Bench-Scale Flotation Tests
3.3. Discussion
4. Conclusions
- Micro-flotation and contact angle measurements indicated that the addition of NaClO followed by FeCl3 increased the hydrophobicity difference between covellite and enargite.
- Bench-scale flotation tests indicated that the bulk copper concentrate could be separated into two products: low- (As grade of 0.46%) and high-arsenic-containing (As grade of 5.18%) copper concentrates.
- XPS and SEM analyses established that the oxidization treatment of NaClO could lead to the accumulation of oxides on the covellite surface, but not on the enargite surface.
- The presence of FeCl3 caused varying precipitation of ferric hydroxide on the surfaces of covellite and enargite, which exacerbated the difference in the hydrophilicity of these minerals.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. Recent Trends in the Processing of Enargite Concentrates. Miner. Process. Extr. Metall. Rev. 2014, 35, 283–367. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.J.; Li, S.M.; Deng, J.S.; Luo, B.; Lai, H. Depression mechanism involving Fe3+ during arsenopyrite flotation. Sep. Purif. Technol. 2019, 222, 109–116. [Google Scholar] [CrossRef]
- Plackowski, C.; Nguyen, A.V.; Bruckard, W.J. A critical review of surface properties and selective flotation of enargite in sulphide systems. Miner. Eng. 2012, 30, 1–11. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Manlapig, E.; Forbes, E.; Bradshaw, D.; Edraki, M. Selective flotation of enargite from copper sulphides in Tampakan deposit. Miner. Eng. 2017, 112, 1–10. [Google Scholar] [CrossRef]
- Fornasiero, D.; Fullston, D.; Li, C.; Ralston, J. Separation of enargite and tennantite from non-arsenic copper sulfide minerals by selective oxidation or dissolution. Int. J. Miner. Process. 2001, 61, 109–119. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.; Bradshaw, D. A review of copper–arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 36, 179–186. [Google Scholar] [CrossRef]
- García-Garnica, R.; Castillo-Magallanes, N.; Rodríguez, I.; Cruz, R.; Lázaro, I. Electrochemical study of enargite within the mixed potential zone attained with different oxidizing reagents in an alkaline medium. Electrochim. Acta 2022, 425, 140719. [Google Scholar] [CrossRef]
- Castro, S.H.; Baltierra, L.; Munoz, P. Depression of enargite by magnesiumammonium mixtures. In Proceedings of the Copper 2003–Cobre 2003 the 5th International Conference vol. III–Mineral Processing, Santiago, Chile, 30 November–3 December 2003; pp. 257–269. [Google Scholar]
- Menacho, J.M.; Aliaga, W.; Valenzuela, R.; Ramos, V.; Olivares, I. Selective flotation of enargite and chalcopyrite. Minerals 1993, 48, 33–39. [Google Scholar]
- Pauporté, T.; Schuhmann, D. An electrochemical study of natural enargite under conditions relating to those used in flflotation of sulphide minerals. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 1–19. [Google Scholar] [CrossRef]
- Asbjornsson, J.; Kelsall, G.H.; Pattrick, R.A.D.; Vaughan, D.J.; Wincott, P.L.; Hope, G.A. Electrochemical and surface analytical studies of enargite in acid solution. J. Electrochem. Soc. 2004, 151, 250–256. [Google Scholar] [CrossRef]
- Ma, Y.I.; Yang, Y.; Skinner, W.; Chen, M. Electrochemical and spectroscopic analysis of enargite (Cu3AsS4) dissolution mechanism in sulfuric acid solution. Hydrometallurgy 2020, 194, 105346. [Google Scholar] [CrossRef]
- Guo, H.; Yen, W.T. Electrochemical study of synthetic and natural enargites. Proc. Int. Miner. Process. Congr. 2008, 24, 1138–1145. [Google Scholar]
- Yepsen, R.; Gutierrez, L. Effect of Eh and pH on the flotation of enargite using seawater. Miner. Eng. 2020, 159, 106612. [Google Scholar] [CrossRef]
- Suyantara, G.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of H2O2 and potassium amyl xanthate on separation of enargite and tennantite from chalcopyrite and bornite using flotation. Miner. Eng. 2020, 152, 106371. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of Na2SO3 on the floatability of chalcopyrite and enargite. Miner. Eng. 2021, 173, 107222. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.B.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107113. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Ishikura, K.; Hirajima, T. Spectroscopic study on oxidative dissolution of chalcopyrite, enargite and tennantite at different pH values. Hydrometallurgy 2010, 100, 144–151. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Manlapig, E.; Forbes, E.; Edraki, M.; Bradshaw, D. Effect of surface oxidation on the flotation response of enargite in a complex ore system. Miner. Eng. 2018, 119, 149–155. [Google Scholar] [CrossRef]
- Deng, R.D.; Yang, X.F.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Ma, Y.Q. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Nowak, P.; Laajalehto, K. Oxidation of galena surface–an XPS study of the formation of sulfoxy species. Appl. Surf. Sci. 2000, 157, 101–111. [Google Scholar] [CrossRef]
- Wu, D.D.; Wen, S.M.; Deng, J.S.; Liu, J.; Mao, Y.B. Study on the sulfidation behavior of smithsonite. Appl. Surf. Sci. 2015, 329, 315–320. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.L.; Wang, B.; Yuan, Y.; Fang, Z.; Cui, Y. Arsenic Removal and Solidification from Enargite. Chin. J. Rare Met. 2018, 42, 1093–1102. [Google Scholar]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; SasakI, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Xiong, X.L.; Hua, X.M.; Zheng, Y.F.; Lu, X.G.; Li, S.G.; Cheng, H.W.; Xu, Q. Oxidation mechanism of chalcopyrite revealed by X-ray photoelectron spectroscopy and first principles studies. Appl. Surf. Sci. 2018, 427, 233–241. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Prarr, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmochim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Gardner, S.D.; Toghiani, H.; Hill, D.O. XPS study of molybdenum sulfide catalyst exposed to CO and H2. J. Colloid Interface Sci. 2004, 270, 123–126. [Google Scholar] [CrossRef]
- Parada, F.; Jeffrey, M.I.; Asselin, E. Leaching kinetics of enargite in alkaline sodium sulphide solutions. Hydrometallurgy 2014, 146, 48–58. [Google Scholar] [CrossRef]
- Rivera-Vasquez, B.F.; Dixon, D. Rapid atmospheric leaching of enargite in acidic ferric sulfate media. Hydrometallurgy 2015, 152, 149–158. [Google Scholar] [CrossRef]
- Nicol, M.J.; Tjandrawan, V.; Zhang, S.C. Cathodic reduction of iron(III) and copper(II) on various sulfide minerals in chloride solutions. Hydrometallurgy 2016, 166, 113–122. [Google Scholar] [CrossRef]
- Yin, Q.; Vaughan, D.J.; England, K.E.R.; Kelsall, G.H. Electrochemical Oxidation of Covellite (CuS) in Alkaline Solution. J. Colloid Interface Sci. 1994, 166, 133–142. [Google Scholar] [CrossRef]
- Deng, R.D.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Yang, Z.G. Adsorption of Fe(III) on smithsonite surfaces and implications for flotation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 308–315. [Google Scholar] [CrossRef]




| Atoms | Fresh | NaClO Treatment | NaClO + FeCl3 Treatment | |||
|---|---|---|---|---|---|---|
| Covellite | Enargite | Covellite | Enargite | Covellite | Enargite | |
| O (1s) | 25.23 | 11.87 | 31.52 | 12.76 | 35.89 | 18.80 |
| S (2p3/2) | 52.46 | 42.18 | 41.98 | 42.64 | 35.48 | 40.21 |
| Cu(2p3/2) | 22.31 | 28.14 | 26.50 | 29.97 | 21.34 | 26.11 |
| As (3d) | 0 | 17.81 | 0 | 15.83 | 0 | 10.57 |
| Fe (2p3/2) | 0 | 0 | 0 | 0 | 7.29 | 3.81 |
| Products | Mass Recovery (%) | Cu Grade (%) | As Grade (%) | Cu Recovery (%) | As Recovery (%) |
|---|---|---|---|---|---|
| Concentrate A | 20.56 | 32.77 | 5.18 | 28.29 | 74.45 |
| Concentrate B | 79.44 | 21.50 | 0.46 | 71.71 | 25.55 |
| Feed | 100.00 | 23.82 | 1.43 | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Deng, R.; Liu, Q. Flotation Separation of Covellite and Enargite via Oxidation Treatment. Minerals 2022, 12, 970. https://doi.org/10.3390/min12080970
Gan Y, Deng R, Liu Q. Flotation Separation of Covellite and Enargite via Oxidation Treatment. Minerals. 2022; 12(8):970. https://doi.org/10.3390/min12080970
Chicago/Turabian StyleGan, Yonggang, Rongdong Deng, and Quanjun Liu. 2022. "Flotation Separation of Covellite and Enargite via Oxidation Treatment" Minerals 12, no. 8: 970. https://doi.org/10.3390/min12080970






