Genesis of the Graphite from the Tugeman Graphite Deposit, Xinjiang, China: Evidence for Carbon Isotope Refining by Fluids Associated with the Ductile Shear Zone
Abstract
:1. Introduction
2. Geological Setting
3. Geology of the Tugeman Graphite Deposit and Petrography Descriptions
4. Analytical Methods
4.1. Carbon Isotope Test
4.2. Raman Spectroscopy Test
5. Results
5.1. Carbon Isotope Compositions of Graphite
5.2. Graphite Raman Spectroscopy
6. Discussion
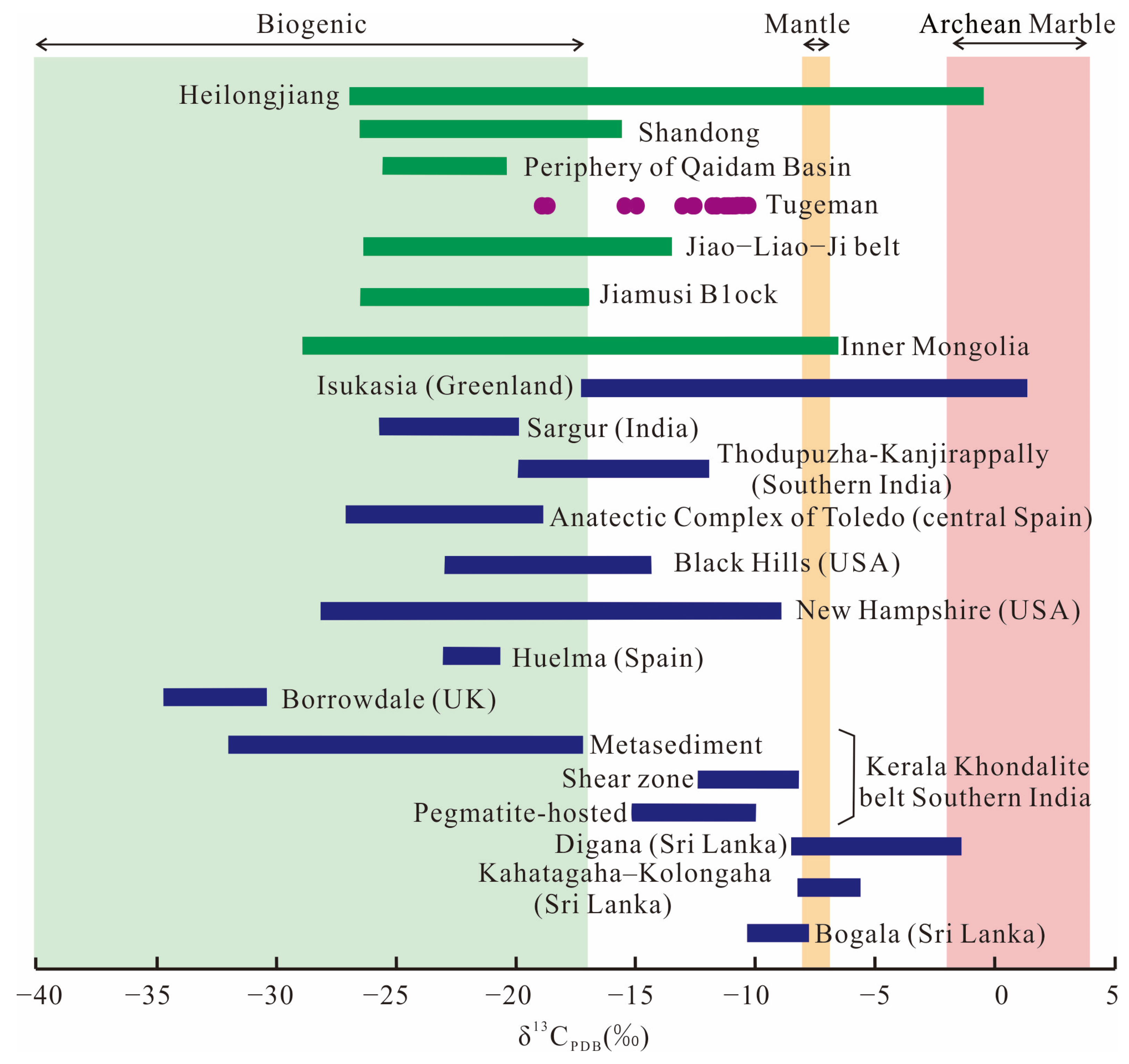
6.1. Carbon Source of the Tugeman Graphite Deposit
6.2. Metallogenic Process of the Tugeman Graphite Deposit
6.3. Degree of Metamorphism in Graphite
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.P.; Mishra, M.; Chandra, A.; Dhawan, S. Graphene oxide/ferrofluid/cement composites for electromagnetic interference shielding application. Nanotechnology 2011, 22, 465701. [Google Scholar] [CrossRef]
- Luque, F.J.; Huizenga, J.M.; Crespo-Feo, E.; Wada, H.; Ortega, L.; Barrenechea, J.F. Vein graphite deposits: Geological settings, origin, and economic significance. Miner. Depos. 2014, 49, 261–277. [Google Scholar] [CrossRef]
- Xiao, K.Y.; Sun, L.; Li, S.Y.; Huang, A. Geological characteristics and mineralization potential of graphite resource in China. Acta Geosci. Sin. 2016, 37, 607–614. [Google Scholar]
- Li, C.; Wang, D.H.; Zhao, H.; Pei, H.X.; Li, X.W.; Zhou, L.M.; Du, A.D.; Qu, W.J. Minerogenetic regularity of graphite deposits in China. Miner. Depos. 2015, 34, 1223–1236. [Google Scholar]
- Liu, J.D.; Xiao, R.G.; Zhang, Y.F.; Liang, S.; Zhao, Q.; Bai, F.J.; Zhang, Y.X.; Wang, J.C.; Yang, P.Q.; Liu, J. Phaneritic Graphite Deposits in the North China; Science Press: Beijing, China, 2017; pp. 1–453. (In Chinese) [Google Scholar]
- Bai, J.K.; Chen, J.L.; Peng, S.X. Characteristics and metallogeny regulation of graphite resources in Xinjiang. Acta Geol. Sin. 2017, 91, 2828–2840. (In Chinese) [Google Scholar]
- Walter, M.J.; Kohn, S.C.; Araujo, D.; Bulanova, G.P.; Smith, C.B.; Gaillou, E.; Wang, J.; Steele, A.; Shirey, S.B. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 2011, 334, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, Y.; Liu, L. Genesis of the Tianping flake graphite deposit at the western margin of Yangtze Block, SW China. Ore Geol. Rev. 2021, 139, 104434. [Google Scholar] [CrossRef]
- Schidlowski, M. Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: Evolution of a concept. Precambrian Res. 2001, 106, 117–134. [Google Scholar] [CrossRef]
- Ueno, Y.; Yurimoto, H.; Yoshioka, H.; Komiya, T.; Maruyama, S. Ion microprobe analysis of graphite from ca. 3.8 Ga metasediments, Isua supracrustal belt, West Greenland: Relationship between metamorphism and carbon isotopic composition. Geochim. Cosmochim. Acta 2002, 66, 1257–1268. [Google Scholar] [CrossRef]
- Van Zuilen, M.A.; Lepland, A.; Teranes, J.; Finarelli, J.; Wahlen, M.; Arrhenius, G. Graphite and carbonates in the 3.8 Ga old Isua Supracrustal Belt, southern West Greenland. Precambrian Res. 2003, 126, 331–348. [Google Scholar] [CrossRef]
- Papineau, D.; De Gregorio, B.T.; Stroud, R.M.; Steele, A.; Pecoits, E.; Konhauser, K.; Wang, J.; Fogel, M.L. Ancient graphite in the Eoarchean quartz-pyroxene rocks from Akilia in southern West Greenland II: Isotopic and chemical compositions and comparison with Paleoproterozoic banded iron formations. Geochim. Cosmochim. Acta 2010, 74, 5884–5905. [Google Scholar] [CrossRef]
- Lepland, A.; Zuilen, M.A.V.; Philippot, P. Fluid-deposited graphite and its geobiological implications in early Archean gneiss from Akilia, Greenland. Geobiology 2011, 9, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Luque, F.J.; Crespo-Feo, E.; Barrenechea, J.F.; Ortega, L. Carbon isotopes of graphite: Implications on fluid history. Geosci. Front 2012, 3, 197–207. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Santosh, M.; Wada, H. Graphite mineralization in Paleoproterozoic khondalites of the North China Craton: A carbon isotope study. Precambrian Res. 2014, 255, 641–652. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, X.D.; Li, H.K.; Zhai, M.G. Revisit and comparative analysis of the typical graphite deposits in the Paleoproterozoic khondalite series, western North China Craton: Implications for genesis, depositional environment and prospecting Potential. Ore Geol. Rev. 2019, 109, 370–380. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, L.F.; Tao, R.B.; Fei, Y.W. The formation of graphite-rich eclogite vein in S.W. Tianshan (China) and its implication for deep carbon cycling in subduction zone. Chem. Geol. 2020, 533, 119430. [Google Scholar] [CrossRef]
- Schidlowski, M. A 3,800-million-year isotopic record of life from carbon insedimentary rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Dunn, S.R.; Valley, J.W. Calcite–graphite isotope thermometry: A test for polymetamorphism in marble, Tudor gabbro aureole, Ontario, Canada. J. Metamorph. Geol. 1992, 10, 487–501. [Google Scholar] [CrossRef]
- Kitchen, N.E.; Valley, J.W. Carbon isotope thermometry in marbles of the Adirondack Mountains, New York. J. Metamorph. Geol. 1995, 13, 577–594. [Google Scholar] [CrossRef]
- Satish-Kumar, M.; Itoh, S.; Cesare, B. Carbon isotope anatomy of a single graphite crystal in a metapelitic migmatite revealed by high-spatial resolution SIMS analysis. Contrib. Mineral. Petrol. 2011, 162, 821–834. [Google Scholar] [CrossRef]
- Satish-Kumar, M.; Wada, H.; Santosh, M. Constraints on the application of carbon isotope thermometry in high- to ultrahigh-temperature metamorphic terranes. J. Metamorph. Geol. 2002, 20, 335–350. [Google Scholar] [CrossRef]
- Satish-Kumar, M.; Jaszczak, J.A.; Hamamatsu, T.; Wada, H. Relationship between structure, morphology and carbon isotopic composition of graphite in marbles: Implications for calcite-graphite carbon isotope thermometry. Am. Mineral. 2011, 96, 470–485. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 53–107. [Google Scholar]
- Weis, P.L.; Friedman, I.; Gleason, J.P. The origin of epigenetic graphite: Evidence from isotopes. Geochim. Cosmochim. Acta 1981, 45, 2325–2332. [Google Scholar] [CrossRef]
- Sharp, Z. Principles of Stable Isotope Geochemistry; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; pp. 1–344. [Google Scholar]
- Chen, Y.J.; Liu, C.Q.; Chen, H.Y.; Zhang, Z.J.; Li, C. Carbon isotope geochemistry of graphite deposits and ore-bearing khondalite series in North China: Implication for several geoscientific problems. Acta Petrol. Sin. 2000, 16, 233–244, (In Chinese with English abstract). [Google Scholar]
- Ai, J.; Lu, X.B.; Li, Z.W.; Wu, Y.L. Genesis of the graphite orbicules in the Huangyangshan graphite deposit, Xinjiang, China: Evidence from geochemical, isotopic and fluid inclusion data. Ore Geol. Rev. 2020, 122, 103505. [Google Scholar] [CrossRef]
- Sun, X.H.; Ren, Y.S.; Sun, J.S.; Wang, C.Y.; Li, Z.W. Geochronology and geochemical properties of the large-scale graphite mineralization associated with the Huangyangshan alkaline pluton, Eastern Junggar, Xinjiang, NW China. Geochemistry 2021, 81, 125820. [Google Scholar] [CrossRef]
- Hong, T.; Zhai, M.G.; Xu, X.W.; Li, H.; Wu, C.; Ma, Y.C.; Niu, L.; Ke, Q.; Wang, C. Tourmaline and quartz in the igneous and metamorphic rocks of the Tashisayi granitic batholith, Altyn Tagh, northwestern China: Geochemical variability constraints on metallogenesis. Lithos 2021, 400−401, 106358. [Google Scholar] [CrossRef]
- Cui, J.W.; Tang, Z.M.; Deng, J.F.; Yue, Y.J.; Meng, L.S.; Yu, Q.F.; Li, J.X.; Lai, S.C.; Qi, L.; Guo, G.C.; et al. Altun Fault System; Geological Publishing House: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Lu, S.N.; Yuan, G.B. Geochronology of early Precambrian magmatic activities in Aketashitage, east Altyn Tagh. Acta Geol. Sin. 2003, 77, 61–68. [Google Scholar]
- Xu, Z.Q.; Yang, J.S.; Zhang, J.X. A comparison between the tectonic units on the two sides of the Altun sinistral strike fault and the mechanism of lithospheric shearing. Acta Geol. Sin. 1999, 73, 193–205. (In Chinese) [Google Scholar]
- Sobel, E.R.; Arnaud, N. A possible middle Paleozoic suture in the Altyn Tagh, NW China. Tectonics 1999, 18, 64–74. [Google Scholar] [CrossRef]
- Ma, T.Q.; Wang, X.H.; Meng, D.B. Characteristic and geology meaning of alkaline calcium invade−rock zone at the SW fringe of Altun massif. Hunan Geol. 2002, 21, 12–16. [Google Scholar]
- Chen, X.H.; Yin, A.; Gehrels, G.E.; Cowgill, E.S.; Grove, M.; Harrison, T.M.; Wang, X.F. Two phases of Mesozoic north−south extension in the eastern Altyn Tagh range, northern Tibetan Plateau. Tectonics 2003, 22, 1053. [Google Scholar] [CrossRef]
- Li, H.B.; Yang, J.S.; Xu, Z.Q.; Sun, Z.M.; Tapponnier, P.; VanDer, W.J.; Meriaux, A.S. The constraint of the Altyn Tagh fault system to the growth and rise of the northern Tibetan plateau. Earth Sci. Front. 2006, 13, 59–79, (In Chinese with English abstract). [Google Scholar]
- Liu, Y.J.; Neubauer, F.; Genser, J.; Ge, X.H.; Takasu, A.; Yuan, S.H.; Chang, L.H.; Li, W.M. Geochronology of the initiation and displacement of the Altyn Strike−Slip Fault, western China. J. Asian Earth Sci. 2007, 29, 243–252. [Google Scholar] [CrossRef]
- Liu, L.; Che, Z.C.; Wang, Y.; Luo, J.H.; Chen, D.L. The metrological characters and geotectonic setting of high−pressure metamorphic rock belts in Altun Mountains. Acta Petrol. Sin. 1999, 15, 58–65. (In Chinese) [Google Scholar]
- Guo, Z.; Zhang, Z.; Jia, C.; Wei, G. Tectonics of Precambrian basement of the Tarim craton. Sci. China Ser. D Earth Sci. 2001, 44, 229–236. [Google Scholar] [CrossRef]
- Yang, J.S.; Shi, R.D.; Wu, C.L.; Su, D.C.; Chen, S.Y.; Wang, X.B.; Wooden, J.L. Petrology and SHRIMP age of the Hongliugou ophiolite at Milan, north Altun, at the margin of the Tibetan plateau. Acta Petrol. Sin. 2008, 24, 1567–1584. (In Chinese) [Google Scholar]
- Liu, L.; Cao, Y.T.; Chen, D.L.; Zhang, C.L.; Yang, W.Q.; Kang, L.; Liao, X.Y. New progresses on the HP−UHP metamorphism in the South Altyn Tagh and the North Qinling. Chin. Sci. Bull. 2013, 58, 2113–2123. [Google Scholar]
- Zhang, J.X.; Yu, S.Y.; Mattinson, C.G. Early Paleozoic polyphase metamorphism in northern Tibet, China. Gondwana Res. 2017, 41, 267–289. [Google Scholar] [CrossRef]
- Gehrels, G.E.; Yin, A.; Wang, X.F. Magmatic history of the northeastern Tibetan plateau. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Han, F.B.; Chen, B.L.; Cui, L.L. Zircon SHRIMP U−Pb age of intermediate−acid intrusive rocks in Kaladawan area, eastern Altun Mountains, NW China, and its implications. Acta Petrol. Sin. 2012, 28, 2277–2291. (In Chinese) [Google Scholar]
- Qi, X.X.; Li, H.B.; Wu, C.L. SHRIMP U−Pb zircon dating for Qiashikansayi granodiorite, the Northern Altyn Tagh Mountains and its geological implications. Chin. Sci. Bull. 2005, 50, 440–445. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.L.; Chen, B.L.; Wang, Y.; Meng, L.T.; He, J.T.; Han, M.M.; Wang, B. Geochronological and geochemical characteristics of the deformed diorite from the North Altyn brittle−ductile shear zone and its constraint on the Early Paleozoic tectonic evolution of the North Altyn Tagh. Acta Petrol. Sin. 2016, 32, 555–570. (In Chinese) [Google Scholar]
- Meng, L.T.; Chen, B.L.; Wang, Y. Timing of Early Paleozoic Tectonic Regime Transition in North Altun: Evidence from Granite. Geotecton. Metallog. 2016, 40, 295–307. (In Chinese) [Google Scholar]
- Liu, Y.S.; Yu, H.F.; Xin, H.T.; Lu, S.N.; Xiu, Q.Y.; Li, Q. Tectonic units division and Precambrian significant geological events in Altyn Tagh mountain, China. Geol. Bull. China 2009, 28, 1430–1438. (In Chinese) [Google Scholar]
- Liu, C.H.; Wu, C.L.; Gao, Y.H.; Lei, M.; Qin, H.P. Age, composition, and tectonic significance of Palaeozoic granites in the Altyn orogenic belt, China. Int. Geol. Rev. 2015, 58, 131–154. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xu, Z.Q.; Yang, J.S.; Zhang, Z.M.; Cui, J.W. Petrology, Geochemistry and Geochronology of Eclogites from the Western Segment of the Altum Tectonic Belt northwestern China. Acta Geol. Sin. 2001, 75, 186–197, (In Chinese with English abstract). [Google Scholar]
- Qin, X.F. The Proterozoic—Early Paleozoic Tectonic Framework and Geological Evolution of the Western Segment of Altyn Tagh Tectonic Belt; Guangzhou Institute of Geochemistry, Chinese Academy of Sciences: Guangdong, China, 2009. (In Chinese) [Google Scholar]
- Cao, Y.T.; Liu, L.; Wang, C.; Kang, L.; Li, D.; Yang, W.Q.; Zhu, X.H. Timing and nature of the partial melting processes during the exhumation of the garnet−bearing biotite gneiss in the southern Altyn Tagh HP/UHP belt, Western China. J. Asian Earth Sci. 2019, 170, 274–293. [Google Scholar] [CrossRef]
- Li, H.; Hong, T.; Yang, Z.Q.; Chen, J.Z.; Liu, X.Z.; Ke, Q.; Wang, X.H.; Niu, L.; Xu, X.W. Comparative studying on zircon, cassiterite and coltan U−Pb dating and 40Ar/39Ar dating of muscovite rare−metal granitic pegmatites—A case study of the Northern Tugeman lithium−beryllium deposit in the middle of Altyn Tagh. Acta Petrol. Sin. 2020, 36, 2869–2892. (In Chinese) [Google Scholar]
- No. 3 Geological Party, Xinjiang Bureau of Geology and Mineral Exploration and Development. Investigation and Evaluation Report of Pegmatite Rare Metal Deposit, Andalusite Deposit and Graphite Ore in Tugeman Area, Ruoqiang County, Xinjiang, China; Xinjiang Bureau of Geology and Mineral Exploration and Development: Xinjiang, China, 2019; pp. 1–210, (In Chinese, unpublished). [Google Scholar]
- Yan, M.Q.; Zhang, D.H.; Huizenga, J.M.; Wei, J.H.; Li, H.; Li, G.M.; Huang, X.K.; Zhang, X.M.; Zhao, S.Q. Mineralogical and isotopic characterization of graphite deposits in the western part of the North Qaidam Orogen and East Kunlun Orogen, northeast Tibetan Plateau, China. Ore Geol. Rev. 2020, 126, 103788. [Google Scholar] [CrossRef]
- Li, S.G.; Zhi, X.C.; Chen, J.F.; Wang, J.X.; Deng, Y.R. Origin of graphite in early precambrian banded iron formation in Anshan, China. Geochimica 1983, 2, 162–169. [Google Scholar] [CrossRef]
- Lu, L.Z.; Xu, X.C.; Liu, F.L. Early Precambrian Khondalite Series in North China; Changchun Publishing House: Changchun, China, 1996; p. 276. (In Chinese) [Google Scholar]
- Lan, X.Y. Characteristics of Precambrian graphite-bearing construction and the genesis of graphite deposits in Nanshu, Shandong. J. Chang. Inst. Geol. 1981, 3, 32–44. [Google Scholar]
- Perry, E.C., Jr.; Ahmad, S.N. Carbon isotope composition of graphite and carbonate minerals from 3.8-AE metamorphosed sediments, Isukasia, Greenland. Earth Planet Sci. Lett. 1977, 36, 280–284. [Google Scholar] [CrossRef]
- Maibam, B.; Sanyal, P.; Bhattacharya, S. Geochronological study of metasediments and carbon isotopes in associated graphites from the Sargur area, Dharwar craton: Constraints on the age and nature of the protoliths. J. Geol. Soc. India 2015, 85, 577–585. [Google Scholar] [CrossRef]
- Baiju, K.R.; Satish-Kumar, M.; Kagi, H.; Nambiar, C.G.; Ravisankar, M. Mineralogical Characterization of Graphite Deposits from Thodupuzha-Kanjirappally Belt, Madurai Granulite Block, Southern India. Gondwana Res. 2005, 8, 223–230. [Google Scholar] [CrossRef]
- Martín-Méndez, I.; Boixereu, E.; Villaseca, C. Mineralogical and isotopic characterization of graphite deposits from the Anatectic Complex of Toledo, central Spain. Miner. Depos. 2016, 51, 575–590. [Google Scholar] [CrossRef]
- Radhika, U.P.; Santosh, M. Shear-zone hosted graphite in southern Kerala, India: Implications for CO2 infiltration. J. Southeast Asian Earth Sci. 1996, 14, 265–273. [Google Scholar] [CrossRef]
- Santosh, M.; Wada, H. A carbon isotope study of graphites from the Kerala Khondalite Belt, southern India: Evidence for CO2 infiltration in granulites. J. Geol. 1993, 101, 643–651. [Google Scholar] [CrossRef]
- Santosh, M.; Wada, H. Microscale isotopic zonation in graphite crystals: Evidence for channeled CO2 influx in granulite. Earth Planet. Sci. Lett 1993, 119, 19–26. [Google Scholar] [CrossRef]
- Barrenechea, J.F.; Luque, F.J.; Millward, D.; Ortega, L.; Beyssac, O.; Rodas, M. Graphite morphologies from the Borrowdale deposit (NW England, UK): Raman and SIMS data. Contrib. Miner. Pet. 2009, 158, 37–51. [Google Scholar] [CrossRef]
- Luque, F.J.; Ortega, L.; Barrenechea, J.F.; Millward, D.; Beyssac, O.; Huizenga, J.M. Deposition of highly crystalline graphite from moderate-temperature fluids. Geology 2009, 37, 275–278. [Google Scholar] [CrossRef]
- Ortega, L.; Millward, D.; Luque, F.J.; Barrenechea, J.F.; Beyssac, O.; Huizenga, J.M.; Rodas, M.; Clarke, S.M. The graphite deposit at Borrowdale (UK): A catastrophic mineralizing event associated with Ordovician magmatism. Geochim. Cosmochim. Acta 2010, 74, 2429–2449. [Google Scholar] [CrossRef]
- Barrenechea, J.F.; Luque, F.J.; Rodas, M.; Pasteris, J.D. Vein-type graphite mineralization in the Jurassic volcanic rocks of the external zone of the Betic Cordillera (Southern Spain). Can. Minera 1997, 35, 1379–1390. [Google Scholar]
- Rumble, D.; Hoering, T.C. Carbon isotope geochemistry of graphite vein deposits from New Hampshire, USA. Geochim. Cosmochim. Acta 1986, 50, 1239–1247. [Google Scholar] [CrossRef]
- Rumble, D.; Duke, E.F.; Hoering, T.C. Hydrothermal graphite in New Hampshire: Evidence of carbon mobility during regional metamorphism. Geology 1986, 14, 452–455. [Google Scholar] [CrossRef]
- Nabelek, P.I.; Wilke, M.; Huff, T.A.; Wopenka, B. Methane, an important component of fluids in graphitic metapelites. Geochim. Cosmochim. Acta Suppl. 2003, 67, 317. [Google Scholar]
- Huff, T.A.; Nabelek, P.I. Production of carbonic fluids during metamorphism of graphitic pelites in a collisional orogendan assessment from fluid inclusions. Geochim. Cosmochim. Acta 2007, 71, 4997–5017. [Google Scholar] [CrossRef]
- Zhu, J.J.; Liu, F.L.; Wang, F.; Xu, W.T.; Liu, F.X.; Shi, C. Carbon isotope and geochemical characteristics of the Paleoproterozoic graphite deposits in the Jiao-Liao-Ji belt, North China Craton: Implications for genesis and depositional environment. Precambrian Res. 2021, 362, 106320. [Google Scholar] [CrossRef]
- Touzain, P.; Balasooriya, N.; Bandaranayake, K.; Descolas-Gros, C. Vein graphite from the Bogala and Kahatagaha-Kolongaha mines, Sri Lanka: A possible origin. Can. Miner. 2010, 48, 1373–1384. [Google Scholar] [CrossRef]
- Dobner, A.; Graf, W.; Hahn-Weinheimer, P.; Hirner, A. Stable carbon isotopes of graphite from Bogala Mine, Sri Lanka. Lithos 1978, 11, 251–255. [Google Scholar] [CrossRef]
- Binu-Lal, S.S.; Kehekpannala, W.; Satish-Kumar, M.; Wada, H. Multistage graphite precipitated through protracted fluid flow in sheared metagranitoid, Digana, Sri Lanka; Evidence from stable isotopes. Chem. Geol. 2003, 197, 253–270. [Google Scholar] [CrossRef]
- Schidlowski, M. Application of stable carbon isotopes to early biochemical evolution on earth. Annu. Rev. Earth Planet. Sci 1987, 15, 47–72. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finitesize crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Zhang, C.; Santosh, M. Coupled laser Raman spectroscopy and carbon stable isotopes of graphite from the khondalite belt of Kerala, southern India. Lithos 2019, 334–335, 245–253. [Google Scholar] [CrossRef]
- Parnell, J.; Brolly, C. Increased biomass and carbon burial 2 billion years ago triggered mountain building. Commun. Earth Environ. 2021, 2, 238. [Google Scholar] [CrossRef]
- Li, Y.; Satish-Kumar, M.; Kiran, S.; Wan, C.; Zheng, J.P. 2.0 Ga orogenic graphite deposits and associated 13C-enriched meta-carbonate rocks from south china craton: Implications for global lomagundi event. Geosci. Front. 2022, 13, 101409. [Google Scholar] [CrossRef]
- Touret, J.L.R.; Huizenga, J.M.; Kehelpannala, K.V.W.; Piccoli, F. Vein-type graphite deposits in Sri Lanka: The ultimate fate of granulite fluids. Chem. Geol. 2019, 508, 167–181. [Google Scholar] [CrossRef]
- Knauth, L.P.; Kennedy, M.J. The late Precambrian greening of the Earth. Nature 2009, 460, 728–732. [Google Scholar] [CrossRef]
- Santosh, M.; Wada, H.; Satish-Kumar, M.; Binu-Lal, S.S. Carbon isotope “stratigraphy” in a single graphite crystal: Implications for crystal growth mechanism of fluid deposited graphite. Amer. Miner. 2003, 88, 1689–1696. [Google Scholar] [CrossRef]
- Mo, R.J.; Liu, S.B.; Huang, C.R.; Zhang, G.R.; Tan, G.M.; Wang, B.X.; Xiao, X.Z. Geology of Graphite Deposits in China; China Architecture & Building Press: Beijing, China, 1989; pp. 65–80. (In Chinese) [Google Scholar]
- Gu, L.X.; Zheng, Y.C.; Tang, X.Q.; Fernando, D.P.; Wu, C.Z.; Tian, Z.M.; Lu, J.J.; Ni, P.; Li, X.; Yang, F.T.; et al. Copper, gold and silver enrichment in ore mylonites within massive sulphide orebodies at Hongtoushan, NE China. Ore Geol. Rev. 2007, 30, 1–29. [Google Scholar] [CrossRef]
- Bellot, J.P. Shear zone-hosted pollymetallic sulfides in the south Limousin area, massif central, France: Remobilized sulfide deposits related to Variscan collisional tectonics and amphibolite facies metamorphism. Econ. Geol. 2004, 99, 819–827. [Google Scholar] [CrossRef]
- Lamb, W.M.; Valley, J.W. C-O-H fluid calculations and granulite genesis. In The Deep Proterozoic Crust in the North Atlantic Provinces; Tobi, A.C., Touret, J.L.R., Eds.; NATO ASI Series; Reidel: Dordrecht, The Netherlands, 1985; Volume 158, pp. 119–131. [Google Scholar]
- Cesare, B. Graphite precipitation in C-O-H fluid inclusions: Closed system compositional and density changes and thermobarometric implications. Contrib. Miner. Petrol. 1995, 122, 25–33. [Google Scholar] [CrossRef]
- Jedwab, J.; Boulegue, J. Graphite crystals in hydrothermal vents. Nature 1984, 310, 41–43. [Google Scholar] [CrossRef]
- Strens, R.G.J. The graphite deposit of Seathwaite in Borrowdale, Cumberland. Geol. Mag. 1965, 102, 393–406. [Google Scholar] [CrossRef]
- Luque, F.J.; Pasteris, J.D.; Wopenka, B.; Rodas, M.; Barrenchea, J.F. Natural fluid-deposited graphite: Mineralogical characteristics and mechanisms of formation. Am. J. Sci. 1998, 298, 471–498. [Google Scholar] [CrossRef]
- Pasteris, J.D. Causes of the uniformly high crystallinity of graphite in large epigenetic deposits. J. Metamorph. Geol. 1999, 17, 779–787. [Google Scholar] [CrossRef]
- Klemd, R.; Brocker, M.; Schramm, J. Characterization of amphibolite-facies fluids of Variscan eclogites from the Orlica Snieznik dome (Sudetes, SW Poland). Chem. Geol. 1995, 119, 101–113. [Google Scholar] [CrossRef]
- Kelley, D.S. Methane-rich fluids in the oceanic crust. J. Geophys. Res. 1996, 101, 2943–2962. [Google Scholar] [CrossRef]
- Morgan, G.B.; Chou, I.M.; Pasteris, J.D.; Oslen, S.N. Re-equilibration of CO2 fluid inclusions at controlled hydrogen fugacities. J. Metamorph. Geol. 1993, 11, 155–164. [Google Scholar]
- Satish-Kumar, M. Graphite-bearing CO2-fluid inclusions in granulites: Insights on graphite precipitation and carbon isotope evolution. Geochim. Cosmochim. Acta 2005, 69, 3841–3856. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Buseck, P.R.; Beyssac, O. From organic matter to graphite: Graphitization. Elements 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Structural characterization of kerogens to granulitefacies graphite: Applicability of Raman microprobe spectroscopy. Am. Miner. 1993, 78, 533–557. [Google Scholar]
- Zhang, C.; Yu, X.Y.; Jiang, T.L. Mineral association and graphite inclusions in nephrite jade from Liaoning, northeast China: Implications for metamorphic conditions and ore genesis. Geosci. Front. 2019, 10, 425–437. [Google Scholar] [CrossRef]
- Kiran, S.; Satish-Kumar, M.; Nakamura, Y.; Hokada, T. Comparison between Raman spectra of carbonaceous material and carbon isotope thermometries in low-medium grade meta-carbonates: Implications for estimation of metamorphic temperature condition. Precambrian Res. 2022, 374, 106656. [Google Scholar] [CrossRef]
- Aoya, M.; Kouketsu, Y.; Endo, S.; Shimizu, H.; Mizukami, T.; Nakamura, D.; Wallis, S. Extending the applicability of the Raman carbonaceous-material geothermometer using data from contact metamorphic rocks. J. Metamorph. Geol 2010, 28, 895–914. [Google Scholar] [CrossRef]
- Stüwe, K. Geodynamics of the Lithosphere: An Introduction; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Wang, S.Q. Characteristics of ore-bearing formation and genesis of the Xinghe graphite deposit in Inner Mongolia. Miner. Depos. 1989, 8, 85–96, (In Chinese with English abstract). [Google Scholar]
- Parnell, J.; Brolly, C.; Boyce, A.J. Graphite from Palaeoproterozoic enhanced carbon burial, and its metallogenic legacy. Geol. Mag. 2021, 158, 1711–1718. [Google Scholar] [CrossRef]
- Taylor, W.R.; Berry, R.F. Origin of the Proterozoic graphite deposits of the southern Eyre Peninsula, South Australia. 10th Australian Geological Convention, February 4–9, 1990, University of Tasmania, Hobart, Abstracts 25. Geol. Soc. Aust. 1990, 230–231. [Google Scholar]
- Karhu, J.A. Paleoproterozoic evolution of the carbon isotope ratios of sedimentary carbonates in the Fennoscandian Shield. Geol. Surv. Finl. Bull. 1993, 371, 1–87. [Google Scholar]
- Rosing-Schow, N.; Bagas, L.; Kolb, J.; Balić-Žunić, T.; Korte, C.F. Hydrothermal flake graphite mineralisation in Paleoproterozoic rocks of south-east Greenland. Miner. Depos. 2017, 52, 769–789. [Google Scholar] [CrossRef]
- Ji, H.; Shimazaki, H.; Hu, S.; Zhao, Y. Occurrence and geochemistry of khondalite series in the Shandong Peninsula, China. Resour. Geol. 1994, 44, 39–49. [Google Scholar]
- Miranda, D.A.; Chaves, A.O.; Campello, M.S.; Ramos, S.L.L.M. Origin and thermometry of graphites from Itapecerica supracrustal succession of the southern Sao Francisco Craton by C isotopes, X-ray diffraction, and Raman spectroscopy. Int. Geol. Rev. 2019, 61, 1864–1875. [Google Scholar] [CrossRef]
- Mackenzie, D. Graphite-bearing ignimbrites and granites at Croydon, Queensland, and their relationship to gold mineralisation. BMR Res. Newsl. 1988, 8, 1–2. [Google Scholar]
- Buchanan, D.L.; Rouse, J.E. Role of contamination in the precipitation of sulphides in the Platreef of the Bushveld Complex. In Sulfide Deposits in Mafic and Ultramafic Rocks; Buchanan, D.L., Jones, M.J., Eds.; Special Publication of the Institution of Mining and Metallurgy: London, UK, 1984; pp. 141–146. [Google Scholar]
- Ripley, E.M.; Taib, N.I. Carbon isotopic studies of metasedimentary and igneous rocks at the Babbitt Cu-Ni deposit, Duluth Complex, Minnesota, U.S.A. Chem. Geol. 1989, 73, 319–342. [Google Scholar] [CrossRef]
- Kyser, T.K.; Wilson, M.R.; Ruhrmann, G. Stable isotope constraints on the role of graphite in the genesis of unconformity-type uranium deposits. Can. J. Earth Sci. 1989, 26, 490–498. [Google Scholar] [CrossRef]
- Satish-Kumar, M.; Wada, H. Carbon isotopic equilibrium between calcite and graphite in Skallen marbles, East Antarctica: Evidence for preservation of peak metamorphic temperatures. Chem. Geol. 2000, 166, 173–182. [Google Scholar] [CrossRef]





| Graphite Ore Body | Long (m) | Thickness (m) | Fixed Carbon Grade | Ore-Bearing Lithology | Occurrence | Types of Graphite | Other Features |
|---|---|---|---|---|---|---|---|
| C1 | 550 | 2.91 | 5.24%–6.35% | Schist and marble | 308°–325°∠75°–78° | Flake-crystalline graphite | Graphite distribution along foliation, accompanied by pyrite in graphite ore |
| C2 | 394 | 3.45 | 4.64%–7.46% | Schist and marble | 304°–325°∠75°–82° | Flake-crystalline graphite | Graphite distribution along foliation, accompanied by pyrite in graphite ore |
| C3 | 386 | 2.64 | 2.95%–3.96% | Schist and marble | 310°–318°∠70°–73° | Flake-crystalline graphite | Graphite distribution along foliation, accompanied by pyrite in graphite ore |
| C4 | 603 | 2.54 | 2.95%–5.59% | Schist and marble | 300°–315°∠71°–77° | Flake-crystalline graphite | Graphite distribution along foliation, accompanied by pyrite in graphite ore |
| Serial No. | Samples No. | Mineral | δ13C ‰ (VPDB) |
|---|---|---|---|
| Samples from the C3 ore body | |||
| 1 | G1 | Graphite | −11.05 |
| 2 | G2 | Graphite | −10.03 |
| 3 | G3 | Graphite | −10.60 |
| 4 | G4 | Graphite | −10.32 |
| 5 | G5 | Graphite | −10.71 |
| 6 | G6 | Graphite | −10.50 |
| 7 | G7 | Graphite | −10.86 |
| 8 | G8 | Graphite | −12.35 |
| 9 | G9 | Graphite | −10.22 |
| 10 | G10 | Graphite | −12.43 |
| 11 | G11 | Graphite | −10.94 |
| 12 | G12 | Graphite | −11.58 |
| Samples from the C1 ore body | |||
| 13 | G13 | Graphite | −18.66 |
| 14 | G14 | Graphite | −14.83 |
| 15 | G15 | Graphite | −15.35 |
| 16 | G16 | Graphite | −11.41 |
| 17 | G17 | Graphite | −18.90 |
| 18 | G18 | Graphite | −11.39 |
| 19 | G19 | Graphite | −12.87 |
| 20 | G20 | Graphite | −18.90 |
| Sample | D1-Bands/cm−1 | G-Band/cm−1 | D2-Bands/cm−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Position | Band Area | FWHM | Peak Value | Peak Position | Band Area | FWHM | Peak Value | Peak Position | Band Area | FWHM | Peak Value | |
| C4-1 | 1352 | 2150 | 52 | 39 | 1576 | 4301 | 27 | 148 | 1622 | 1175 | 71 | 16 |
| C4-2 | 1349 | 649 | 54 | 11 | 1579 | 1365 | 24 | 54 | 1612 | 230 | 34 | 6 |
| C4-3 | 1350 | 441 | 48 | 9 | 1580 | 759 | 25 | 29 | 1616 | 139 | 29 | 4 |
| C4-4 | 1350 | 841 | 47 | 17 | 1579 | 1441 | 24 | 56 | 1615 | 264 | 34 | 7 |
| C4-6 | 1346 | 2714 | 56 | 46 | 1571 | 12,985 | 29 | 417 | 1610 | 1243 | 30 | 39 |
| C3-2-4 | 1348 | 25,045 | 53 | 441 | 1578 | 43,385 | 23 | 1762 | 1612 | 7434 | 32 | 220 |
| C3-2-1 | 1349 | 2482 | 49 | 48 | 1579 | 4347 | 23 | 175 | 1612 | 741 | 29 | 24 |
| C3-3-1 | 1347 | 13,307 | 54 | 233 | 1577 | 24,681 | 25 | 933 | 1612 | 3699 | 29 | 118 |
| S2-band/cm−1 | ID/IG | D1/(G + D1 + D2) | S2/G | Peak Metamorphic Temperature/°C | ||||||||
| Peak Position | Band Area | FWHM | Peak Value | R1 Intensity Ratio | R2 Area Ratio | Area Ratio | Formula (1) | Formula (3) | ||||
| 2719 | 5051 | 75 | 63 | 0.26 | 0.28 | 1.17 | 516 | 527 | ||||
| 2705 | 1697 | 80 | 20 | 0.21 | 0.29 | 1.24 | 512 | 523 | ||||
| 2704 | 1336 | 78 | 16 | 0.30 | 0.33 | 1.76 | 494 | 503 | ||||
| 2702 | 2599 | 81 | 30 | 0.30 | 0.33 | 1.80 | 494 | 503 | ||||
| 2697 | 7209 | 67 | 102 | 0.11 | 0.16 | 0.56 | 570 | 590 | ||||
| 2701 | 57,821 | 82 | 663 | 0.25 | 0.33 | 1.33 | 494 | 503 | ||||
| 2704 | 6075 | 77 | 74 | 0.27 | 0.33 | 1.40 | 495 | 504 | ||||
| 2698 | 29,031 | 82 | 331 | 0.25 | 0.32 | 1.18 | 499 | 508 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hong, T.; Liu, S.; Ke, Q.; Yang, Z.; Ma, Y.; Wang, X.; Niu, L.; Kang, K.; Xu, X. Genesis of the Graphite from the Tugeman Graphite Deposit, Xinjiang, China: Evidence for Carbon Isotope Refining by Fluids Associated with the Ductile Shear Zone. Minerals 2023, 13, 1328. https://doi.org/10.3390/min13101328
Li H, Hong T, Liu S, Ke Q, Yang Z, Ma Y, Wang X, Niu L, Kang K, Xu X. Genesis of the Graphite from the Tugeman Graphite Deposit, Xinjiang, China: Evidence for Carbon Isotope Refining by Fluids Associated with the Ductile Shear Zone. Minerals. 2023; 13(10):1328. https://doi.org/10.3390/min13101328
Chicago/Turabian StyleLi, Hang, Tao Hong, Shanke Liu, Qiang Ke, Zhiquan Yang, Yince Ma, Xuehai Wang, Lei Niu, Kai Kang, and Xingwang Xu. 2023. "Genesis of the Graphite from the Tugeman Graphite Deposit, Xinjiang, China: Evidence for Carbon Isotope Refining by Fluids Associated with the Ductile Shear Zone" Minerals 13, no. 10: 1328. https://doi.org/10.3390/min13101328








