Effects of Short-Term Acidification on the Adsorption of Dissolved Organic Matter by Soil Minerals and Its Mechanism of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Mineral Acidification Culture
2.3. Isothermal Adsorption and Adsorption Kinetic Tests
2.3.1. Isothermal Adsorption Test
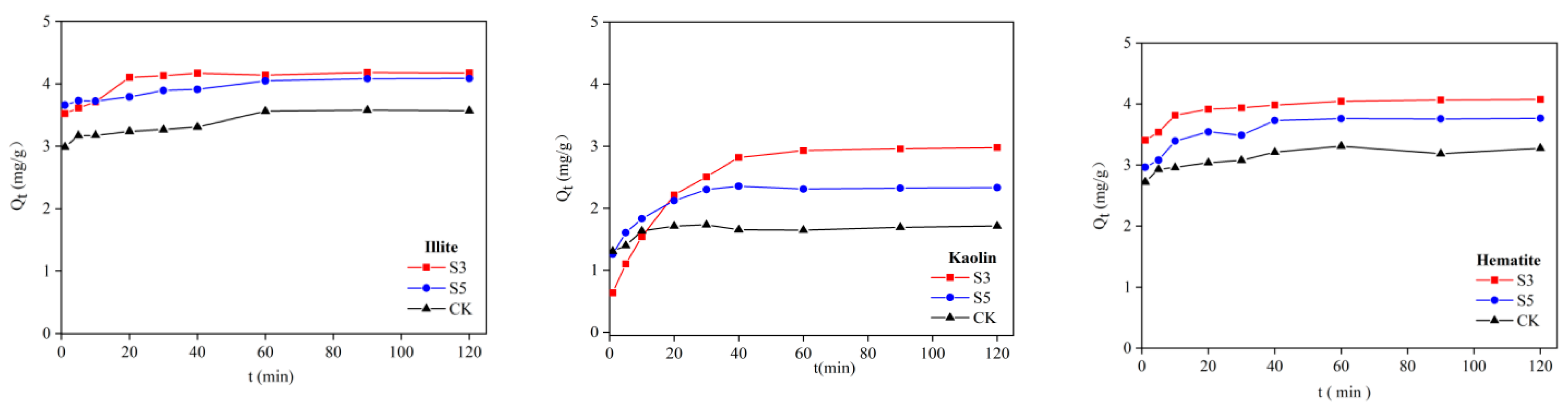
2.3.2. Adsorption Kinetic Test
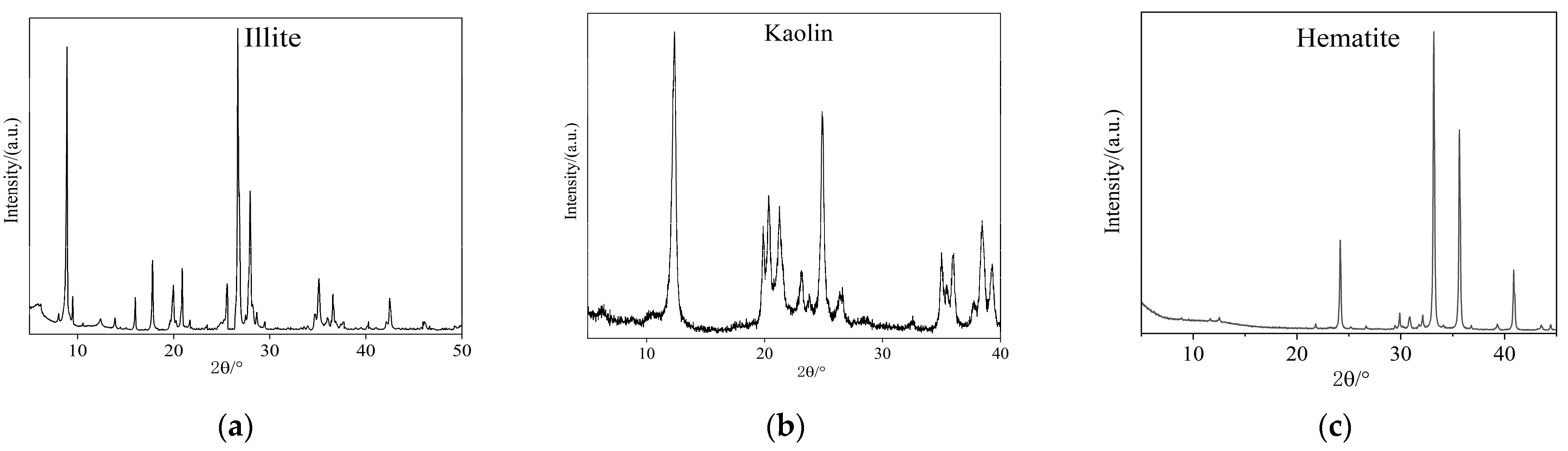
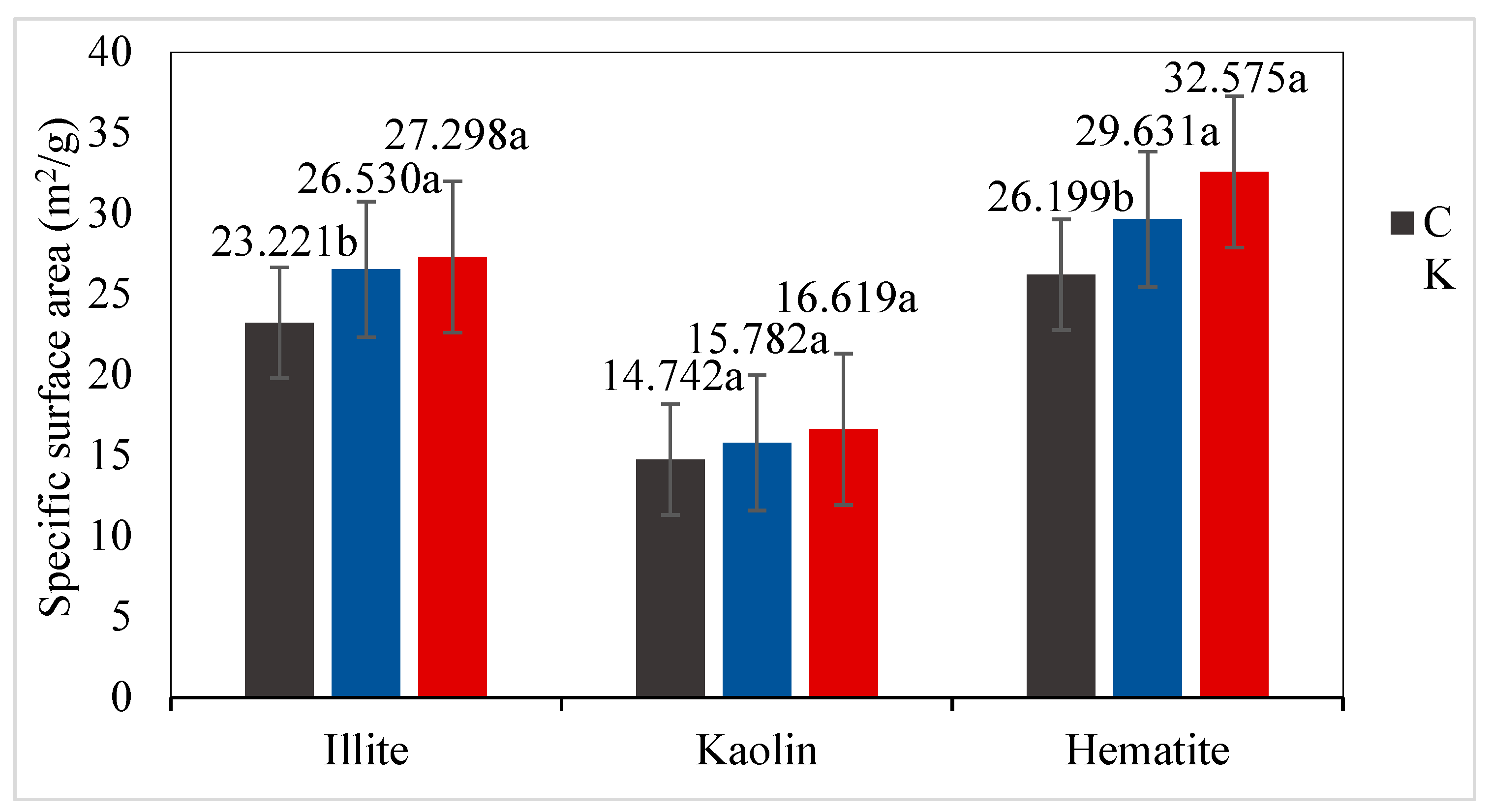
2.4. Analysis of Mineral Characterization
2.5. Data Processing
3. Results and Analysis
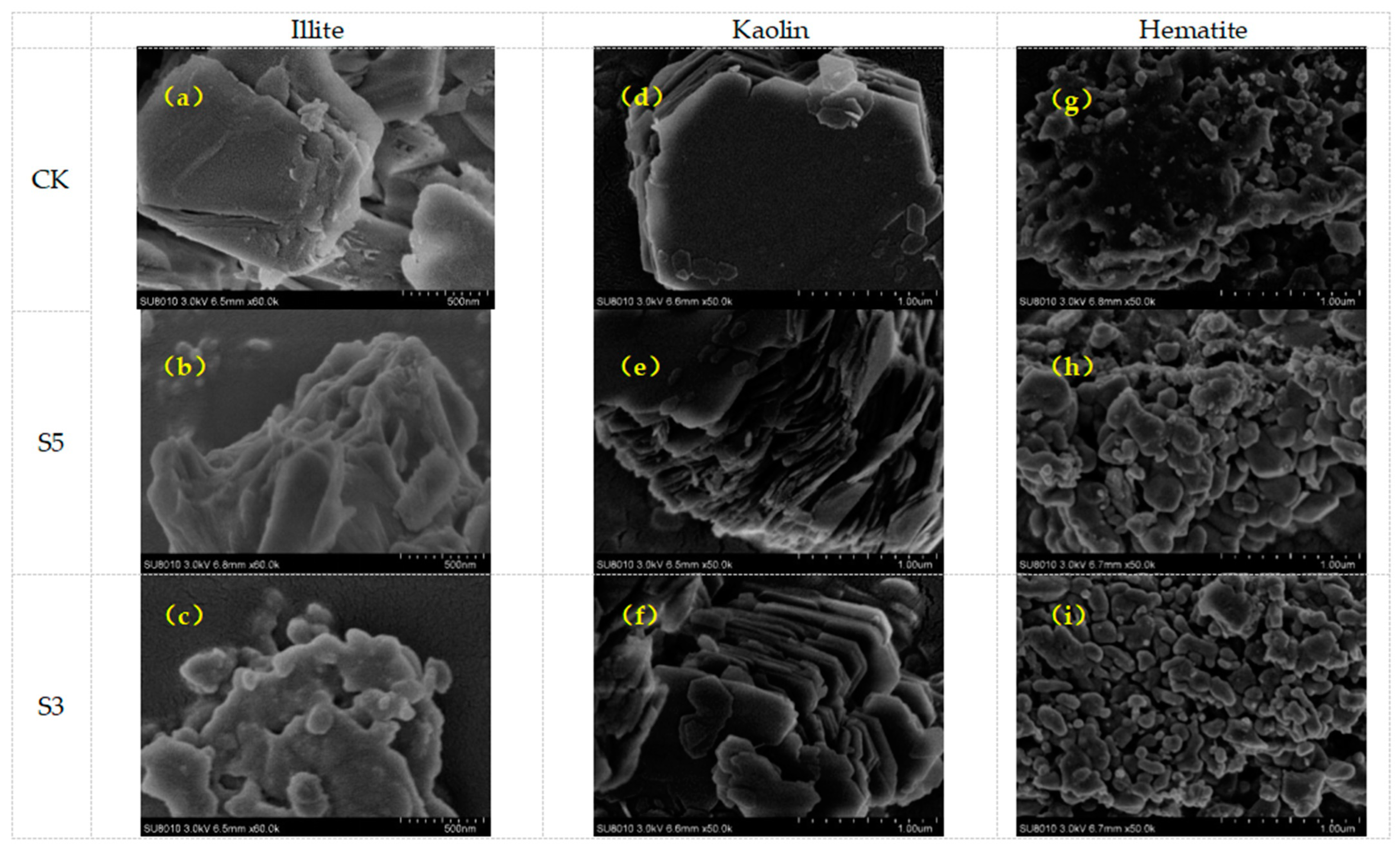
3.1. Microscopic Characterization of Soil Minerals
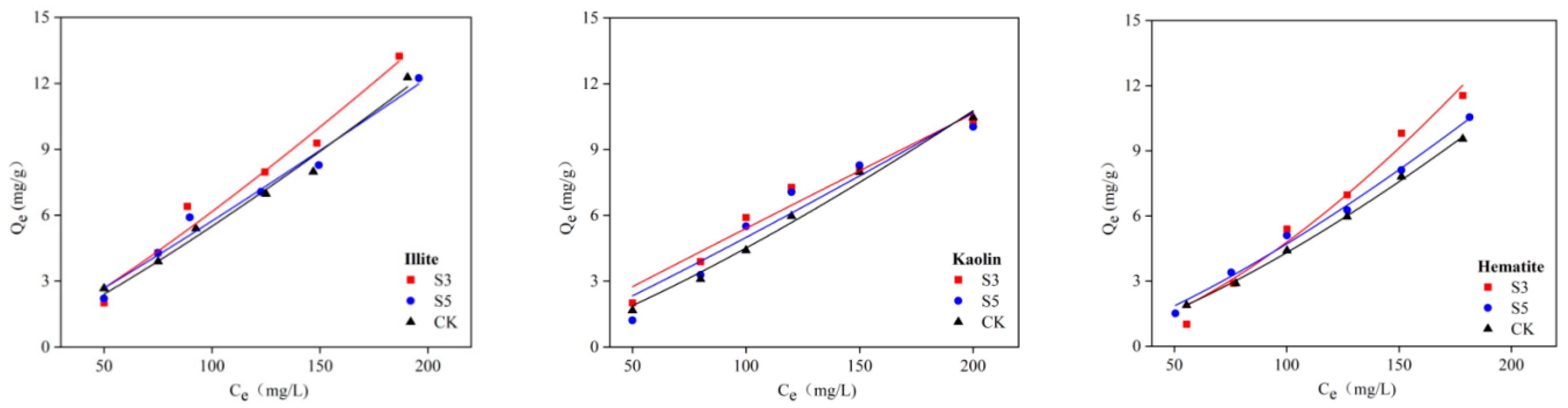
3.2. Effect of Short-Term Acidification on the Adsorption of Glucose by Soil Minerals
3.2.1. Isothermal Adsorption Characterization of Glucose Adsorption by soil Minerals
3.2.2. Kinetic Characterization of Glucose Adsorption by Soil Minerals
3.3. Effect of Short-Term Acidification on the Adsorption of Tannic Acid by Soil Minerals
3.3.1. Isothermal Adsorption Characterization of Tannic Acid Adsorption by Soil Minerals
3.3.2. Kinetic Characterization of Tannic Acid Adsorption by Soil Minerals
4. Discussion
4.1. Influence of Soil Minerals on Glucose and Tannic Acid Adsorption
4.2. Mechanism of the Effect of Acidification on the Adsorption of Glucose and Tannic Acid by Soil Minerals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikari, D.; Zhao, Q.; Das, K.; Mejia, J.; Huang, R.; Wang, X.; Poulson, S.R.; Tang, Y.; Roden, E.E.; Yang, Y. Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction. Geochim. Cosmochim. Acta 2017, 212, 221–233. [Google Scholar] [CrossRef]
- Williams, C.F.; Agassi, M.; Letey, J.; Farmer, W.J.; Nelson, S.D.; Ben-Hur, M. Facilitated transport of napropamide by dissolved organic matter through soil columns. Soil Sci. Soc. Am. J. 2000, 64, 590–594. [Google Scholar] [CrossRef]
- Hoyle, F.C.; Murphy, D.V.; Brookes, P.C. Microbial response to the addition of glucose in low-fertility soils. Biol. Fert. Soils 2008, 44, 571–579. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Schmidt, M.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kgel-Knabner, I.; Lehmann, J.; Manning, D. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Guggenberger, G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 2000, 31, 711–725. [Google Scholar] [CrossRef]
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J. The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide. Geoderma 2013, 209–210, 15–21. [Google Scholar] [CrossRef]
- Shaker, A.M.; Komy, Z.R.; Heggy, S.E.; Mohamed, E.S. Kinetic study for adsorption humic acid on soil minerals. J. Phys. Chem. A 2012, 116, 10889–10896. [Google Scholar] [CrossRef]
- Ulrich, B. An ecosystem approch to soil acidification. Soil Acidity 1991, 28–79. [Google Scholar]
- Xu, R.K.; Zhao, A.Z.; Jiang, J. Effect of acidification on CEC and mineral compositions of yellow brown soils in tea gardens. Ecol. Environ. Sci. 2011, 20, 1395–1398. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.M.; Liu, J.D. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef]
- Alekseeva, T.; Alekseev, A.; Xu, R.K.; Zhao, A.Z.; Kalinin, P. Effect of soil acidification induced by a tea plantation on chemical and mineralogical properties of Alfisols in eastern China. Environ. Geochem. Health 2011, 33, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ying, R.R.; Shi, J.Q.; Long, T.; Lin, Y.S. Advancement in study on adsorption of organic matter on soil minerals and its mechanism. Acta Pedol. Sin. 2017, 54, 805–818. [Google Scholar]
- Allen, S.J.; McKay, G.; Khader, K.Y.H. Intraparticle diffusion of a basic dye during adsorption onto sphagnum peat. Environ. Pollut. 1989, 56, 39–50. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Kleber, M.; Sollins, P.; Sutton, R.; Sollins, P.; Swanston, C.; Kramer, M. A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Mayes, M.A.; Heal, K.R.; Brandt, C.C.; Phillips, J.R.; Jardine, P.M. Relation between soil order and sorption of dissolved organic carbon in temperate subsoils. Soil Sci. Soc. Am. J. 2012, 76, 1027–1037. [Google Scholar] [CrossRef]
- Sollins, P.; Swanston, C.; Kleber, M.; Filley, T.; Kramer, M.; Crow, S.; Caldwell, B.A.; Lajtha, K.; Bowden, R. Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biol. Biochem. 2006, 38, 3313–3324. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lin, D.H.; Xing, B.S. Interactions of humic acid with nanosized inorganic oxides. Langmuir 2009, 25, 3571–3576. [Google Scholar] [CrossRef]
- Manash, D.R.; Sekh, M. The influence of functionality on the adsorption of p-hydroxy benzoate and phthalate at the hematite-electrolyte interface. J. Colloid Interf. Sci. 2007, 306, 205–215. [Google Scholar]
- Mietek, J.; Leonid, S. Improvement of the Kruk-Jaroniec-Sayari method for pore size analysis of ordered silicas with cylindrical mesopores. Langmuir 2006, 22, 6757–6760. [Google Scholar]
- Mortland, M.M. Formation and properties of clay-polymer complexes. Geoderma 1980, 23, 225–226. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhu, J.; Shi, C.; Shang, L.L.; Ma, R. Modification of kaolin and its adsorption properties on Cr(VI). Res. Environ. Sci. 2013, 26, 561–568. [Google Scholar]
- Yin, H.R.; Wu, L.H.; Chen, H.; Ma, L.; Qi, F.Y. Research and application of Nanokaolin. Mater. Rep. 2006, S1, 196–199. [Google Scholar]
- Min, F.F.; Zhao, Q.; Li, H.L.; Peng, C.L. Study of electrokinetic properties of kaolinite in coal slime. J. China Univ. Min. Technol. 2013, 42, 284–290. [Google Scholar] [CrossRef]
- Gu, B.H.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCathy, J.F. Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta 1995, 59, 219–229. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Wang, G.; Huang, G. Adsorption of humic acid from aqueous solution by hematite: Effects of pH and ionic strength. Environ. Earth Sci. 2015, 73, 4011–4017. [Google Scholar] [CrossRef]

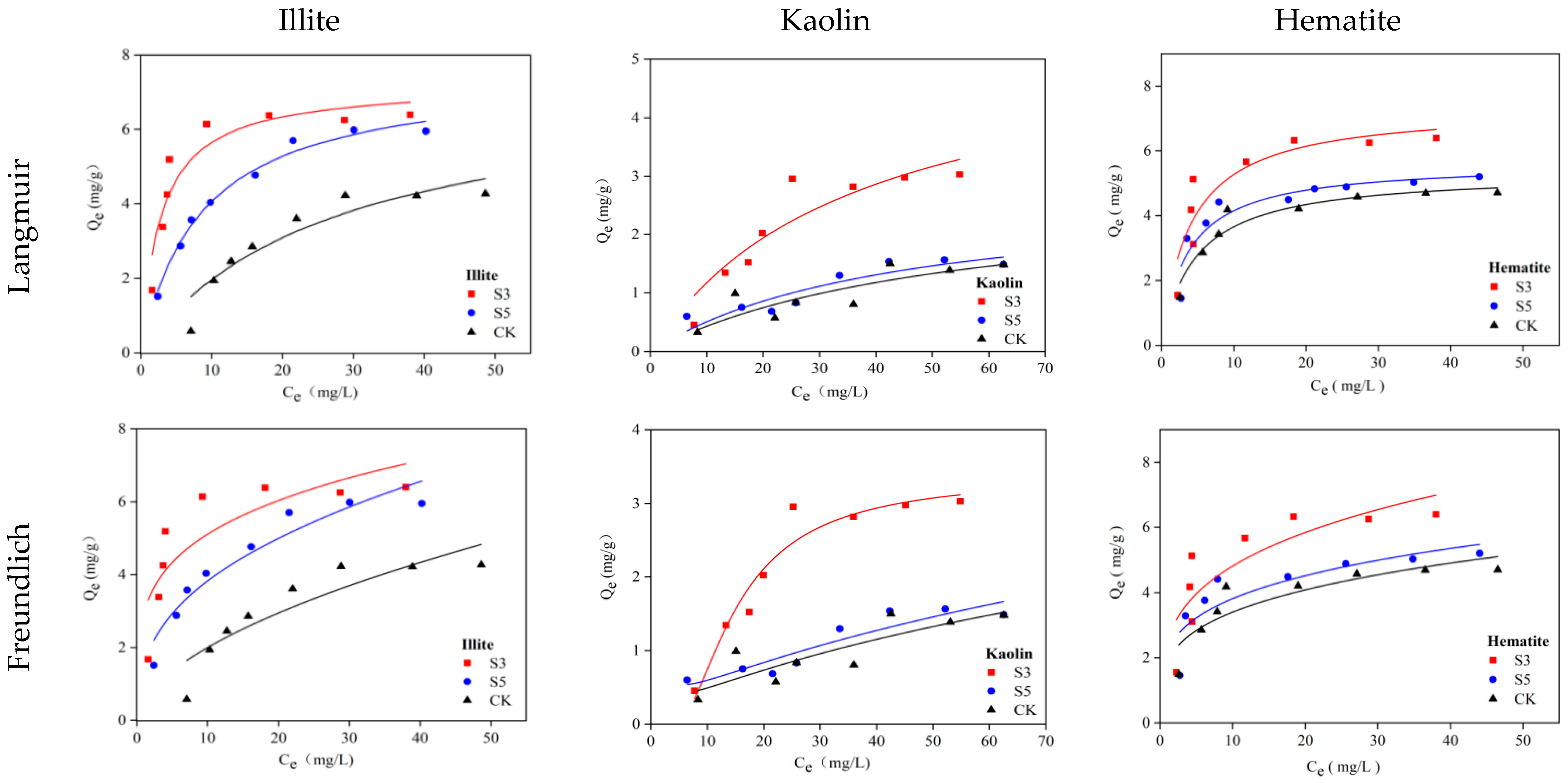
| Mineral | Treatment | Langmuir Fit | Freundlich Fit | ||||
|---|---|---|---|---|---|---|---|
| KL(L/mg) | Qm(mg/g) | R2 | KF [(mg/g)·(mg/L)n] | n | R2 | ||
| Illite | CK | 2.288 | 1.346 | 0.956 | 0.022 | 1.089 | 0.979 |
| S5 | 1.427 | 8.488 | 0.964 | 0.037 | 1.094 | 0.971 | |
| S3 | 6.051 | 3.992 | 0.942 | 0.024 | 1.196 | 0.969 | |
| Kaolin | CK | 2.374 | 1.184 | 0.945 | 0.013 | 1.055 | 0.986 |
| S5 | 1.251 | 6.450 | 0.933 | 0.031 | 1.098 | 0.977 | |
| S3 | 1.020 | 4.139 | 0.941 | 0.060 | 0.995 | 0.958 | |
| Hematite | CK | 1.593 | 7.823 | 0.923 | 0.007 | 1.390 | 0.998 |
| S5 | 2.903 | 1.540 | 0.932 | 0.009 | 1.343 | 0.991 | |
| S3 | 4.889 | 2.859 | 0.844 | 0.003 | 1.589 | 0.973 | |
| Mineral | Treatment | Qe Measured Value (mg/g) | Proposed Secondary Model | ||
|---|---|---|---|---|---|
| Qe Theoretical Value (mg/g) | k [g/(mg·h)] | R2 | |||
| Illite | CK | 11.640 | 12.289 | 0.089 | 0.997 |
| S5 | 12.945 | 13.517 | 0.097 | 0.994 | |
| S3 | 7.312 | 7.472 | 0.149 | 0.995 | |
| Kaolin | CK | 11.783 | 10.022 | 0.080 | 0.960 |
| S5 | 11.592 | 10.754 | 0.088 | 0.966 | |
| S3 | 11.759 | 10.828 | 0.065 | 0.931 | |
| Hematite | CK | 10.060 | 10.217 | 0.222 | 0.995 |
| S5 | 11.056 | 11.337 | 0.403 | 0.999 | |
| S3 | 12.394 | 13.326 | 0.393 | 0.997 | |
| Mineral | Treatment | Langmuir Fit | Freundlich Fit | ||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF[(mg/g)·(mg/L)n] | n | R2 | ||
| Illite | CK | 27.053 | 7.270 | 0.935 | 0.551 | 0.558 | 0.790 |
| S5 | 8.565 | 7.537 | 0.983 | 1.561 | 0.388 | 0.914 | |
| S3 | 2.780 | 7.519 | 0.920 | 2.947 | 0.239 | 0.662 | |
| Kaolin | CK | 49.839 | 2.650 | 0.454 | 0.146 | 0.561 | 0.896 |
| S5 | 43.630 | 2.734 | 0.794 | 0.016 | 0.558 | 0.984 | |
| S3 | 36.770 | 5.495 | 0.833 | 0.310 | 0.597 | 0.970 | |
| Hematite | CK | 4.612 | 5.336 | 0.916 | 1.860 | 0.262 | 0.760 |
| S5 | 4.198 | 5.768 | 0.859 | 1.898 | 0.284 | 0.730 | |
| S3 | 4.205 | 7.435 | 0.850 | 2.275 | 0.313 | 0.723 | |
| Mineral | Treatment | Qe Measured Value (mg/g) | Proposed Secondary Model | ||
|---|---|---|---|---|---|
| Qe Theoretical Value (mg/g) | k [g/(mg·h)] | R2 | |||
| Illite | CK | 3.571 | 3.509 | 0.265 | 0.995 |
| S5 | 4.086 | 3.896 | 0.959 | 0.999 | |
| S3 | 4.183 | 4.212 | 0.340 | 0.998 | |
| Kaolin | CK | 1.734 | 1.779 | 0.675 | 0.998 |
| S5 | 2.356 | 2.467 | 0.168 | 0.995 | |
| S3 | 2.819 | 2.778 | 0.036 | 0.964 | |
| Hematite | CK | 3.159 | 3.134 | 0.539 | 0.999 |
| S5 | 3.346 | 3.349 | 0.483 | 0.999 | |
| S3 | 3.306 | 3.347 | 0.210 | 0.998 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, Y.; Wang, X.; Luan, Y.; Dai, W. Effects of Short-Term Acidification on the Adsorption of Dissolved Organic Matter by Soil Minerals and Its Mechanism of Action. Minerals 2023, 13, 1448. https://doi.org/10.3390/min13111448
Chen Y, Wang Y, Wang X, Luan Y, Dai W. Effects of Short-Term Acidification on the Adsorption of Dissolved Organic Matter by Soil Minerals and Its Mechanism of Action. Minerals. 2023; 13(11):1448. https://doi.org/10.3390/min13111448
Chicago/Turabian StyleChen, Yueting, Yue Wang, Xuqin Wang, Yaning Luan, and Wei Dai. 2023. "Effects of Short-Term Acidification on the Adsorption of Dissolved Organic Matter by Soil Minerals and Its Mechanism of Action" Minerals 13, no. 11: 1448. https://doi.org/10.3390/min13111448





