A Novel Chalcopyrite Depressant for Selective Separation of Molybdenite from Cu-Mo Sulfide Ores and Its Interaction Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flotation Tests
2.3. Contact Angle Measurements
2.4. Zeta-Potential Measurements
2.5. FTIR Spectral Measurements
2.6. XPS Measurements
3. Results and Discussion
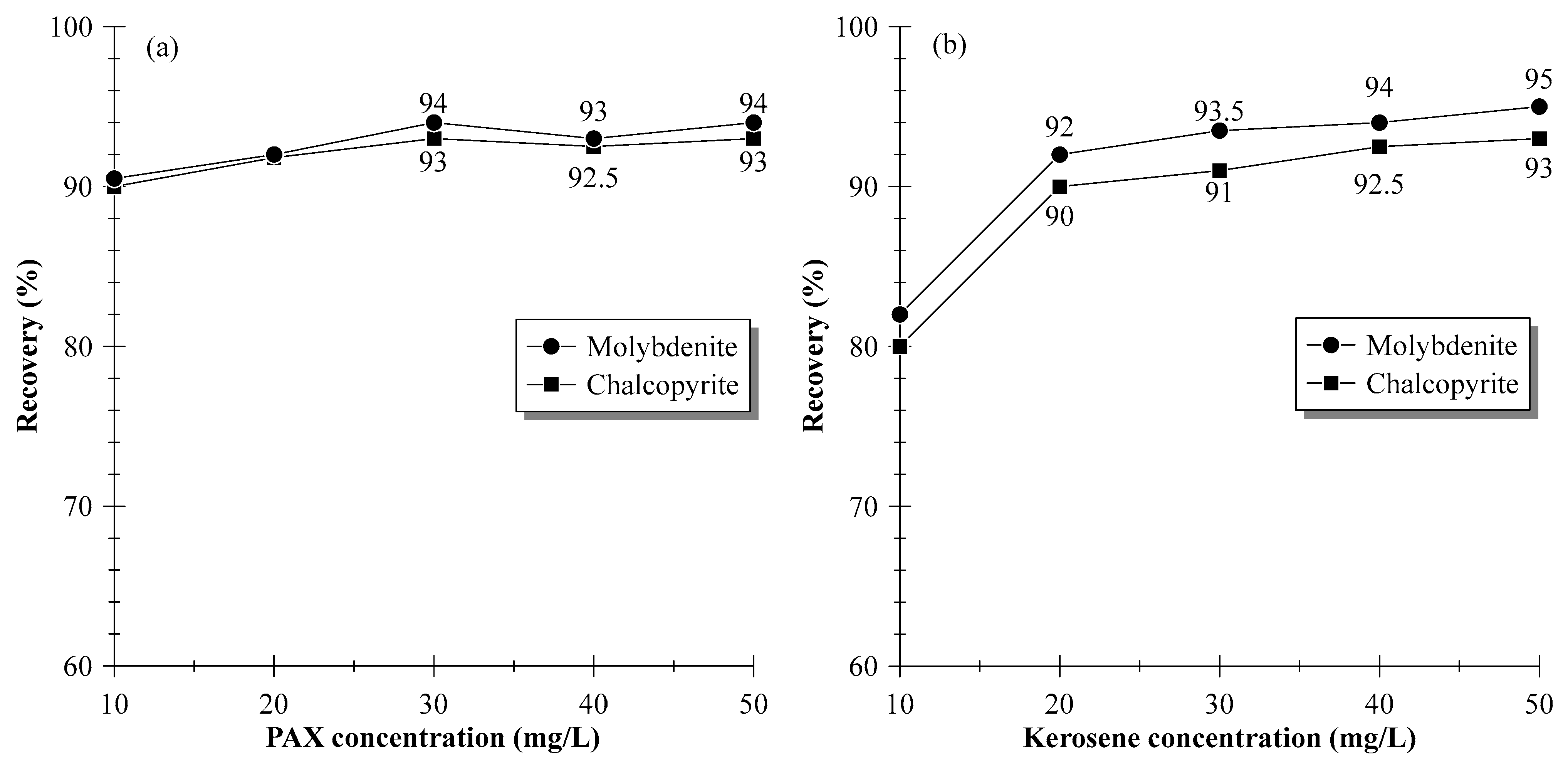
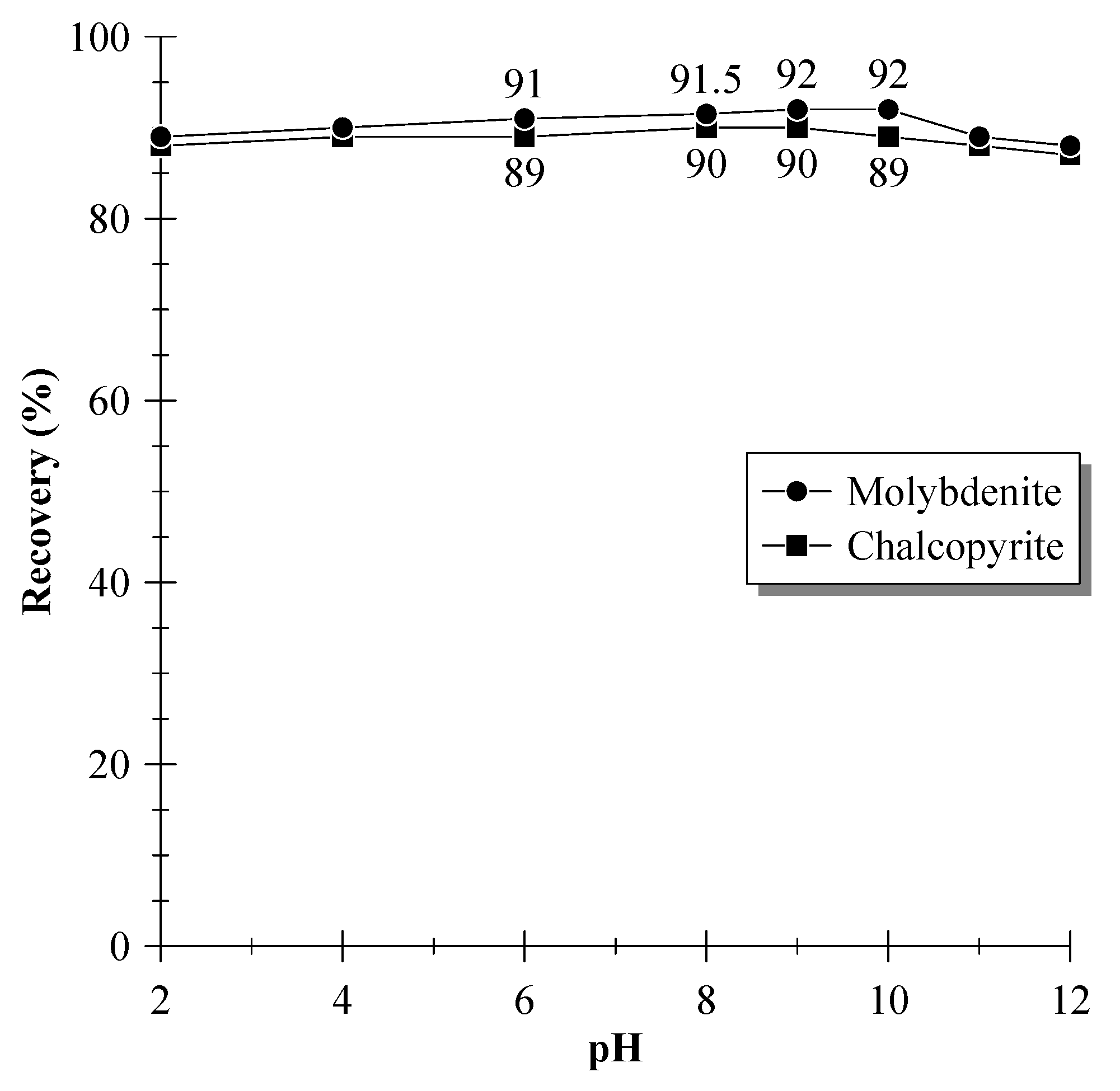
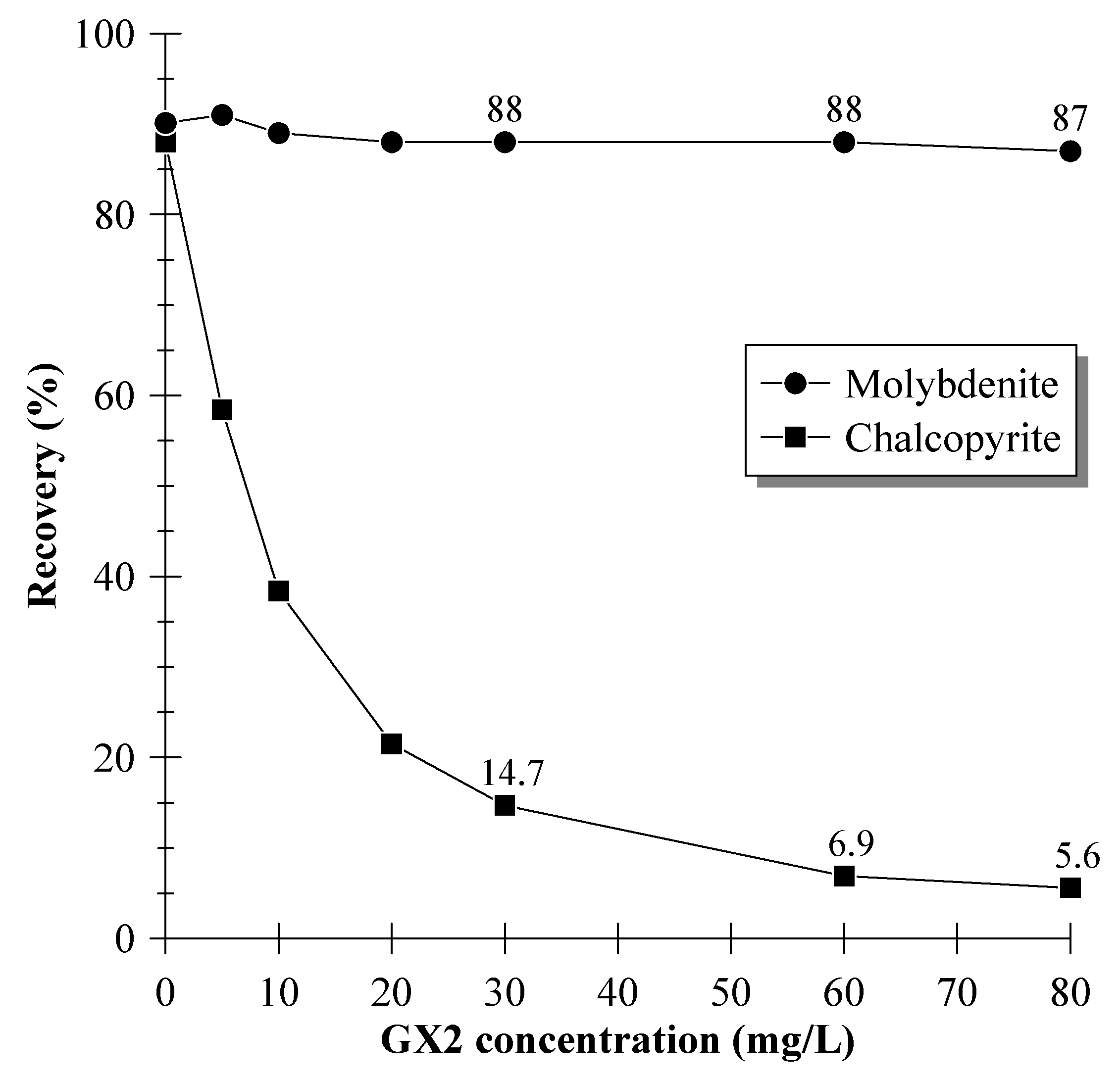
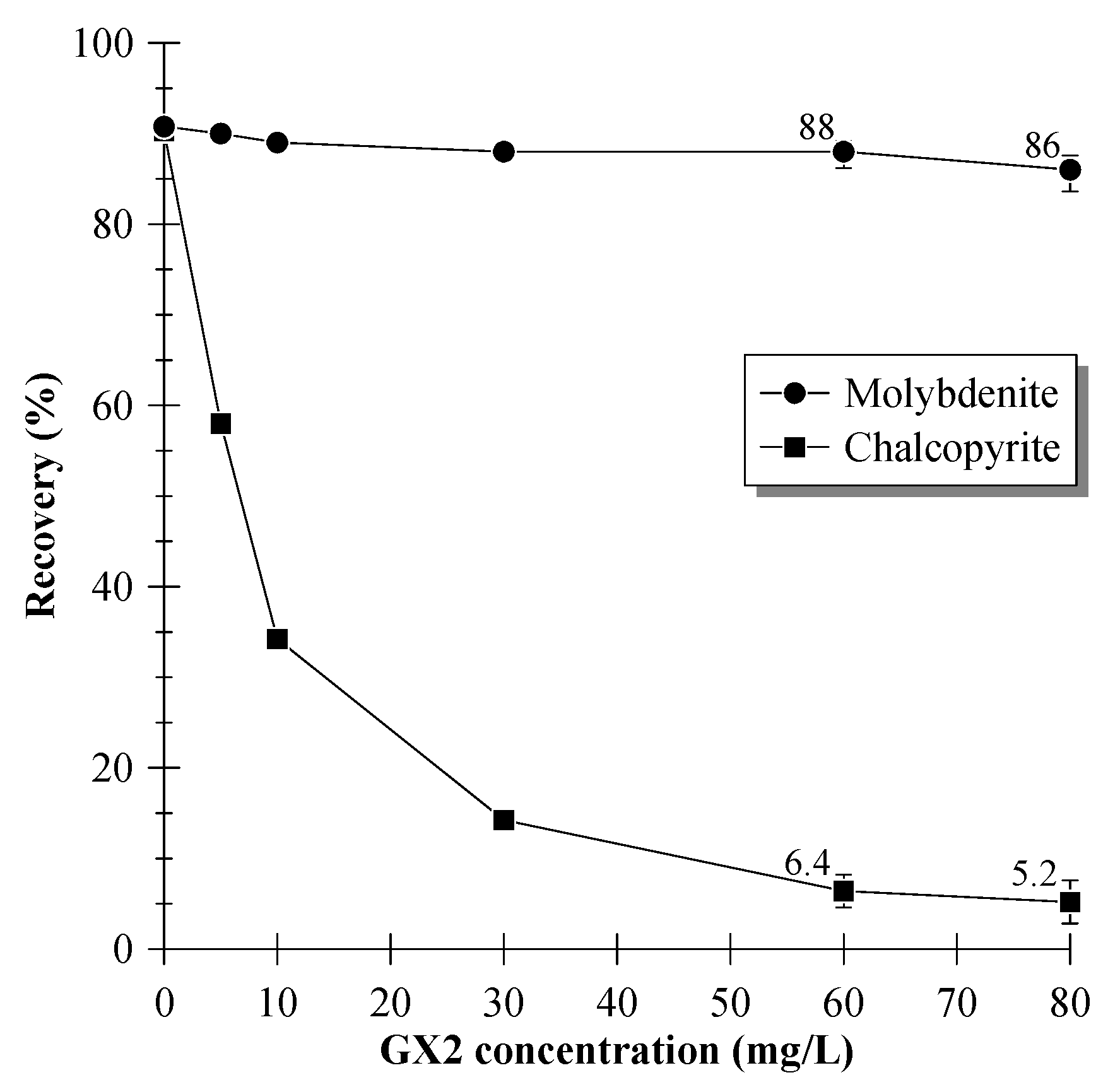
3.1. Single-Mineral Flotation
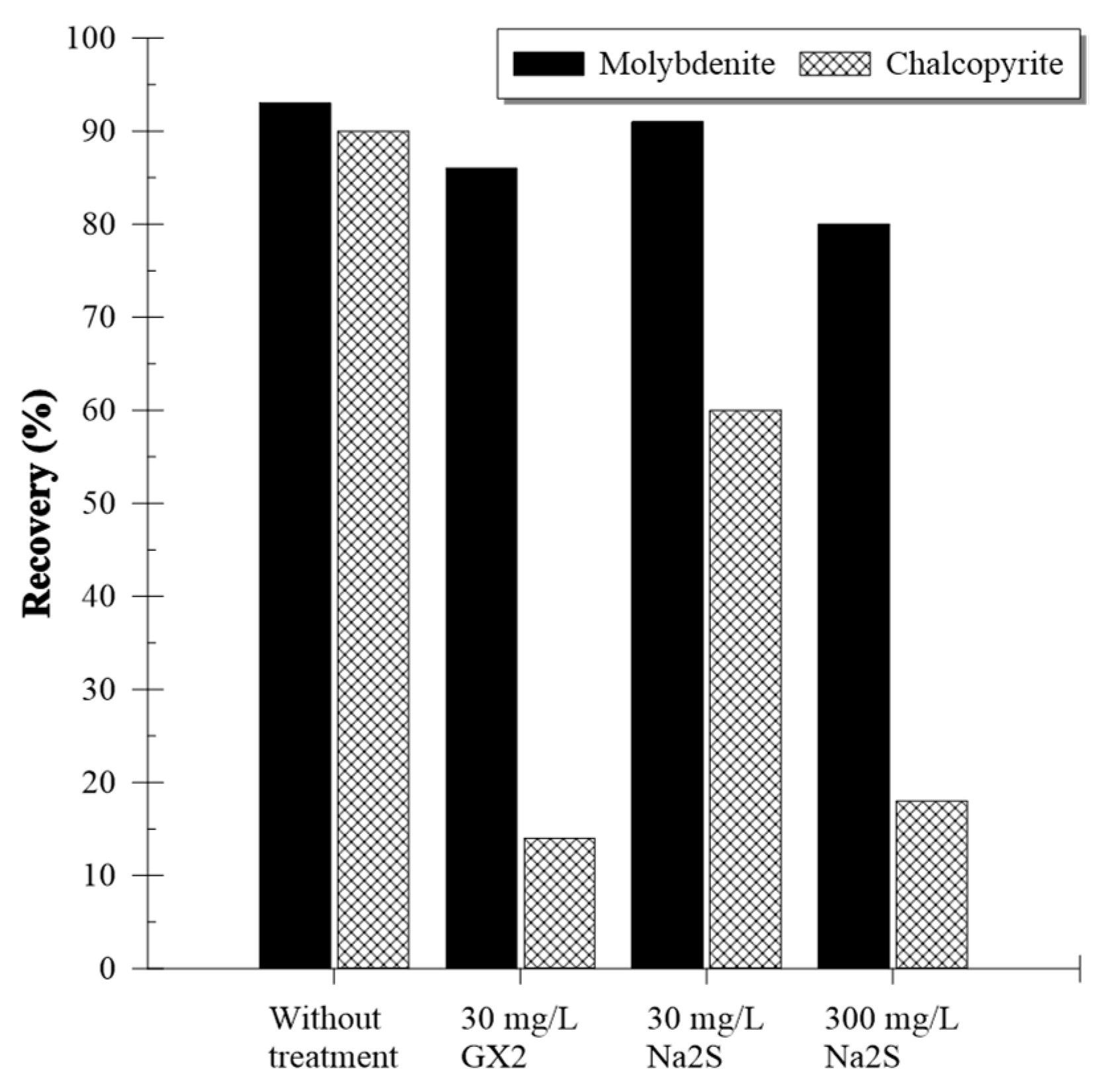
3.2. Artificially Mixed Mineral Flotation
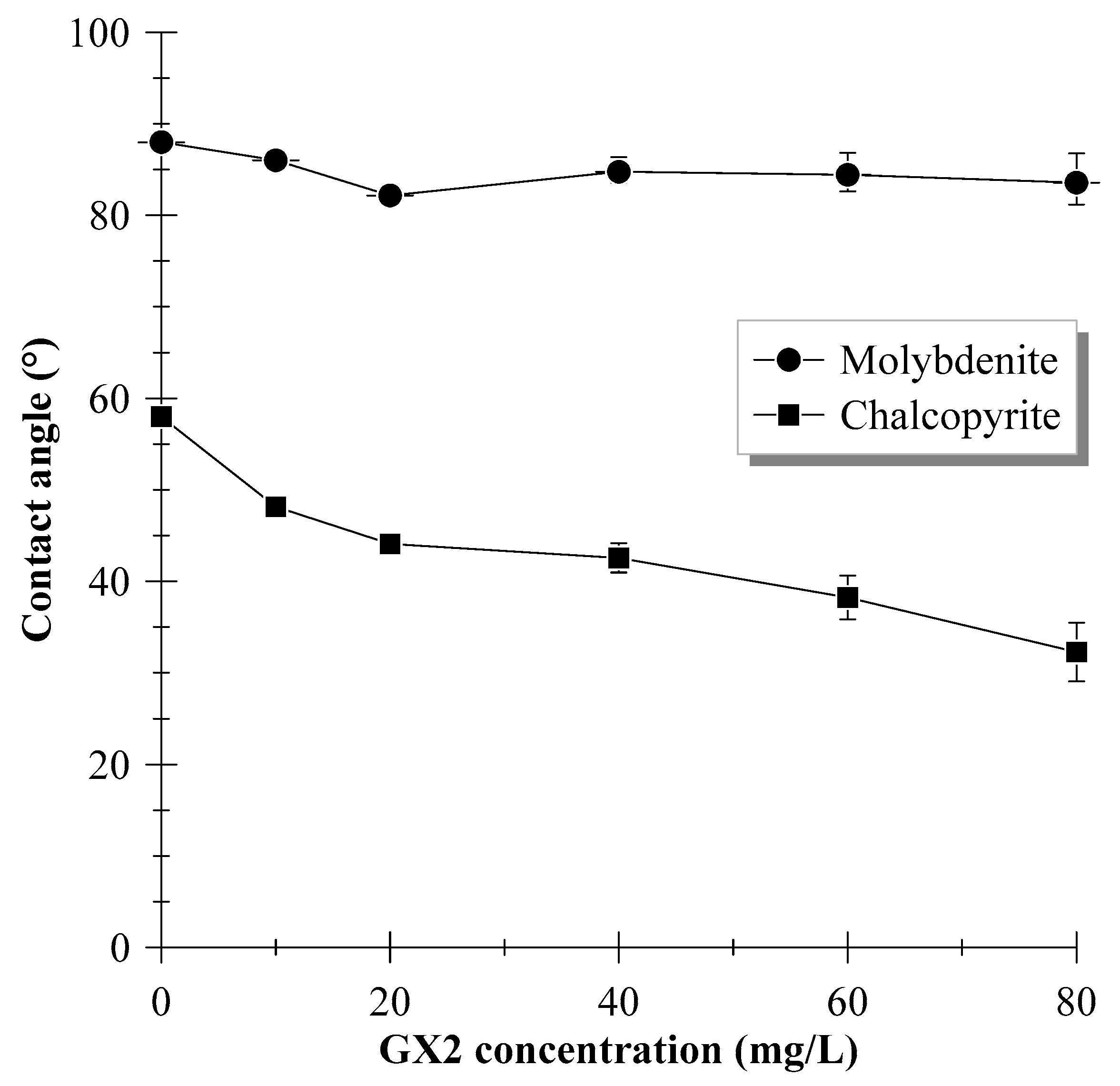
3.3. Contact Angle Measurements
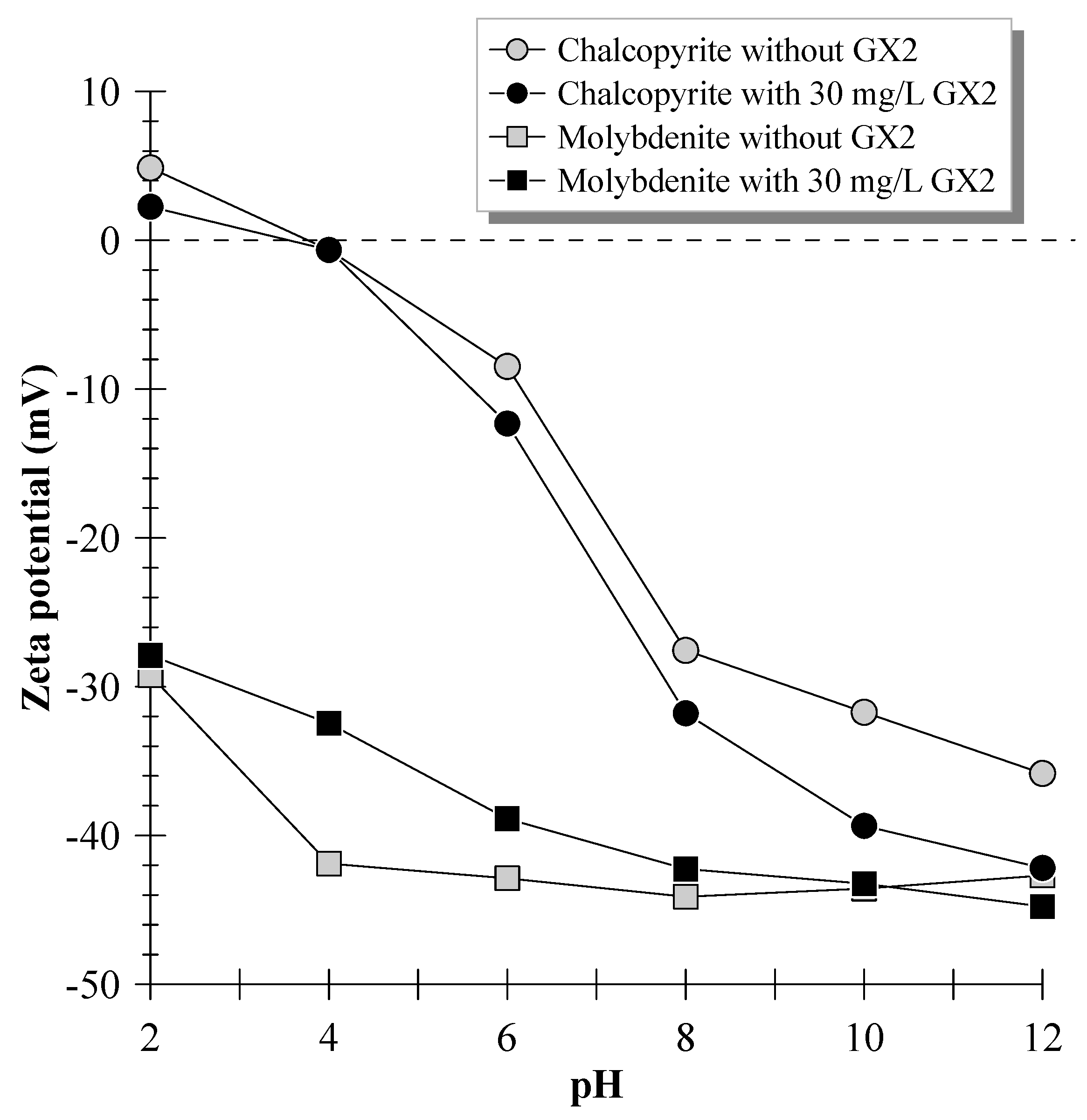
3.4. Zeta-Potential Measurements
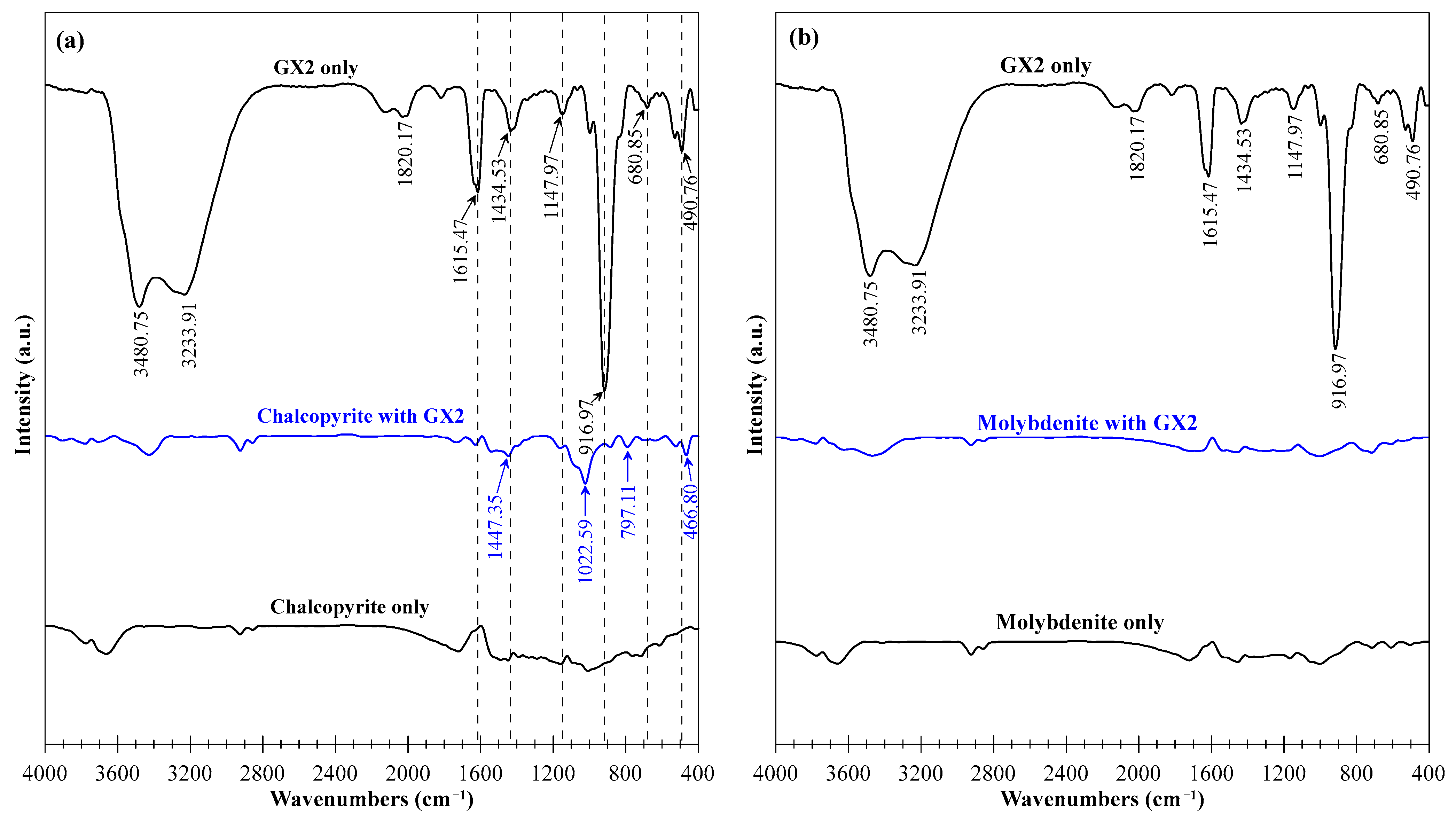
3.5. FTIR Measurements
3.6. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Effect of Fenton-like oxidation reagent on hydrophobicity and floatability of chalcopyrite and molybdenite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 34–48. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Cao, Y.; Yang, J.; Che, W.; Liu, J. The flotation separation of molybdenite from chalcopyrite using a polymer depressant and insights to its adsorption mechanism. Chem. Eng. J. 2020, 395, 125137. [Google Scholar] [CrossRef]
- Yang, B.; Yan, H.; Zeng, M.; Huang, P.; Jia, F.; Teng, A. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151, 106309. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. [Google Scholar] [CrossRef]
- Xu, H.; Ye, T.; Zhang, X.; Lu, L.; Xiong, W.; Zhu, Y. Insights into the adsorption mechanism of N-thiourea-maleamic acid on chalcopyrite surface in the flotation separation of Cu-Mo sulfide ores. J. Mol. Liq. 2022, 350, 118554. [Google Scholar] [CrossRef]
- Wu, D.; Peng, H.; Abdalla, M. Effect of sodium sulfide waste water recycling on the separation of chalcopyrite and molybdenite. Physicochem. Probl. Miner. Process. 2017, 54, 629–638. [Google Scholar] [CrossRef]
- Mweene, L.; Khanal, G.P.; Kawala, J.; Subramanian, S. Investigations into the flotation of molybdenite in the presence of chalcopyrite using (3S,4S,5S,6R)-3,4,5,6-tetrahydroxyoxane-2-carboxylate acid as a novel selective depressant: An experimental and theoretical perspective. J. Mol. Liq. 2022, 368, 120661. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P.; Tian, M. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Liu, R.; Jiang, W.; Zhang, C.; Guan, Q.; Zhang, C. Synthesis of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium and its application on the flotation separation of molybdenite from galena. J. Ind. Eng. Chem. 2017, 52, 82–88. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jin, X.; Feng, Z.T.; Zheng, R.J.; Cao, J.; Chen, J.; Sun, W.; Xu, S.H.; Gao, Z.Y. Collectorless flotation separation of molybdenite from complex sulfide minerals employing a bi-carbonyl depressant. Sep. Purif. Technol. 2023, 322, 124207. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Liu, J.; Zhu, Y.; Han, Y. Dithiouracil, a highly efficient depressant for the selective separation of molybdenite from chalcopyrite by flotation: Applications and mechanism. Miner. Eng. 2022, 175, 107287. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wei, D.-Z.; Shen, Y.-B.; Liu, W.-G.; Gao, S.-L.; Liang, G.-Q. Selective depression effect in flotation separation of copper–molybdenum sulfides using 2,3-disulfanylbutanedioic acid. Trans. Nonferrous Met. Soc. China 2015, 25, 3126–3132. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Guan, J. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Yin, Z.-G.; Sun, W.; Hu, Y.-H.; Guan, Q.-J.; Zhang, C.-H.; Gao, Y.-S.; Zhai, J.-H. Depressing behaviors and mechanism of disodium bis (carboxymethyl) trithiocarbonate on separation of chalcopyrite and molybdenite. Trans. Nonferrous Met. Soc. China 2017, 27, 883–890. [Google Scholar] [CrossRef]
- Yin, Z.G.; Hu, Y.H.; Sun, W.; Zhang, C.Y.; He, J.Y.; Xu, Z.J.; Zou, J.X.; Guan, C.P.; Zhang, C.H.; Guan, Q.J.; et al. Adsorption Mechanism of 4-Amino-5-mercapto-1,2,4-triazole as Flotation Reagent on Chalcopyrite. Langmuir 2018, 34, 4071–4083. [Google Scholar] [CrossRef]
- Guan, C.; Yin, Z.; Ahmed Khoso, S.; Sun, W.; Hu, Y. Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation. Minerals 2018, 8, 142. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lan, L.-H.; Liao, X.-J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper–molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Fu, J.; Li, W.; Hu, C. New insights into the beneficial roles of dispersants in reducing negative influence of Mg2+ on molybdenite flotation. RSC Adv. 2020, 10, 27401–27406. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Escudey, M.; Vyhmeister, E.; Higueras, P.; Godoy-Faúndez, A.; Salazar, J.L.; Valdés-González, H.; Wolf-Sepúlveda, G.; Herrera-Urbina, R. Adsorption of biosolids and their main components on chalcopyrite, molybdenite and pyrite: Zeta potential and FTIR spectroscopy studies. Miner. Eng. 2015, 78, 128–135. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, S.; Xu, Z.; Zhang, C.; He, J.; Zou, J.; Chen, D.; Sun, W. Flotation separation of molybdenite from chalcopyrite using an environmentally-efficient depressant L-cysteine and its adsoption mechanism. Miner. Eng. 2020, 156, 106438. [Google Scholar] [CrossRef]
- Yan, H.; Yang, B.; Zeng, M.; Huang, P.; Teng, A. Selective flotation of Cu-Mo sulfides using xanthan gum as a novel depressant. Miner. Eng. 2020, 156, 106486. [Google Scholar] [CrossRef]
- Chen, A.L.; Zhao, Z.W.; Chen, X.Y.; Liu, X.H.; Cao, C.F. Decoppering capability of nickel thiocarbonate in nickel electrolyte. Hydrometallurgy 2014, 144, 23–26. [Google Scholar] [CrossRef]
- Burke, J.M.; Fackler, J.P., Jr. Vibrational spectra of the thiocarbonate complexes of nickel (II), palladium (II), and platinum (II). Inorg. Chem. 1972, 11, 2744–2749. [Google Scholar] [CrossRef]
- Sahibed-Dine, A.; Aboulayt, A.; Bensitel, M.; Mohammed-Saad, A.B.; Daturi, M.; Lavalley, J.C. IR study of CS2 adsorption on metal oxides: Relation with their surface oxygen basicity and mobility. J. Mol. Catal. A Chem. 2000, 162, 125–134. [Google Scholar] [CrossRef]
- Kardouche, N.G.; Owen, L.N. Dithiols. Part XXVII. Conversion of aliphatic and alicyclic carbonates and thiocarbonates into trithiocarbonates. J. Chem. Soc. Perkin Trans. 1975, 1, 754–761. [Google Scholar] [CrossRef]
- Chimonyo, W.; Wiese, J.; Corin, K.; O’Connor, C. The use of oxidising agents for control of electrochemical potential in flotation. Miner. Eng. 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Peng, Y. The depression of molybdenite flotation by sodium metabisulphite in fresh water and seawater. Miner. Eng. 2021, 168, 106939. [Google Scholar] [CrossRef]
- Chen, G.B.; Sun, J.R.; Yang, H.Y.; Ma, P.C.; Gao, S.X. Rapid Atmospheric Leaching of Chalcopyrite Using a Novel Reagent of Trichloroisocyanuric Acid. Minerals 2021, 11, 1012. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar] [CrossRef]
- Miki, H.; Hirajima, T.; Muta, Y.; Suyantara, G.; Sasaki, K. Effect of Sodium Sulfite on Floatability of Chalcopyrite and Molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Chanturia, V.A.; Bunin, I.Z.; Ryazantseva, M.V.; Khabarova, I.A. X-ray photoelectron spectroscopy-based analysis of change in the composition and chemical state of atoms on chalcopyrite and sphalerite surface before and after the nanosecond electromagnetic pulse treatment. J. Min. Sci. 2013, 49, 489–498. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Wang, H.W.; Skeldon, P.; Thompson, G.E. XPS studies of MoS2 formation from ammonium tetrathiomolybdate solutions. Surf. Coat. Technol. 1997, 91, 200–207. [Google Scholar] [CrossRef]
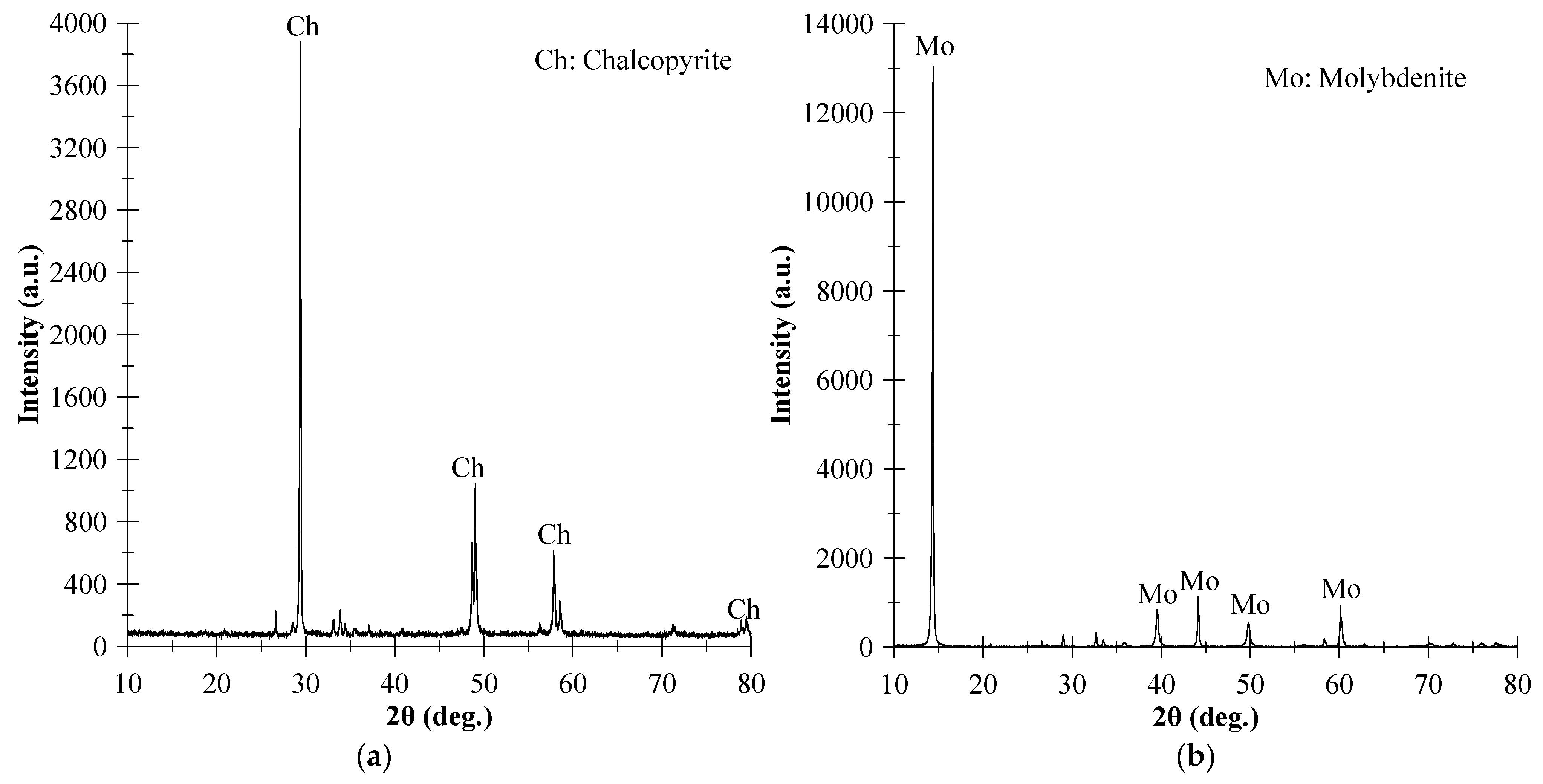



| Elements | Chalcopyrite | Molybdenite |
|---|---|---|
| Cu | 30.50 | - |
| Fe | 29.18 | - |
| S | 33.30 | 48.52 |
| Mo | - | 56.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xu, W.; Jiang, L.; Han, B.; Yang, M. A Novel Chalcopyrite Depressant for Selective Separation of Molybdenite from Cu-Mo Sulfide Ores and Its Interaction Mechanisms. Minerals 2023, 13, 1548. https://doi.org/10.3390/min13121548
Lin Y, Xu W, Jiang L, Han B, Yang M. A Novel Chalcopyrite Depressant for Selective Separation of Molybdenite from Cu-Mo Sulfide Ores and Its Interaction Mechanisms. Minerals. 2023; 13(12):1548. https://doi.org/10.3390/min13121548
Chicago/Turabian StyleLin, Yuemeng, Wentao Xu, Lishuai Jiang, Baisui Han, and Mengyue Yang. 2023. "A Novel Chalcopyrite Depressant for Selective Separation of Molybdenite from Cu-Mo Sulfide Ores and Its Interaction Mechanisms" Minerals 13, no. 12: 1548. https://doi.org/10.3390/min13121548





