Intercalation of Nontronite Clays from Santa Elena, Ecuador, Using Different Surfactant Hydrophobicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organoclay Preparation
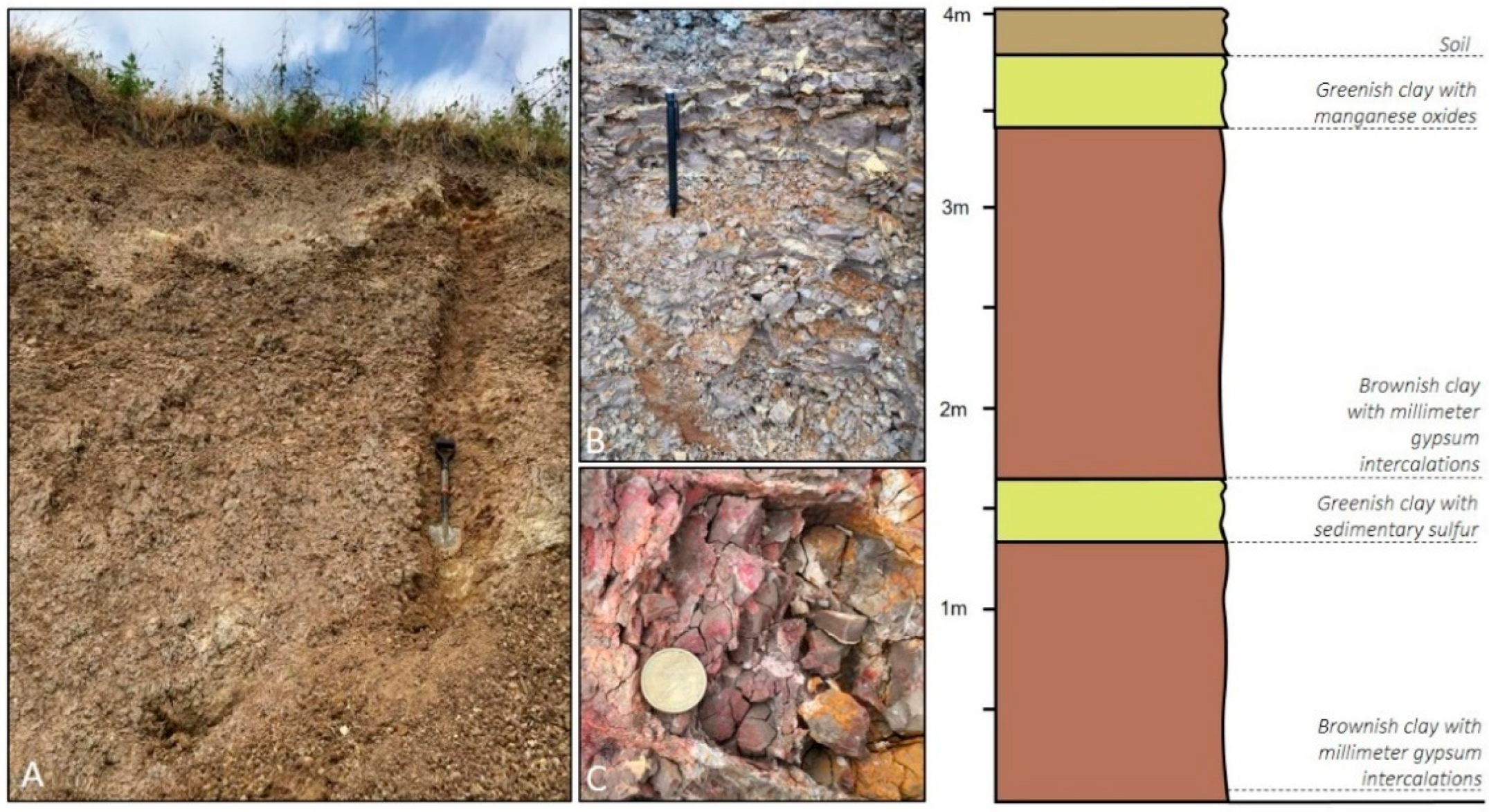
2.1.1. Organic Matter Removal
2.1.2. Carbonate Removal
2.1.3. Gravimetric Separation
2.1.4. Cation Exchange Process
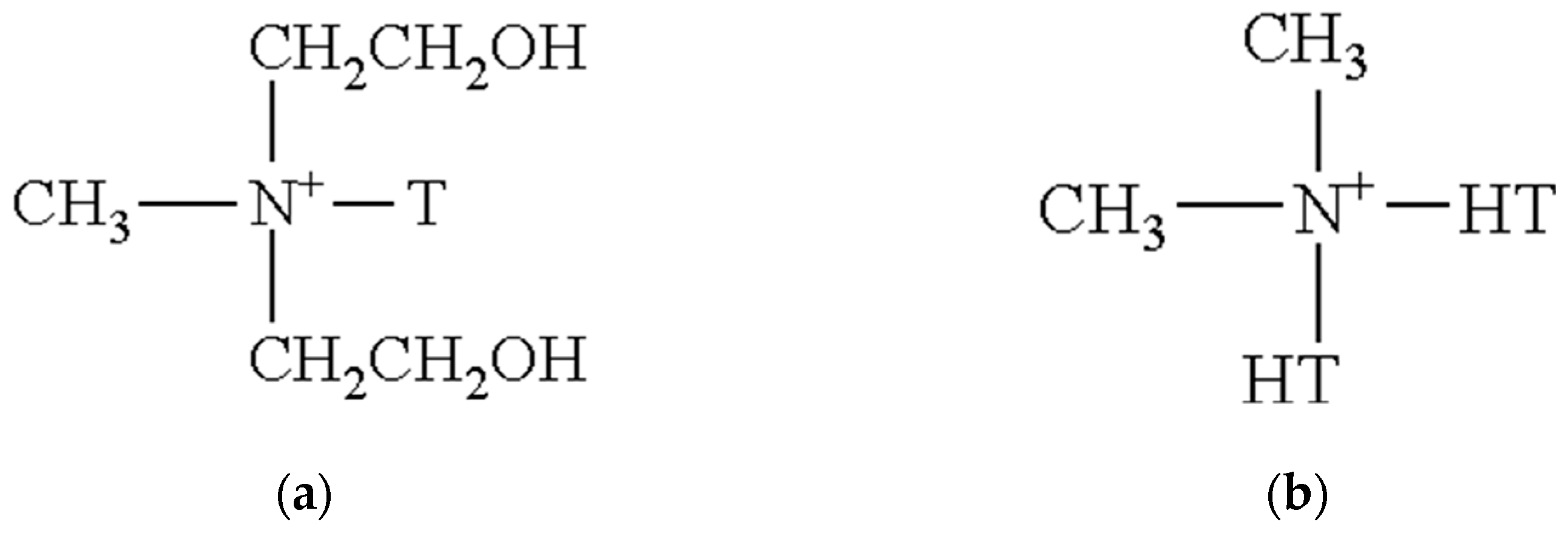
2.1.5. Preparation of the Modified Organoclays
2.1.6. Determination of Cation Exchange Capacity (CEC)
2.2. Characterization
2.2.1. Quantitative X-ray Diffractometry (QXRD)
2.2.2. Fourier Transform Infrared Spectrometry (FTIR)
2.2.3. Thermo-Gravimetric Analysis (TGA)
2.2.4. Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. Cation Exchange Capacity
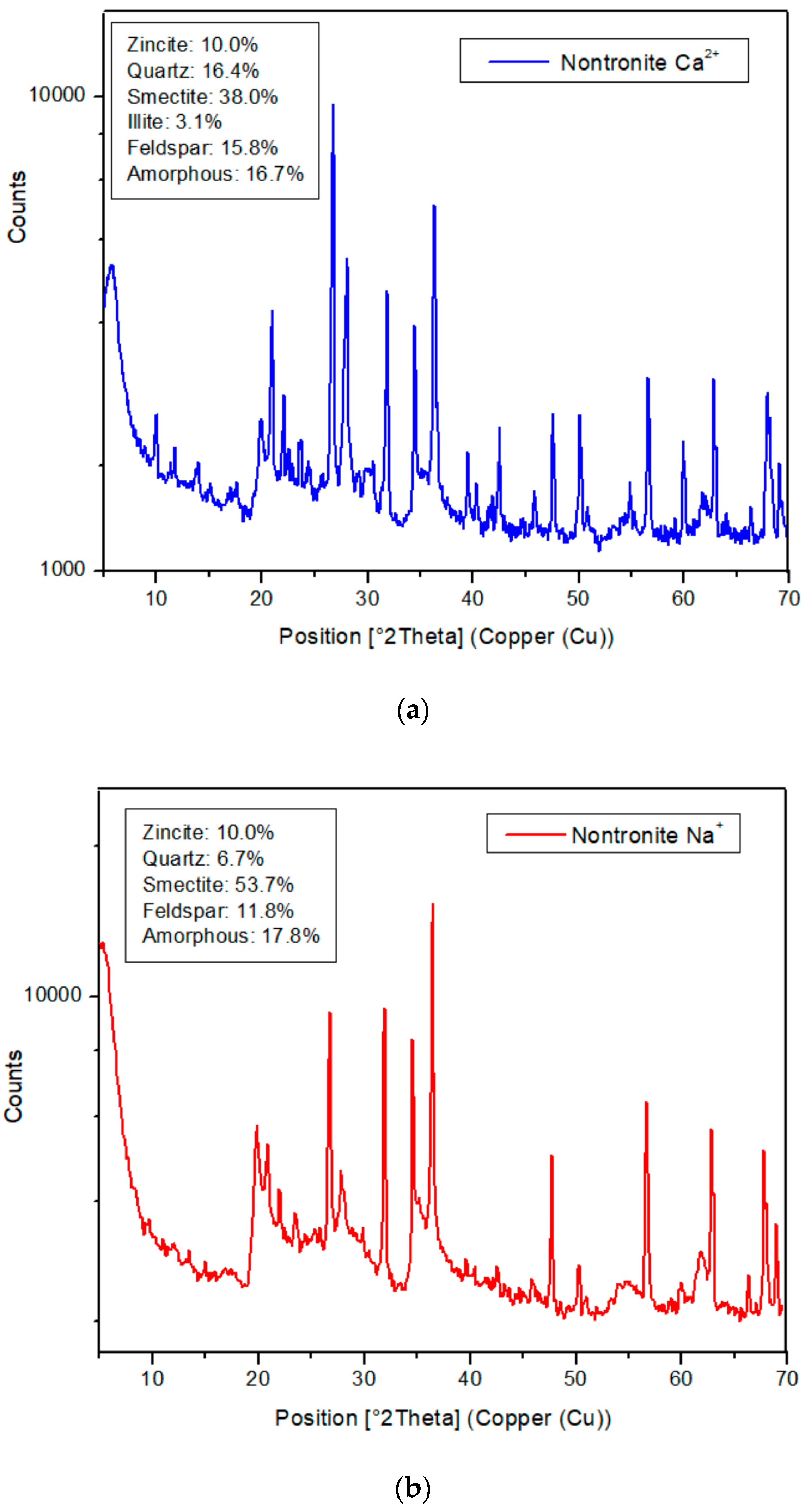
3.2. X-ray Diffractometry (XRD)
3.3. Fourier Transform Infrared Spectrometry (FTIR)
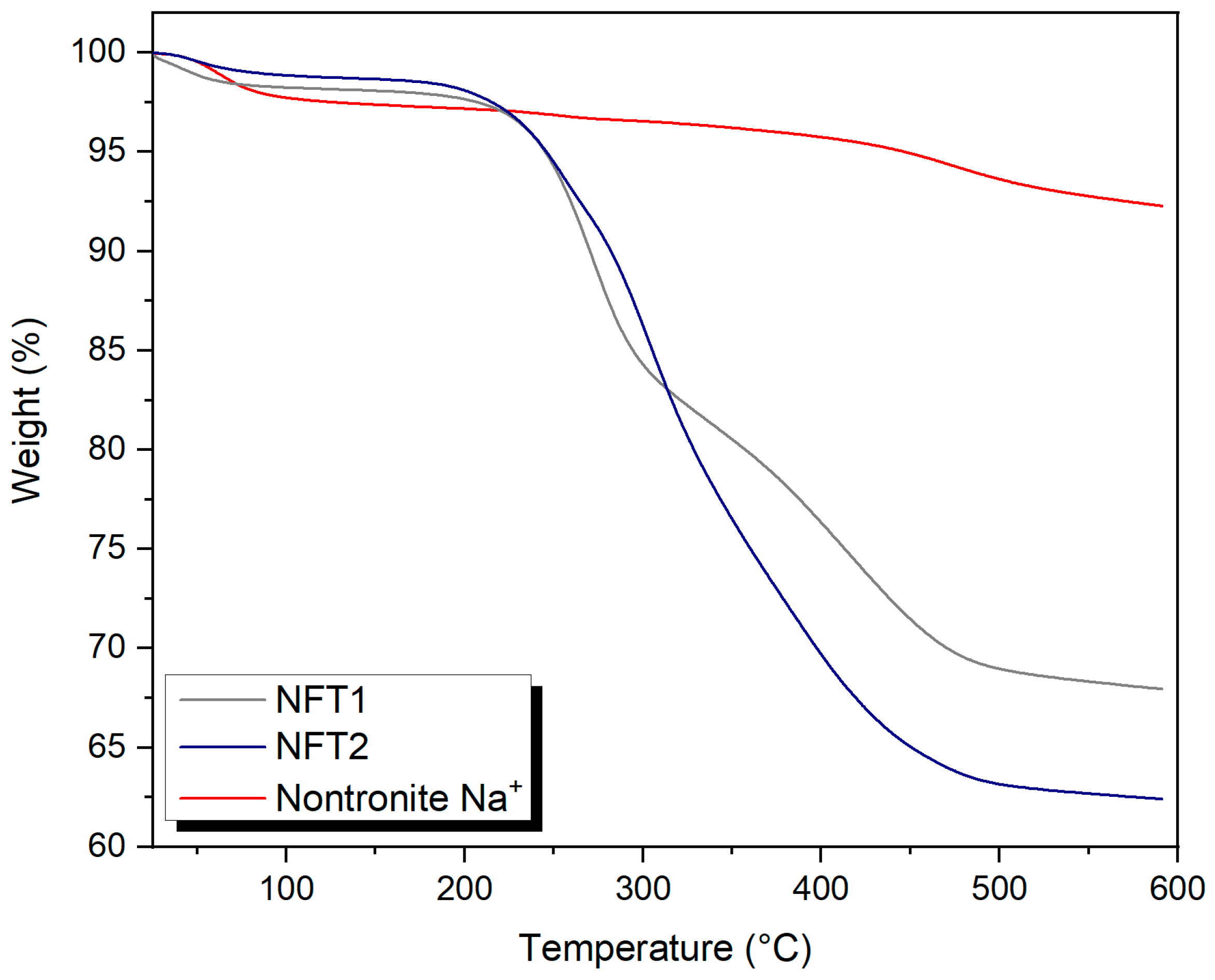
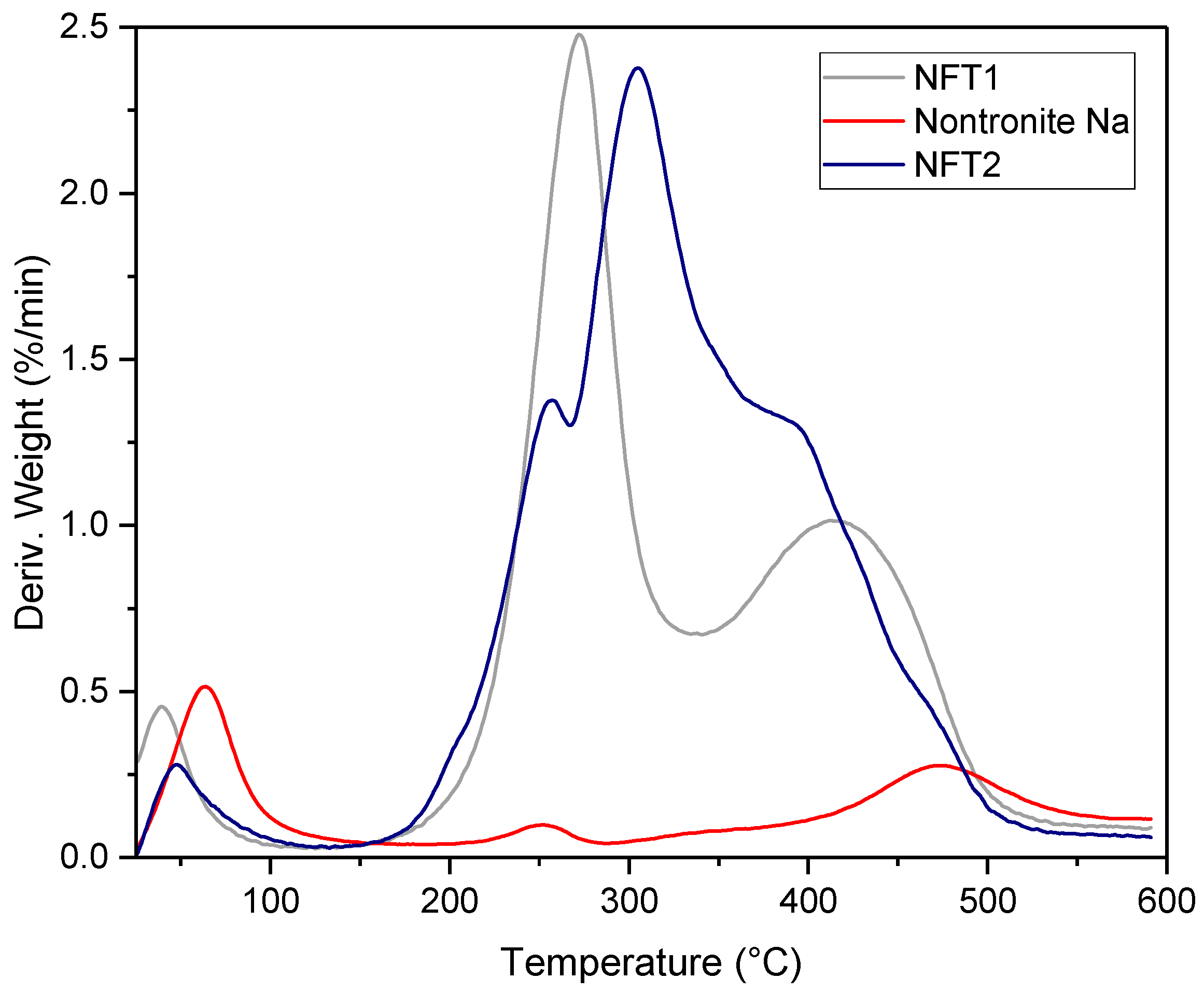
3.4. Thermo-Gravimetric Analysis (TGA)
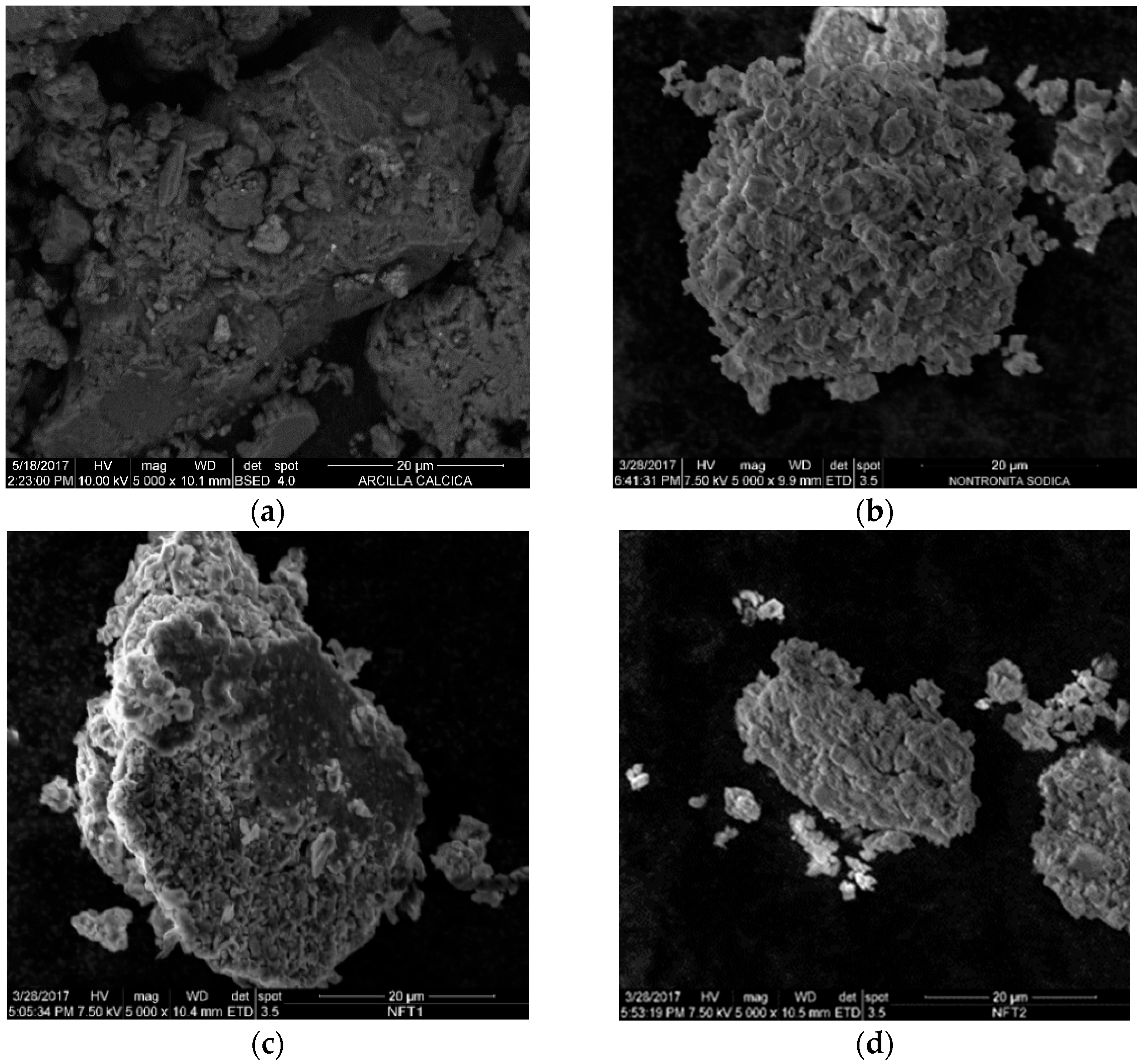
3.5. Scanning Electron Microscope (SEM)
4. Conclusions
5. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Global Organoclay Market Research Report 2022. Available online: https://www.industryresearch.co/global-organoclay-market-20779636) (accessed on 12 October 2022).
- Awasthi, A.; Jadhao, P.; Kumari, K. Clay nano-adsorbent: Structures, applications and mechanism for water treatment. SN Appl. Sci. 2019, 1, 1076. [Google Scholar] [CrossRef] [Green Version]
- Garzón, E.; Pérez-Villarejo, L.; Eliche-Quesada, D.; Martínez-Martínez, S.; Sánchez-Soto, P.J. Vitrification rate and estimation of the optimum firing conditions of ceramic materials from raw clays: A Review. Ceram. Int. 2022, 48, 15889–15898. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.; Medina, F. Synthesis of the zntio3 /tio2 nanocomposite supported in ecuadorian clays for the adsorption and photocatalytic removal of methylene blue dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The synthesis of organoclays based on clay minerals with different structural expansion capacities. Minerals 2021, 11, 707. [Google Scholar] [CrossRef]
- de Paiva, L.B.; Morales, A.; Díaz, F.R.V. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Santos, E.P.; Fook, M.; Malta, O.; de Lima, S.; Leite, I.F. Role of surfactants in the properties of poly(ethylene terephthalate)/purified clay nanocomposites. Materials 2018, 11, 1397. [Google Scholar] [CrossRef] [Green Version]
- Gamoudi, S.; Frini-Srasra, N.; Srasra, E. Influence of synthesis method in preparation of HDTMA ± and HDPy ± illites/smectites. Appl. Clay Sci. 2015, 116–117, 78–84. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K. Intercalation of montmorillonite with dialkylammonium cationic surfactants. J. Mol. Struct. 2022, 1256, 132468. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Zhu, J.; Frost, R.; Theng, B.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Morales Carrera, A.M.; Varajão, A.F.D.C.; Gonçalves, M.A. Mineralogical charaterization of the clays of the Santa Elena Peninsula, Ecuador. Rev. Esc. Minas 2008, 61, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Morales-Carrera, A.M.; Varajão, A.; César-Mendes, J.; Carrión, P.C.M. Estado del arte de la arcilla de la provincia del Guayas y su proyección a la península de Santa Elena, Ecuador. Bol. Geol. Min. 2006, 117, 723–736. [Google Scholar]
- Bahranowski, K.; Serwicka, E.M. Textural effects in powdered montmorillonite induced by freeze-drying and ultrasound pretreatment. Appl. Clay Sci. 2006, 32, 64–72. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.K. Characterization of organobentonite used for polymer nanocomposites. Mater. Chem. Phys. 2004, 85, 410–415. [Google Scholar] [CrossRef]
- Çiftçi, H.; Ersoy, B.; Evcin, A. Synthesis, characterization and Cr(VI) adsorption properties of modified magnetite nanoparticles. Acta Phys. Pol. A 2017, 132, 564–569. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini surfactant modified clays: Effect of surfactant loading and spacer length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.; Benítez-Guerrero, M.; Lorente, M. Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl. Clay Sci. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Sklute, E.C.; Michalski, J.R. Structural and spectroscopic changes to natural nontronite induced by experimental impacts between 10 and 40 GPa. J. Geophys. Res. Planets 2015, 120, 888–912. [Google Scholar] [CrossRef]
- Christidis, G.E.; Skarpelis, N. Clay Mineralogy Of The Sedimentary Iron-Nickel Ore Of Agios Ioannis, Ne Boeotia: New Data And Implication For Diagenetic Modifications. Bull. Geol. Soc. Greece 2010, 43, 2553–2561. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, B.; Megharaj, M.; Xi, Y.; Naidu, R. Structural characterisation of Arquad® 2HT-75 organobentonites: Surface charge characteristics and environmental application. J. Hazard. Mater. 2011, 195, 155–161. [Google Scholar] [CrossRef]
- Shehab-ElDin, A.N.; Sobh, R.; Rabie, A.; Mohamed, W.; Nasr, H.E. Polyacrylamide Grafted Electrospun Polyamide 6 Nanocomposite Fibers for Drug Delivery Application. Egypt. J. Chem. 2022, 65, 33–47. [Google Scholar] [CrossRef]
- Vaia, R.A.; Teukolsky, R.; Giannelis, E.P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 2022, 6, 1017–1022. [Google Scholar] [CrossRef]
- Petit, S.; Madejova, J.M. Fourier Transform Infrared Spectroscopy. Dev. Clay Sci. 2013, 5, 213–231. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Jankovič, L.; Škorňa, P.; Rodriguez, D.; Scholtzová, E. Preparation, characterization and adsorption properties of tetraalkylphosphonium organobeidellites. Appl. Clay Sci. 2021, 204, 106243. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Shabanian, M. The effect of organo-modified silicate nanolayers on the oxygen and water vapor barrier properties of poly(lactic acid). J. Chin. Chem. Soc. 2018, 65, 1340–1346. [Google Scholar] [CrossRef]







| Sample | Formulation |
|---|---|
| Nontronite Ca2+ | Natural clay (untreated) |
| Nontronite Na+ | Purified natural clay with cationic exchange |
| NFT1 | Nontronite Na+ + Oleylmethyl [ethoxylated (2)] |
| NFT2 | Nontronite Na+ + Di (hydrogenated tallow alkyl) |
| Sample | CEC (meq/100 g) |
|---|---|
| Nontronite Ca2+ | 56 |
| Nontronite Na+ | 67 |
| Sample | Quartz | Feldspars | Smectite | Illite | Amorphous |
|---|---|---|---|---|---|
| Nontronite Ca2+ | 16.4 | 15.8 | 38 | 3.1 | 16.7 |
| Nontronite Na+ | 6.7 | 11.8 | 53.7 | 0 | 17.8 |
| Nontronite Ca2+ | Nontronite Na+ | NFT1 | NFT2 | Assignment |
|---|---|---|---|---|
| 3619 | 3623 | 3624 | 3699 | Stretching vibrations of Fe(OH)Fe bonds |
| 3457 | 3440 | 3328 | 3420 | Stretching vibrations of Fe(OH)Fe bonds |
| 1638 | 1637 | 2926 | 2921 | Asymmetric stretching vibration of CH2 bands |
| - | - | 2854 | 2852 | Symmetric stretching vibration of CH2 bands |
| - | - | 1635 | 1644 | Bending vibration of the OH bonds |
| - | - | 1467 | 1469 | Scissoring bending vibrations of the CH2 bands |
| 1032 | 1027 | 1039 | 1031 | Stretch vibrations of the SiO |
| 917 | 918 | 916 | 918 | Flexural vibration of the Al2OH bands |
| 790 | 874 | 852 | 845 | Flexural vibration of the AlMgOH strips |
| 686 | 698 | 695 | 697 | Vibrations of the bands corresponding to the FeO group |
| 526 | 526 | 526 | 523 | Bending vibrations of the Si-O-Al strips |
| Sample | Mass Loss (%) | T1 (°C) | Mass Loss (%) | T2 (°C) | Mass Loss (%) | T3 (°C) | Mass Loss (%) | T4 (°C) |
|---|---|---|---|---|---|---|---|---|
| Nontronite Na+ | 1.14 | 64 | 3.14 | 252 | 5.60 | 474 | -- | -- |
| NFT1 | 0.65 | 41 | 10.49 | 272 | 26.17 | 421 | -- | -- |
| NFT2 | 0.37 | 49 | 63.8 | 256 | 14.88 | 304 | 29.74 | 393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigail-Cedeño, A.F.; Cornejo, M.H.; Cáceres-Zambrano, J.A.; Alava-Rosado, J.S.; García-Mejía, G. Intercalation of Nontronite Clays from Santa Elena, Ecuador, Using Different Surfactant Hydrophobicity. Minerals 2023, 13, 272. https://doi.org/10.3390/min13020272
Rigail-Cedeño AF, Cornejo MH, Cáceres-Zambrano JA, Alava-Rosado JS, García-Mejía G. Intercalation of Nontronite Clays from Santa Elena, Ecuador, Using Different Surfactant Hydrophobicity. Minerals. 2023; 13(2):272. https://doi.org/10.3390/min13020272
Chicago/Turabian StyleRigail-Cedeño, Andres F., Mauricio H. Cornejo, Julio A. Cáceres-Zambrano, Johanna S. Alava-Rosado, and Gladys García-Mejía. 2023. "Intercalation of Nontronite Clays from Santa Elena, Ecuador, Using Different Surfactant Hydrophobicity" Minerals 13, no. 2: 272. https://doi.org/10.3390/min13020272






