Microstructure and Mineral Phase Evolution of Vanadium Slag by Modulating the CaO/V2O5 Ratio
Abstract
:1. Introduction
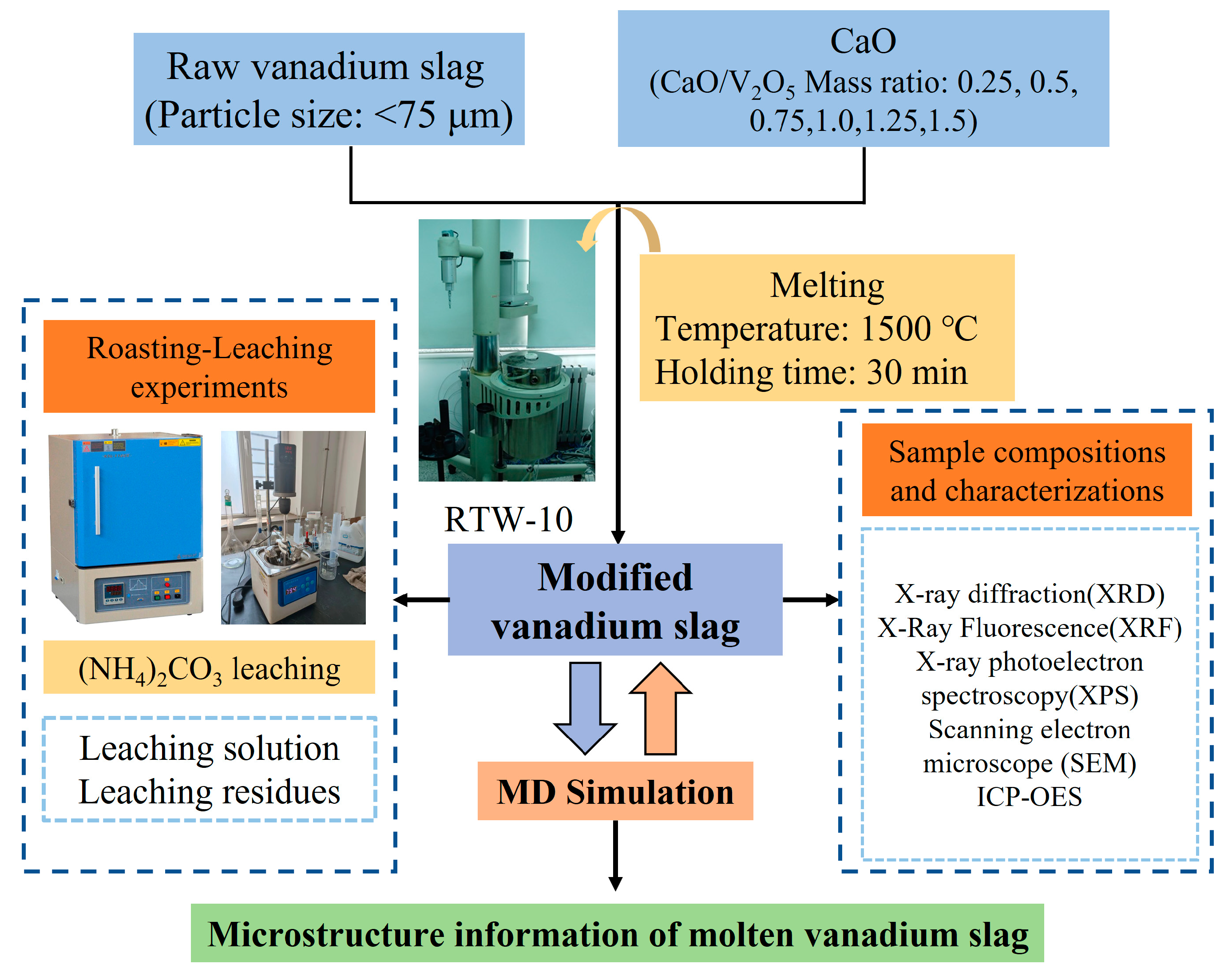
2. Materials and Methods
2.1. Materials
2.2. Melting Calcification and Non-Salt Roasting–Leaching Experimental Process
2.2.1. CaO Modification of Vanadium Slag
2.2.2. Non-Salt Roasting-(NH4)2CO3 Leaching
2.3. Analysis Methods
2.4. MD Simulation Methods
3. Results and Discussion
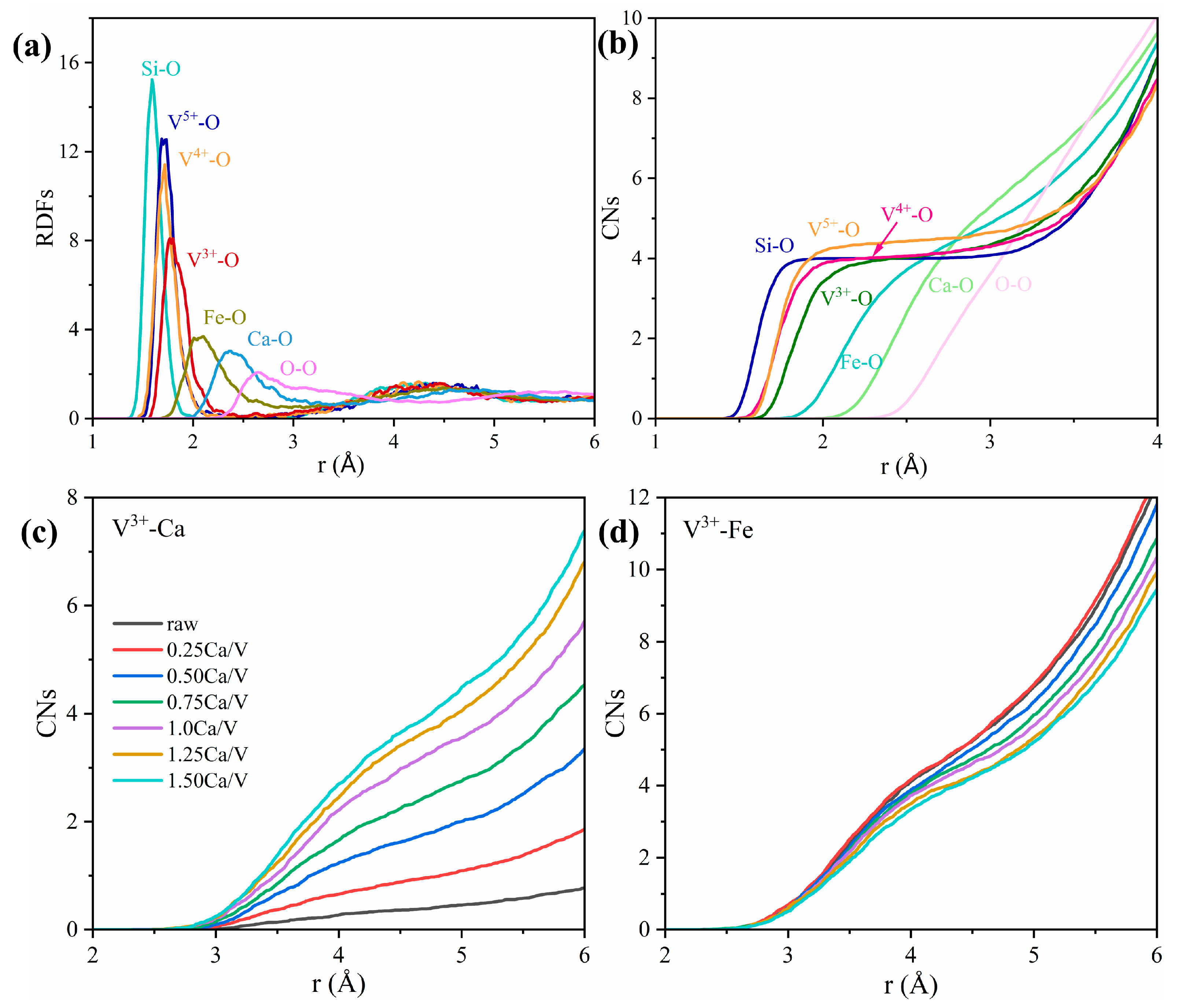
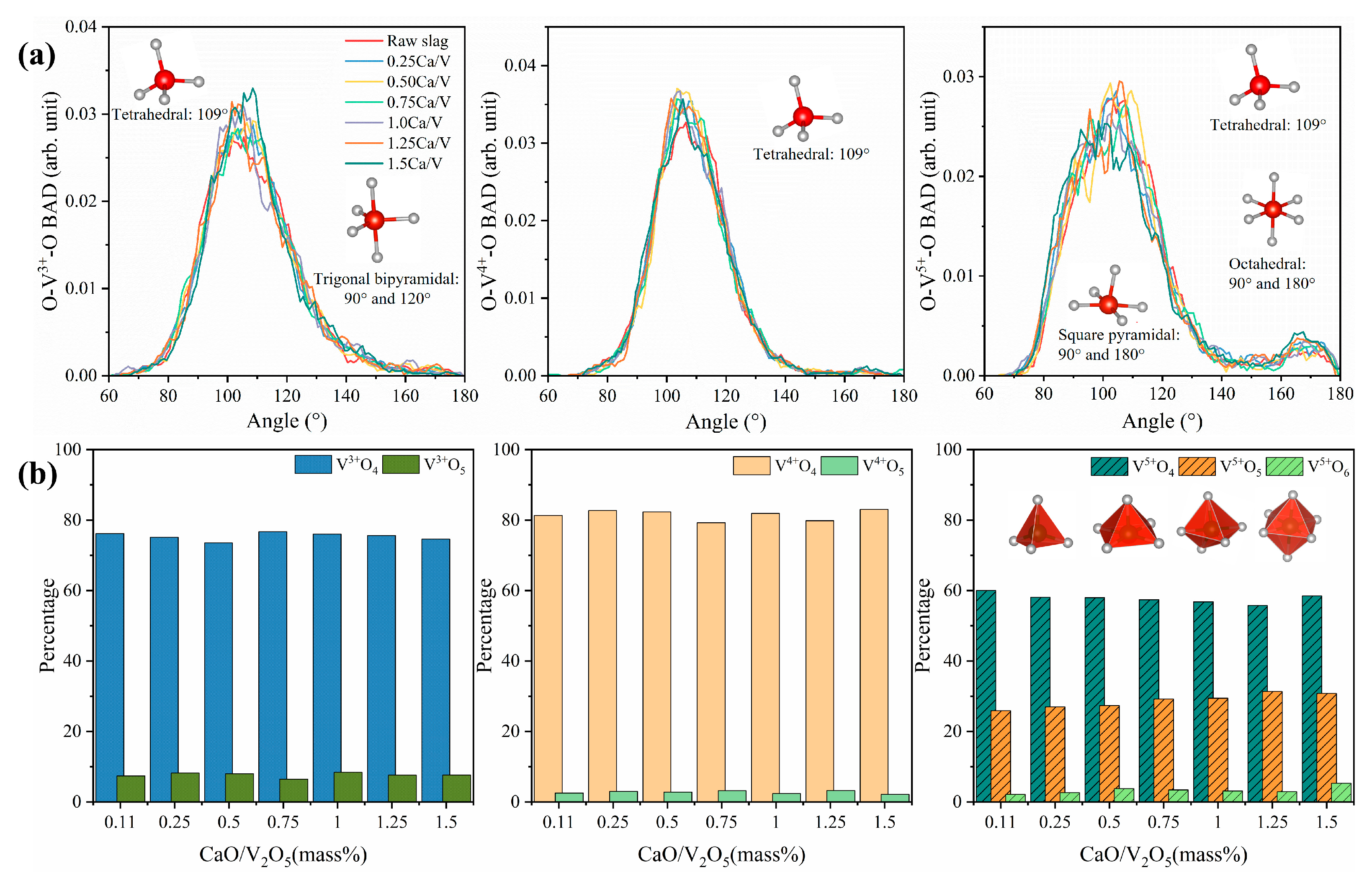
3.1. Short Range Order of Slag: Bond Lengths, Coordination Numbers and Bond Angle Distributions
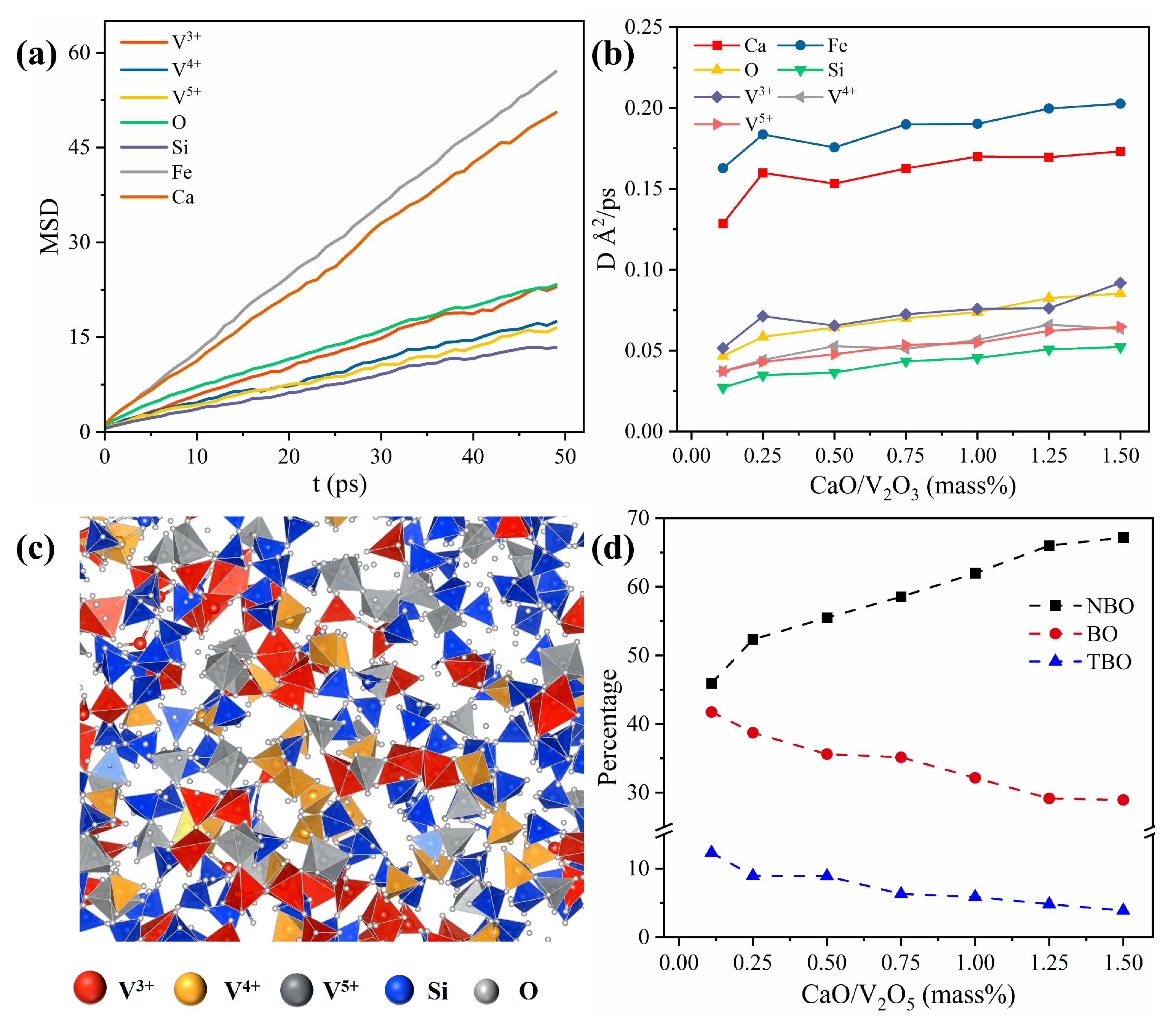
3.2. The Effect of CaO on Transmission Characteristics
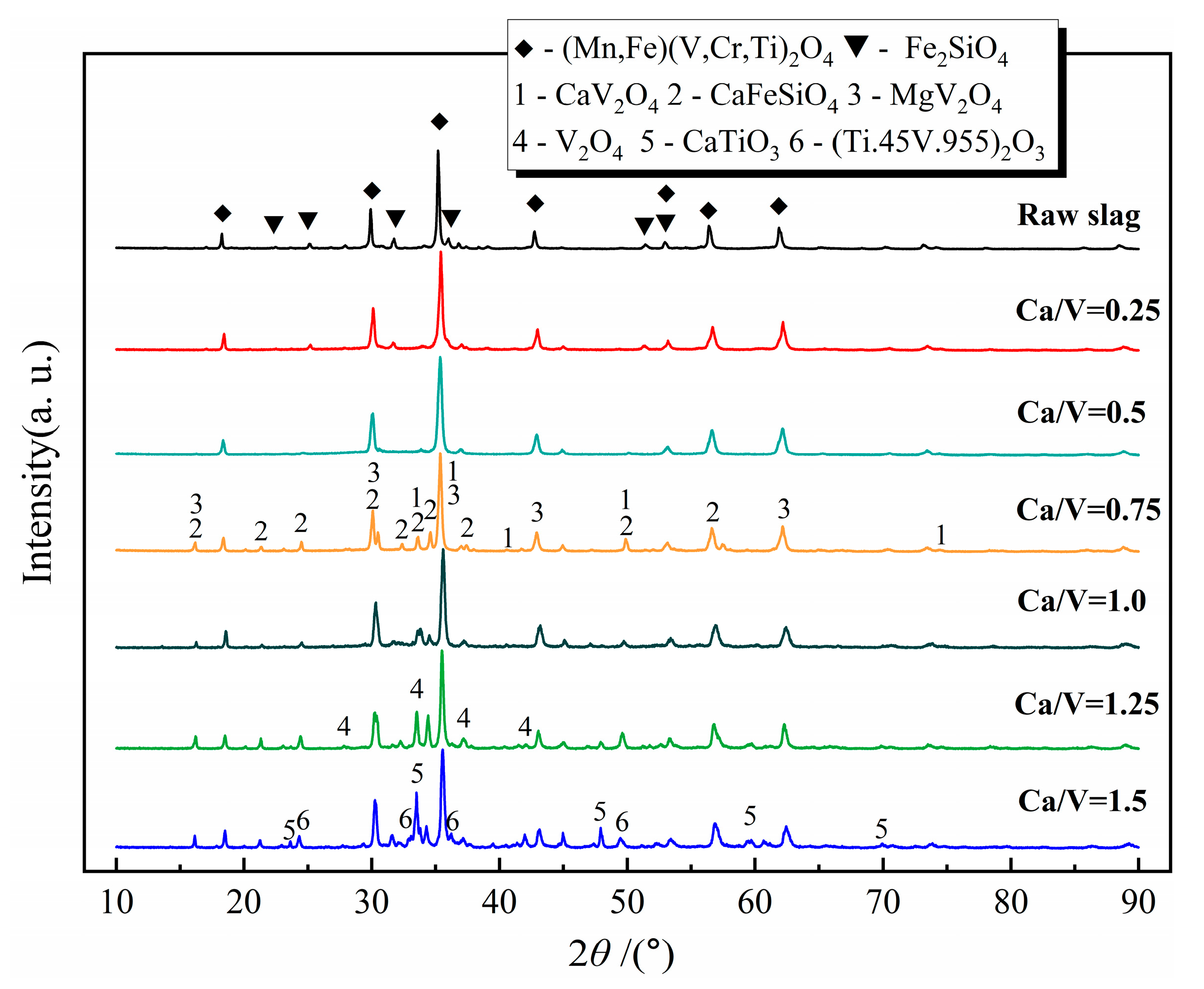
3.3. Mineral Phase Evolution Caused by CaO
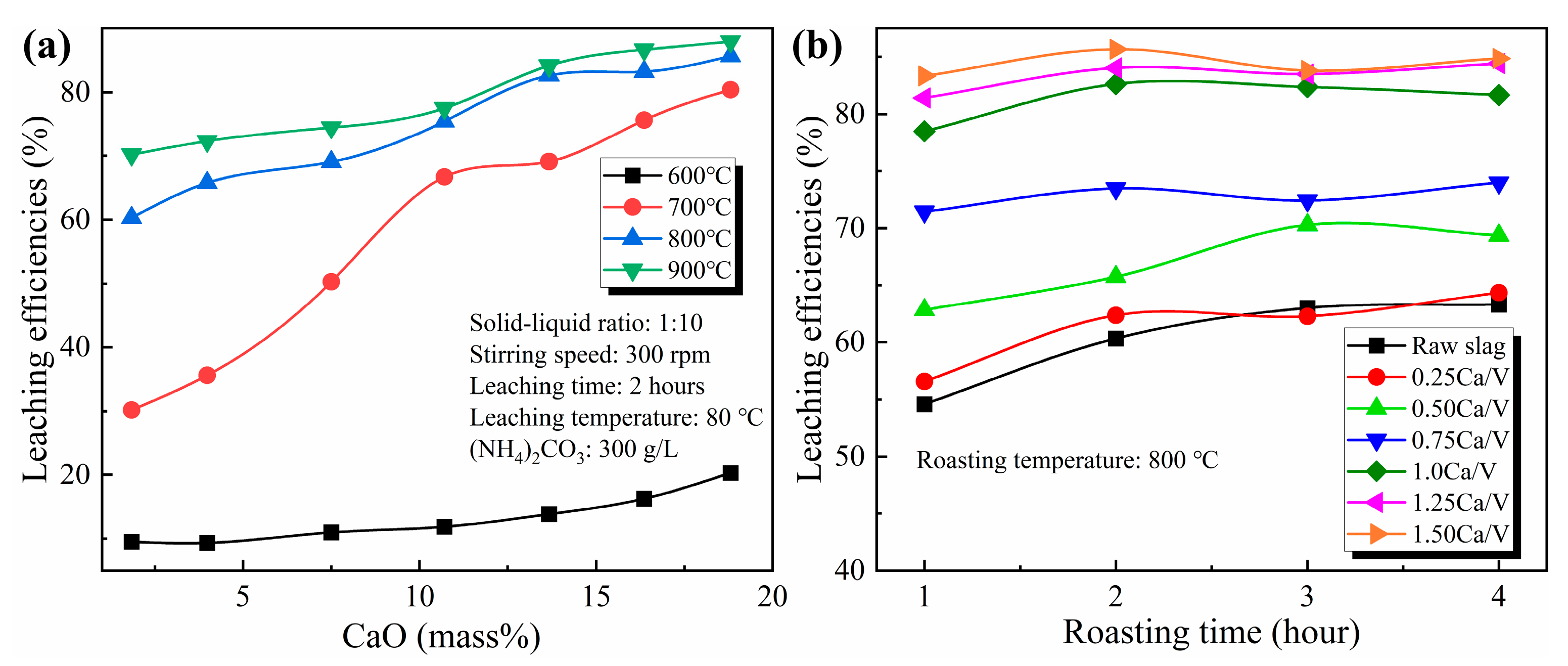
3.4. Effect of CaO/V2O5 Ratio on the Roasting–Leaching Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.C.; Kurniawan; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Gao, M.L.; Xue, X.X.; Li, L.J.; Yang, H.; Chen, D.H. Leaching behavior and kinetics of vanadium extraction from vanadium-bearing steel slag. Metall. Res. Technol. 2019, 116, 407. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, C.; Lin, M.; Guo, Y.; Xie, B. Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag. Powder Technol. 2020, 360, 503–508. [Google Scholar] [CrossRef]
- Smirnov, L.A.; Zhuchkov, V.I.; Zayakin, O.V.; Mikhailova, L.Y. Complex Vanadium-Containing Ferroalloys. Metallurgist 2021, 64, 1249–1255. [Google Scholar] [CrossRef]
- Chen, L.; Diao, J.; Wang, G.; Qiao, Y.; Xie, B. Experimental study on slag splashing with modified vanadium slag. Ironmak. Steelmak. 2019, 46, 165–168. [Google Scholar] [CrossRef]
- Fang, H.-X.; Li, H.-Y.; Zhang, T.; Liu, B.-S.; Xie, B. Influence of CaO on Existence form of Vanadium-containing Phase in Vanadium Slag. ISIJ Int. 2015, 55, 200–206. [Google Scholar] [CrossRef]
- Chen, L.; Diao, J.; Wang, G.; Xie, B. Assessment of Dephosphorization During Vanadium Extraction Process in Converter. JOM 2018, 70, 963–968. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, B.; Tan, W.-F.; Diao, J.; Zhang, X.; Li, H.-Y. Non-isothermal Crystallization Kinetics of Spinels in Vanadium Slag with High CaO Content. JOM 2016, 68, 2520–2524. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.; Li, Y.; Ye, Q.; Yu, X.; Chen, Z. Effect of CaO on the Phase Evolution of Vanadium Slag during Crystallization and Roasting–Leaching Processes for Selective Extraction of Vanadium. Crystals 2022, 12, 927. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Metall. Rev. 2022, 43, 466–488. [Google Scholar] [CrossRef]
- Broglia, G.; Mugoni, C.; Du, J.C.; Siligardi, C.; Montorsi, M. Lithium vanado-phosphate glasses: Structure and dynamics properties studied by molecular dynamics simulations. J. Non-Cryst. Solids 2014, 403, 53–61. [Google Scholar] [CrossRef]
- Deng, L.; Du, J.C. Development of boron oxide potentials for computer simulations of multicomponent oxide glasses. J. Am. Ceram. Soc. 2019, 102, 2482–2505. [Google Scholar] [CrossRef]
- Deng, L.; Lu, X.; Vienna, J.D.; Du, J. Structures of Vanadium-Containing Silicate and Borosilicate Glasses: Vanadium Potential Development and MD Simulations. J. Non-Cryst. Solids 2022, 575, 121223. [Google Scholar] [CrossRef]
- Sun, W.; Du, J. Interfacial structures of spinel crystals with borosilicate nuclear waste glasses from molecular dynamics simulations. J. Am. Ceram. Soc. 2019, 102, 4583–4601. [Google Scholar] [CrossRef]
- Saiko, I.A.; Saetova, N.S.; Raskovalov, A.A.; Il’ina, E.A.; Molchanova, N.G.; Kadyrova, N.I. Hopping conductivity in V2O5-P2O5 glasses: Experiment and non-constant force field molecular dynamics. Solid State Ion. 2020, 345, 115180. [Google Scholar] [CrossRef]
- Gao, L.; Liu, X.; Bai, J.; Kong, L.; Bai, Z.; Li, W. Structure and flow properties of coal ash slag using ring statistics and molecular dynamics simulation: Role of CaO/Na2O in SiO2–Al2O3–CaO–Na2O. Chem. Eng. Sci. 2021, 231, 116285. [Google Scholar] [CrossRef]
- Lin, Z.; Bai, L.; Zhang, X.; Dong, H.; Wu, F. Na-vacancies-induced magnetism in NaxMnO2. J. Magn. Magn. Mater. 2018, 468, 164–167. [Google Scholar] [CrossRef]
- Yue, H.-R.; Xue, X.-X. Generated compounds at the V-slag/CaO diffusion surface and diffusion characteristics of V and Ca in calcium vanadate. J. Hazard. Mater. 2020, 393, 122368. [Google Scholar] [CrossRef]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Liang, W.; Sun, M.; Wang, Z. The effect of CaO(MgO) on the structure and properties of aluminosilicate system by molecular dynamics simulation. J. Mol. Liq. 2018, 268, 762–769. [Google Scholar] [CrossRef]
- Johnston, W.D. Optical Spectra of the Various Valence States of Vanadium in Na2O.2SiO2 Glass. J. Am. Ceram. Soc. 1965, 48, 608–611. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Li, M.; Wang, R.; Wu, G.; Yang, J.; Han, W.; Cao, S.; Zhao, L. Phase composition and valence of pulsed laser deposited vanadium oxide thin films at different oxygen pressures. Surf. Coat. Technol. 2007, 201, 5344–5347. [Google Scholar] [CrossRef]
- Laorodphan, N.; Kidkhunthod, P.; Khajonrit, J.; Montreeuppathum, A.; Chanlek, N.; Pinitsoontorn, S.; Maensiri, S. Effect of B2O3 content on structure-function of vanadium-lithium-borate glasses probed by synchrotron-based XAS and vibrating sample magnetrometry technique. J. Non-Cryst. Solids 2018, 497, 56–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.-A.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, K.; Hua, W.-H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zheng, X.; Wang, J.; Cao, J.; Zhou, M. Efficient separation of chromium and vanadium by calcification roasting–sodium carbonate leaching from high chromium vanadium slag and V2O5 preparation. Sep. Purif. Technol. 2020, 230, 115881. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhang, L.; Gu, S. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, T.; Wen, J.; Sun, H.; Li, M.; Peng, Y. Effect of chemical composition on the element distribution, phase composition and calcification roasting process of vanadium slag. Int. J. Min. Met. Mater. 2022, 29, 2144–2151. [Google Scholar] [CrossRef]

| Constituent | CaO | V2O5 | TFe | TiO2 | MnO | Cr2O3 | MgO | SiO2 | Al2O3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw slag | 1.84 | 16.63 | 26.24 | 12.04 | 8.64 | 1.55 | 2.34 | 15.03 | 4.00 | 0.11 |
| 0.25Ca/V | 3.98 | 15.87 | 26.96 | 11.44 | 8.29 | 1.62 | 2.24 | 13.19 | 4.39 | 0.10 |
| 0.5Ca/V | 7.50 | 15.29 | 25.62 | 11.29 | 7.93 | 1.43 | 2.17 | 13.03 | 4.37 | 0.10 |
| 0.75Ca/V | 10.71 | 15.04 | 24.50 | 10.82 | 7.42 | 1.44 | 2.11 | 12.98 | 4.17 | 0.11 |
| 1.0Ca/V | 13.67 | 14.32 | 23.36 | 10.44 | 7.13 | 1.42 | 1.93 | 12.21 | 4.42 | 0.10 |
| 1.25Ca/V | 16.36 | 13.17 | 22.95 | 9.46 | 6.98 | 1.42 | 1.84 | 12.63 | 3.93 | 0.09 |
| 1.5Ca/V | 18.81 | 13.04 | 21.66 | 8.72 | 6.69 | 1.38 | 1.82 | 12.26 | 4.68 | 0.08 |
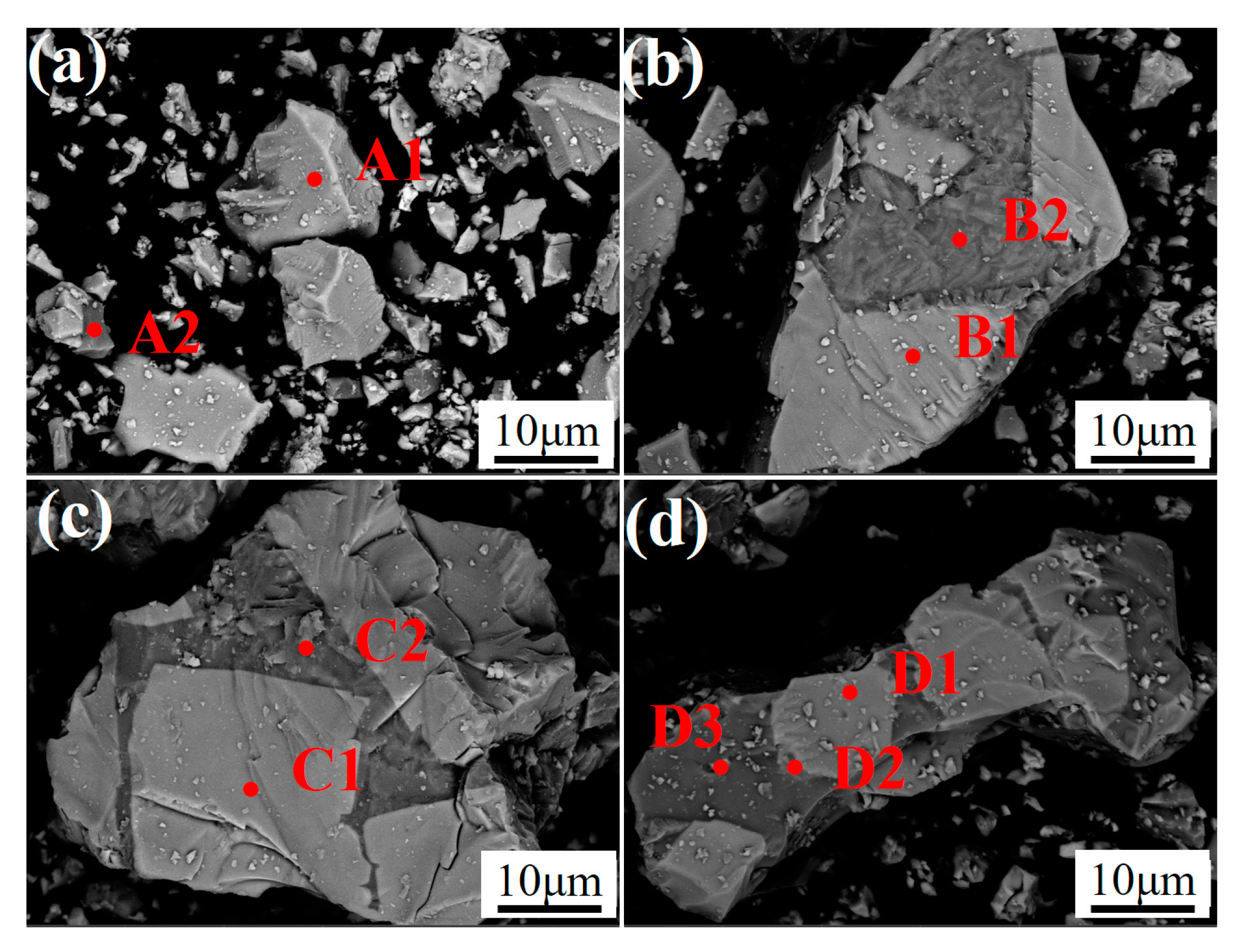
| Point | Fe | V | Ca | Ti | Cr | Mn | Si | Mg | O |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 31.36 | 17.00 | 0.08 | 11.71 | 1.92 | 6.62 | 0.43 | 0.35 | 29.5 |
| A2 | 15.39 | 2.45 | 0.81 | 2.54 | 0.23 | 10.82 | 19.26 | 5.92 | 39.24 |
| B1 | 30.32 | 21.96 | 0.21 | 8.09 | 3.0 | 6.73 | 0.17 | 1.83 | 24.44 |
| B2 | 17.16 | 0.39 | 13.9 | 1.10 | / | 6.14 | 17.43 | 1.43 | 38.84 |
| C1 | 28.78 | 30.05 | 0.87 | 6.9 | 5.46 | 8.2 | 0.22 | 0.93 | 14.56 |
| C2 | 13.71 | 0.42 | 33.26 | 0.67 | 0.02 | 3.59 | 17.56 | 2.0 | 27.95 |
| D1 | 27.77 | 29.40 | 1.23 | 7.25 | 4.38 | 10.21 | 0.19 | 0.94 | 12.58 |
| D2 | 26.97 | 17.36 | 4.79 | 6.41 | 2.78 | 7.80 | 2.23 | 2.71 | 26.78 |
| D3 | 11.51 | 1.02 | 28.82 | 11.55 | 0.14 | 3.84 | 9.35 | 0.34 | 33.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Liu, Y.; Wan, X.; Zhang, T.; Lin, S.; Wang, K.; Li, X. Microstructure and Mineral Phase Evolution of Vanadium Slag by Modulating the CaO/V2O5 Ratio. Minerals 2023, 13, 628. https://doi.org/10.3390/min13050628
Yang H, Liu Y, Wan X, Zhang T, Lin S, Wang K, Li X. Microstructure and Mineral Phase Evolution of Vanadium Slag by Modulating the CaO/V2O5 Ratio. Minerals. 2023; 13(5):628. https://doi.org/10.3390/min13050628
Chicago/Turabian StyleYang, Han, Yan Liu, Xingyuan Wan, Tingan Zhang, Shengnan Lin, Kun Wang, and Xiaolong Li. 2023. "Microstructure and Mineral Phase Evolution of Vanadium Slag by Modulating the CaO/V2O5 Ratio" Minerals 13, no. 5: 628. https://doi.org/10.3390/min13050628





