High-Speed Dynamic Camera Analysis of the Hematite Floc–Bubble Mineralization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Observation Device
2.3. Image Processing
3. Floc–Bubble Mineralization Process
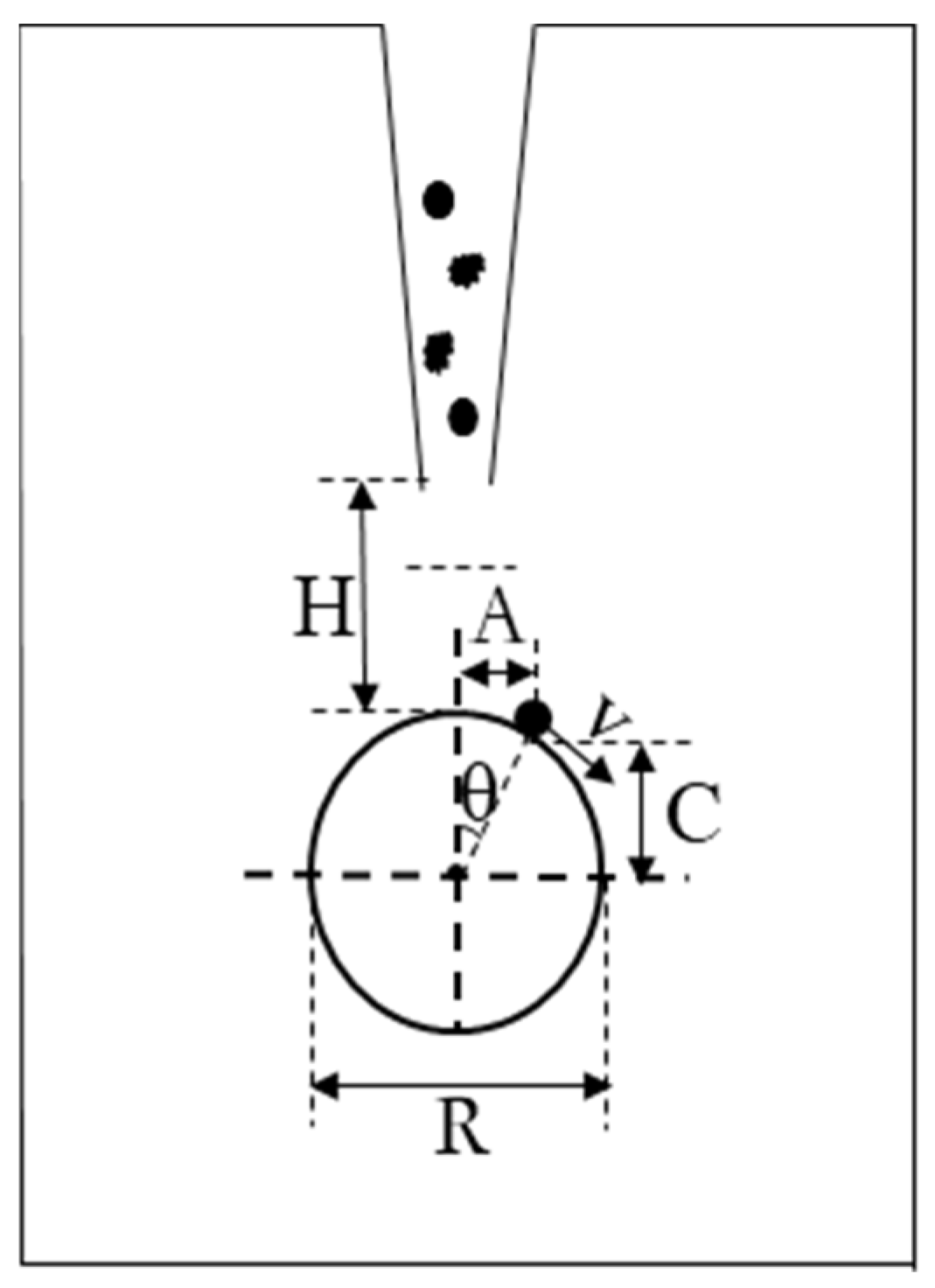
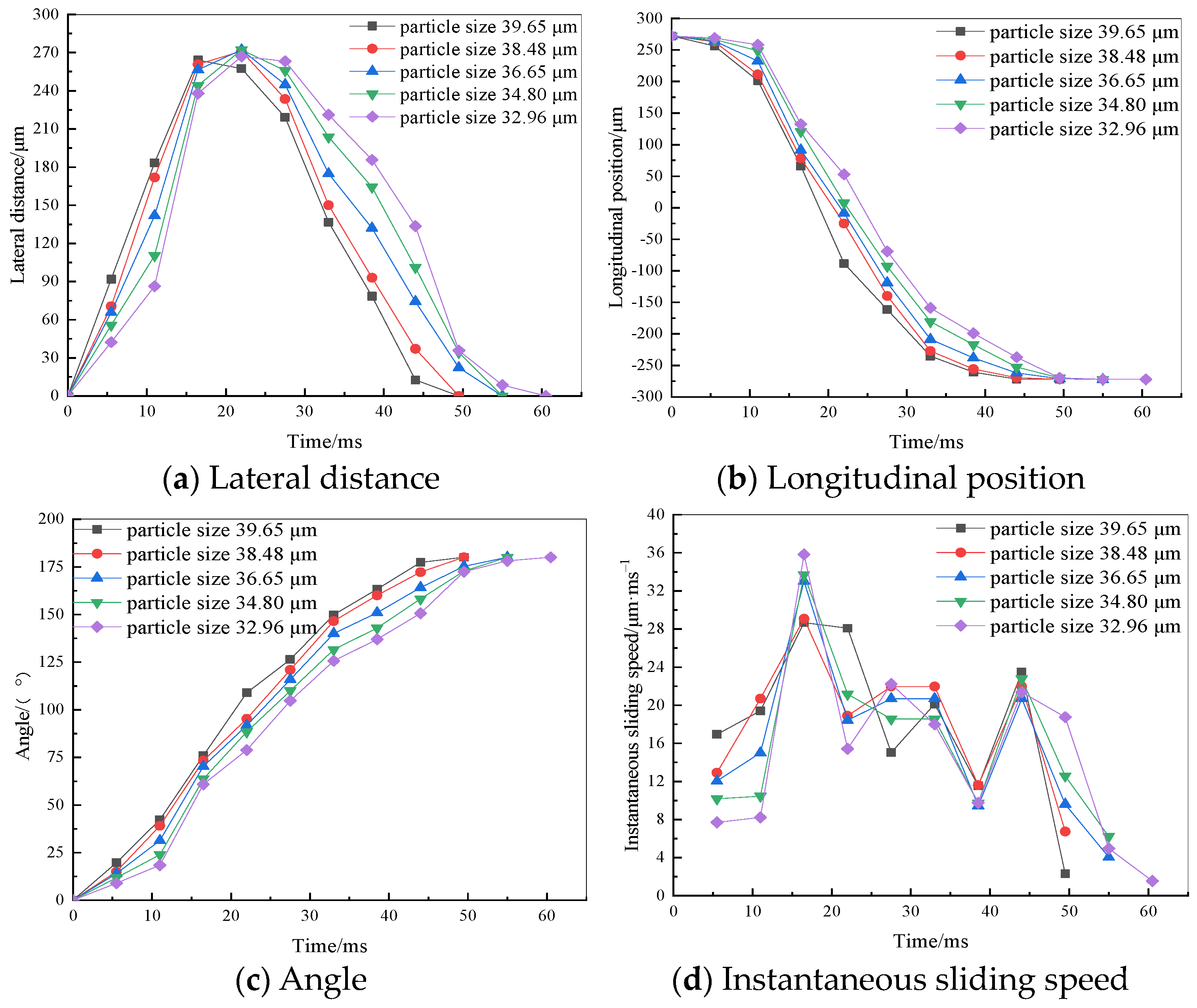
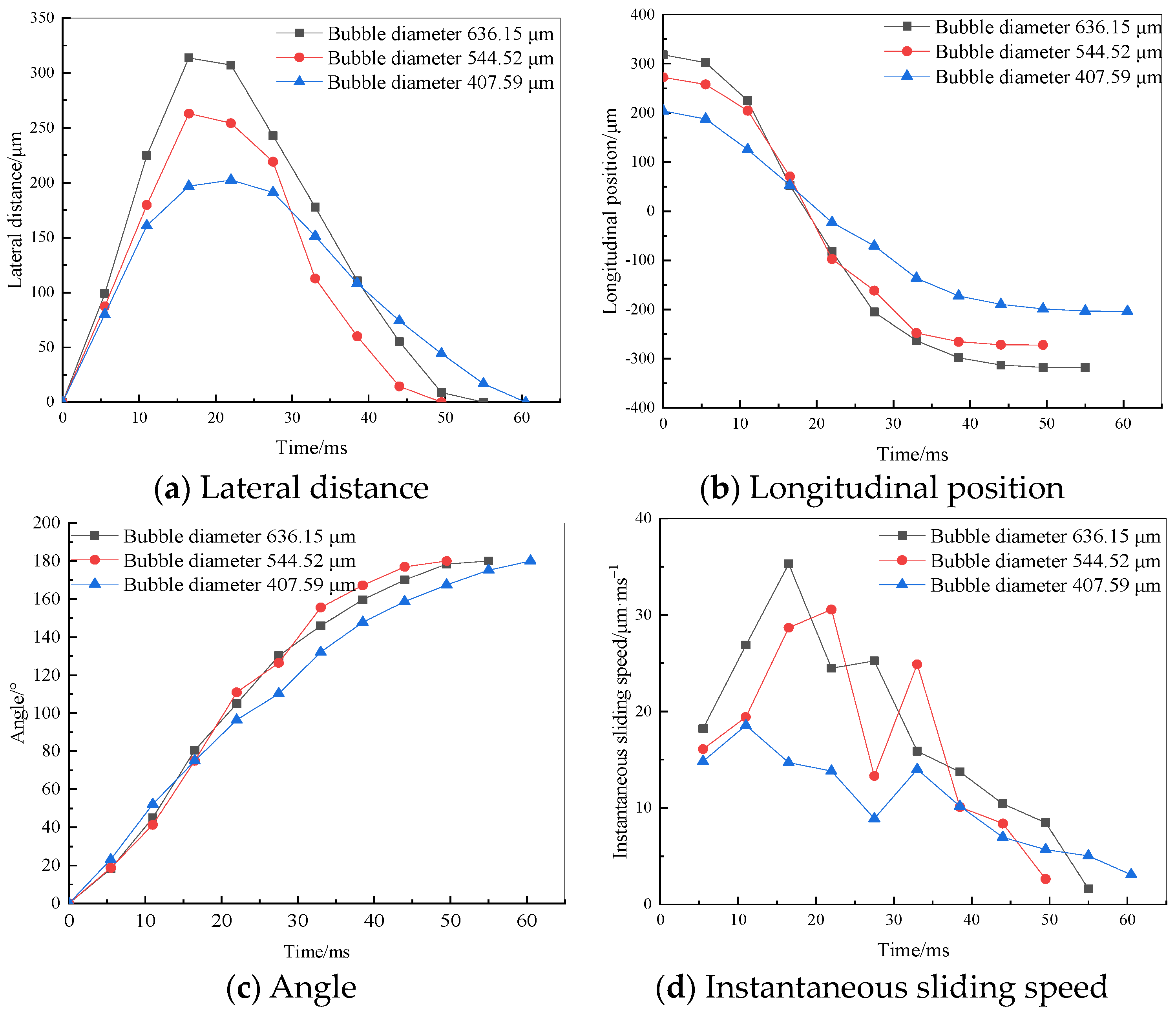
3.1. Variation in the Position of Flocs of Different Particle Sizes on the Bubble Surface
3.2. Variation in Floc Position on the Surface of Bubbles with Different Diameters
4. Study on the Mechanism of Hematite Flocculant–Bubble Mineralization
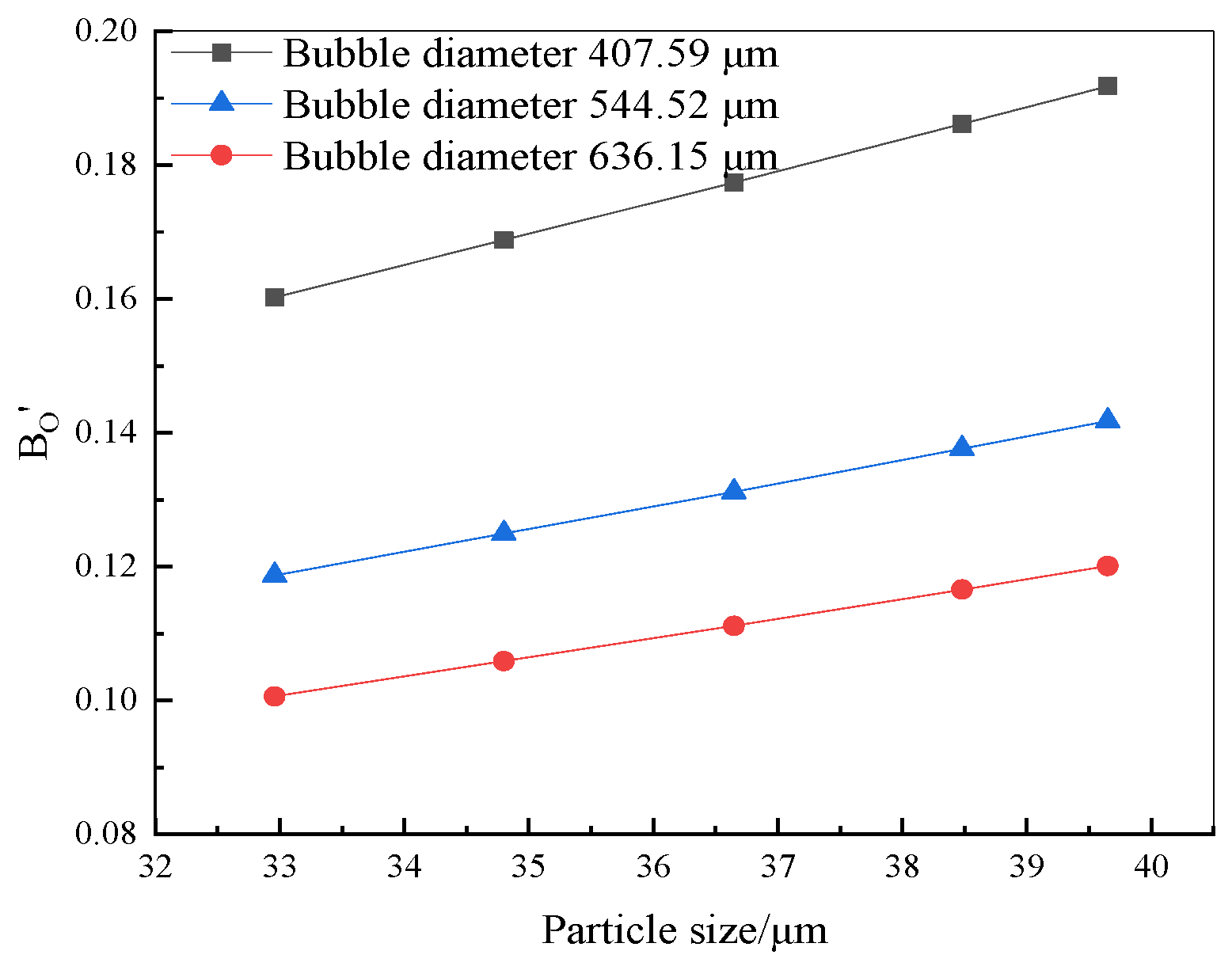
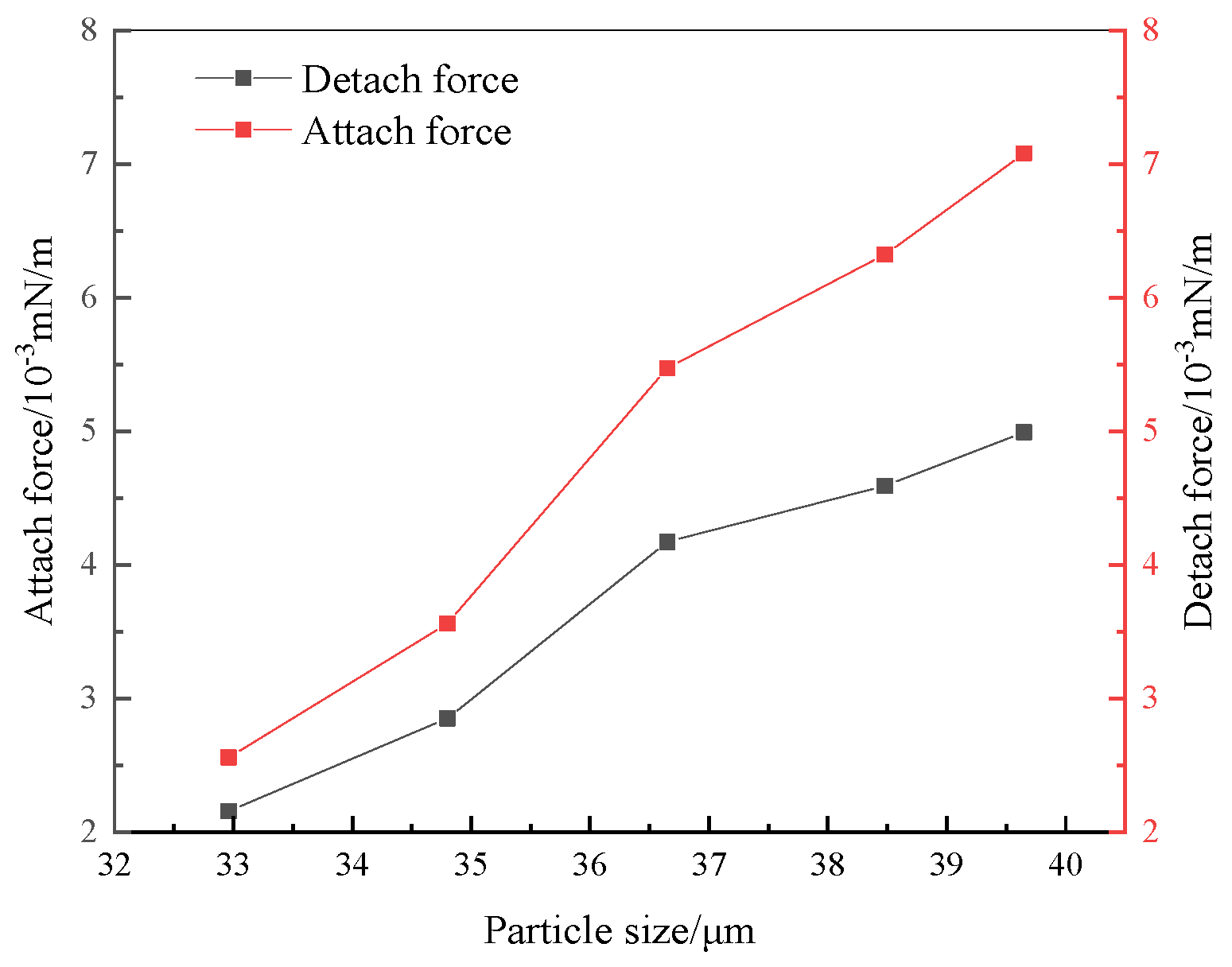
4.1. Analysis of the Forces on the Surface of the Flocs on the Bubble
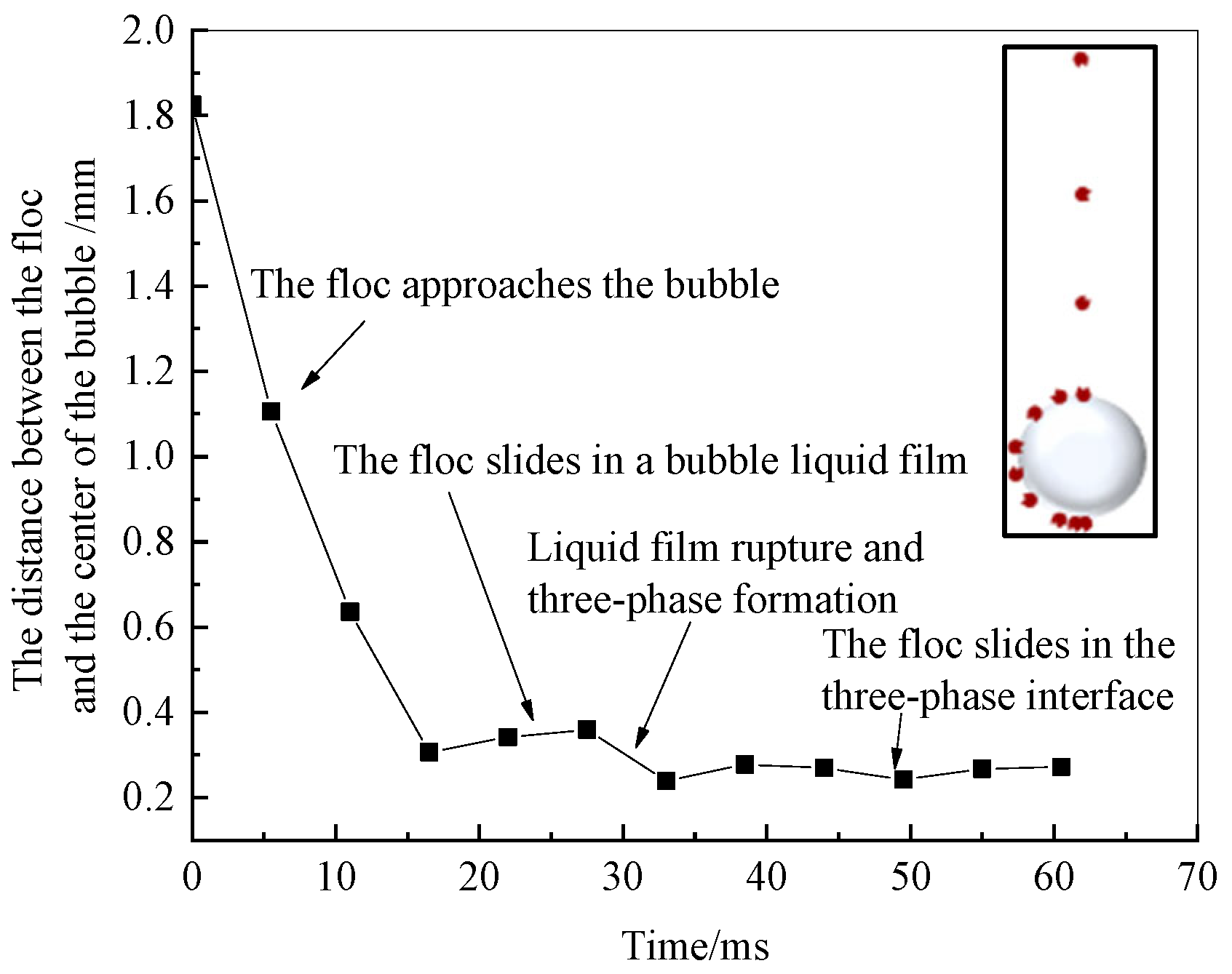
4.2. Collision–Adhesion Process of Flocs and Bubbles
5. Conclusions
- At a fixed bubble diameter of 544.52 μm, as the floc diameter increases, the time it takes for the floc to slide to the bottom of the bubble and stabilize decreases, and the instantaneous dynamic velocity changes more gradually, which is more conducive to the floc adhering to the bubble surface. The floc’s diameter reached 49.5 ms, and its highest instantaneous velocity was 28.67 μm·ms−1.
- The time taken for the floc to adhere to the bottom of the bubble is the shortest and the fluctuation range of the instantaneous sliding velocity of the floc is the smallest with a fixed floc size of 39.65 μm and bubble diameter of 544.52 μm, which make it more likely for the floc to adhere to the bubble surface after the collision.
- After looking into the process of flocs on bubble surfaces, it was discovered that floc particle size RP and bubble width RB are the two key variables influencing the force of particles on bubble surfaces. The desorption force on the flocs is reduced and the Bond constant Bo′ is increased with increasing floc particle size. Conversely, the adhesion force on the flocs increases and the desorption force increases with growing floc particle size.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panda, L.; Venugopal, R.; Mandre, N.R. Selective flocculation of chromite tailings. Trans. Indian Inst. Met. 2021, 74, 619–628. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, M.; Li, Y.; Lau, E. Improving micro-fine mineral flotation via micro/nano technologies. Sep. Sci. Technol. 2023, 58, 520–537. [Google Scholar] [CrossRef]
- Yu, P.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Surface modification of ilmenite by introducing copper-ammonia ion and its response to flotation in H2SO4-H2O2 system. Miner. Eng. 2021, 171, 107102. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Wan, D.; Yi, X.; Sun, X.; Ji, L.; Wang, G. A lattice Boltzmann study of the collisions in a particle-bubble system under turbulent flows. Powder Technol. 2020, 361, 759–768. [Google Scholar] [CrossRef]
- Dianyu, E.; Zhou, P.; Guo, S.; Zeng, J.; Cui, J.; Jiang, Y.; Kuang, S. Particle shape effect on hydrodynamics and heat transfer in spouted bed: A CFD-DEM study. Particuology 2022, 10, 10–21. [Google Scholar]
- Hassanzadeh, A.; Azizi, A.; Kouachi, S.; Karimi, M.; Celik, M.S. Estimation of flotation rate constant and particle-bubble interactions considering key hydrodynamic parameters and their interrelations. Miner. Eng. 2019, 141, 105836. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, L.; Yang, H.; Gui, X.; Schönherr, H.; Kappl, M.; Cao, Y.; Xing, Y. Recent advances for understanding the role of nanobubbles in particles flotation. Adv. Colloid Interface Sci. 2021, 291, 102403. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, A.A. Mineralization kinetics of air bubbles with coarse sphalerite particles in brackish solutions of sulfhydryl collectors. J. Min. Sci. 2021, 57, 1025–1032. [Google Scholar] [CrossRef]
- Samygin, V.D. Mineralization kinetics of air bubbles allowing for the particle detachment and time of buoying of aggregates. Russ. J. Non-Ferr. Met. 2016, 57, 389–394. [Google Scholar] [CrossRef]
- Nikolaev, A.A.; Petrova, A.A.; Goryachev, B.E. Pyrite grain and air bubble attachment kinetics in agitated pulp. J. Min. Sci. 2016, 52, 352–359. [Google Scholar] [CrossRef]
- Dai, Z.F.; Fornasiero, D.; Ralston, J. Particle–bubble collision models—A review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, Y.; Yan, X.; Wang, L.; Xu, Y. A study of bubble mineralization by modified glass microspheres based on a high-speed dynamic microscopic test system. Minerals 2019, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Nazari, M.; Kayhani, M.H.; Ahmadi, G. Experimental study and visualization of the particle-bubble collision process. J. Appl. Mech. Tech. Phys. 2022, 63, 903–913. [Google Scholar] [CrossRef]
- Arriagad, S.; Jaques, A.; Vinnett, L.; Acuña, C. A modified Yoon and Luttrell model for predicting the efficiency of particle-bubble collision. Powder Technol. Int. J. Sci. Technol. Wet Dry Part. Syst. 2020, 361, 990–994. [Google Scholar] [CrossRef]
- Yao, N.; Liu, J.; Sun, X.; Liu, Y.; Chen, S.; Wang, G. A rational interpretation of the role of turbulence in particle-bubble interactions. Minerals 2021, 11, 1006. [Google Scholar] [CrossRef]
- Brozek, M. Probability of particle-bubble collision in pneumo-mechanical flotation cell. Arch. Metall. Mater. 2010, 55, 293–304. [Google Scholar]
- Hassanzadeh, A.; Kouachi, S.; Hasanzadeh, M.; Çelik, M. A new insight to the role of bubble properties on inertial effect in particle–bubble interaction. J. Dispers. Sci. Technol. 2016, 38, 953–960. [Google Scholar] [CrossRef]
- Heindel, T.J. The fundamentals of flotation deinking. Atlanta Ga. Inst. 1999, 82, 23–36. [Google Scholar]

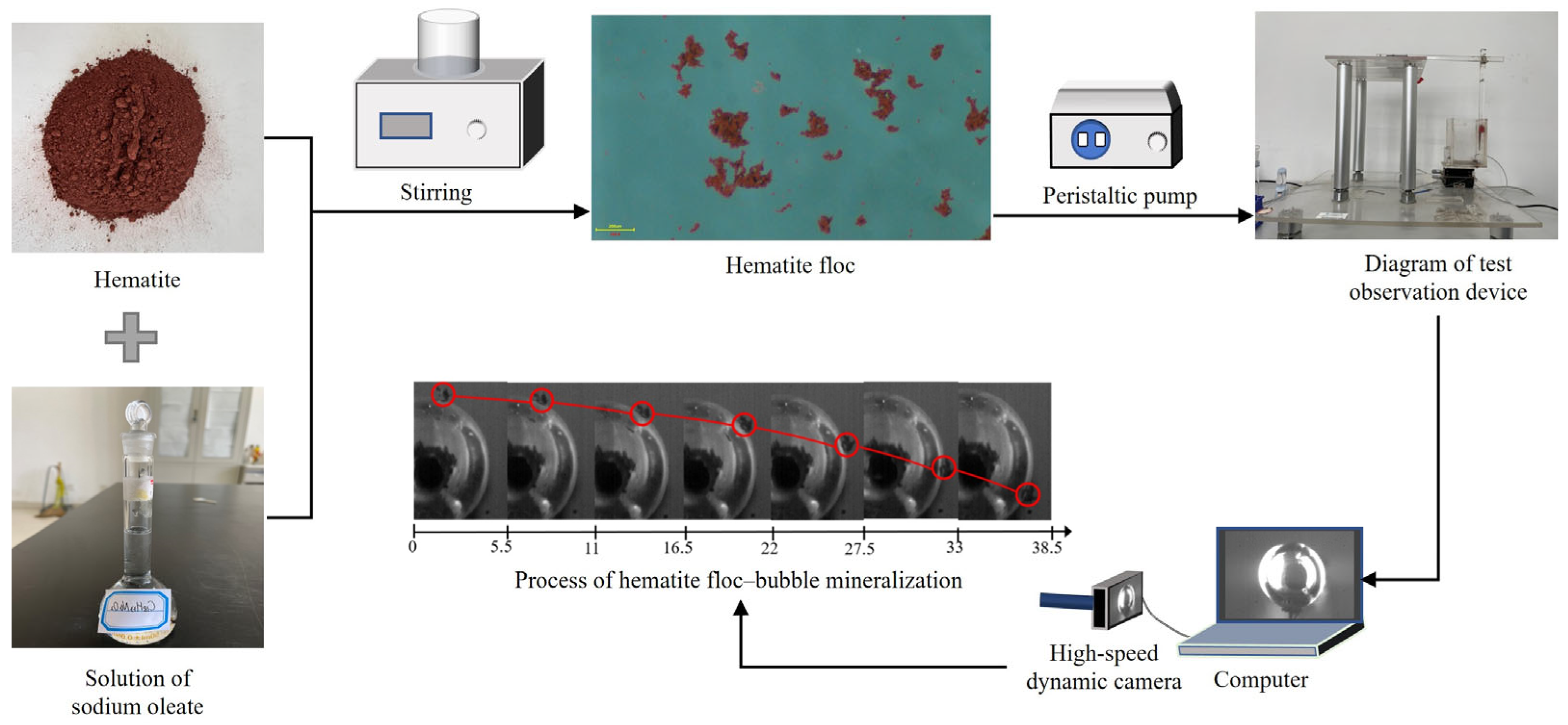


| Ingredients | Fe | Fe2O3 | K2O | MgO | Al2O3 | CaO | TiO2 |
|---|---|---|---|---|---|---|---|
| Content | 67.90 | 98.42 | 0.04 | 0.53 | 0.78 | 0.05 | 0.18 |
| Solution of Sodium Oleate/mg·L−1 | Particle Size/μm | Fractal Dimension | Aperture/nm | Contact Angle/° | Specific Surface Area/cm2·g−1 |
|---|---|---|---|---|---|
| 120 | 32.96 | 1.8283 | 19.979 | 80.21 | 3.500 |
| 140 | 34.80 | 1.8251 | 20.520 | 92.25 | 3.350 |
| 160 | 36.65 | 1.8239 | 21.090 | 116.31 | 3.269 |
| 180 | 38.48 | 1.8204 | 21.528 | 125.17 | 3.144 |
| 200 | 39.65 | 1.8172 | 21.716 | 126.63 | 3.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, F.; Chen, Y.; Zhang, J.; Chang, Z. High-Speed Dynamic Camera Analysis of the Hematite Floc–Bubble Mineralization Process. Minerals 2023, 13, 964. https://doi.org/10.3390/min13070964
Niu F, Chen Y, Zhang J, Chang Z. High-Speed Dynamic Camera Analysis of the Hematite Floc–Bubble Mineralization Process. Minerals. 2023; 13(7):964. https://doi.org/10.3390/min13070964
Chicago/Turabian StyleNiu, Fusheng, Yuying Chen, Jinxia Zhang, and Zhenjia Chang. 2023. "High-Speed Dynamic Camera Analysis of the Hematite Floc–Bubble Mineralization Process" Minerals 13, no. 7: 964. https://doi.org/10.3390/min13070964








