Timurid, Ottoman, Safavid and Qajar Ceramics: Raman and Composition Classification of the Different Types of Glaze and Pigments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artefacts
2.2. Techniques
3. Results
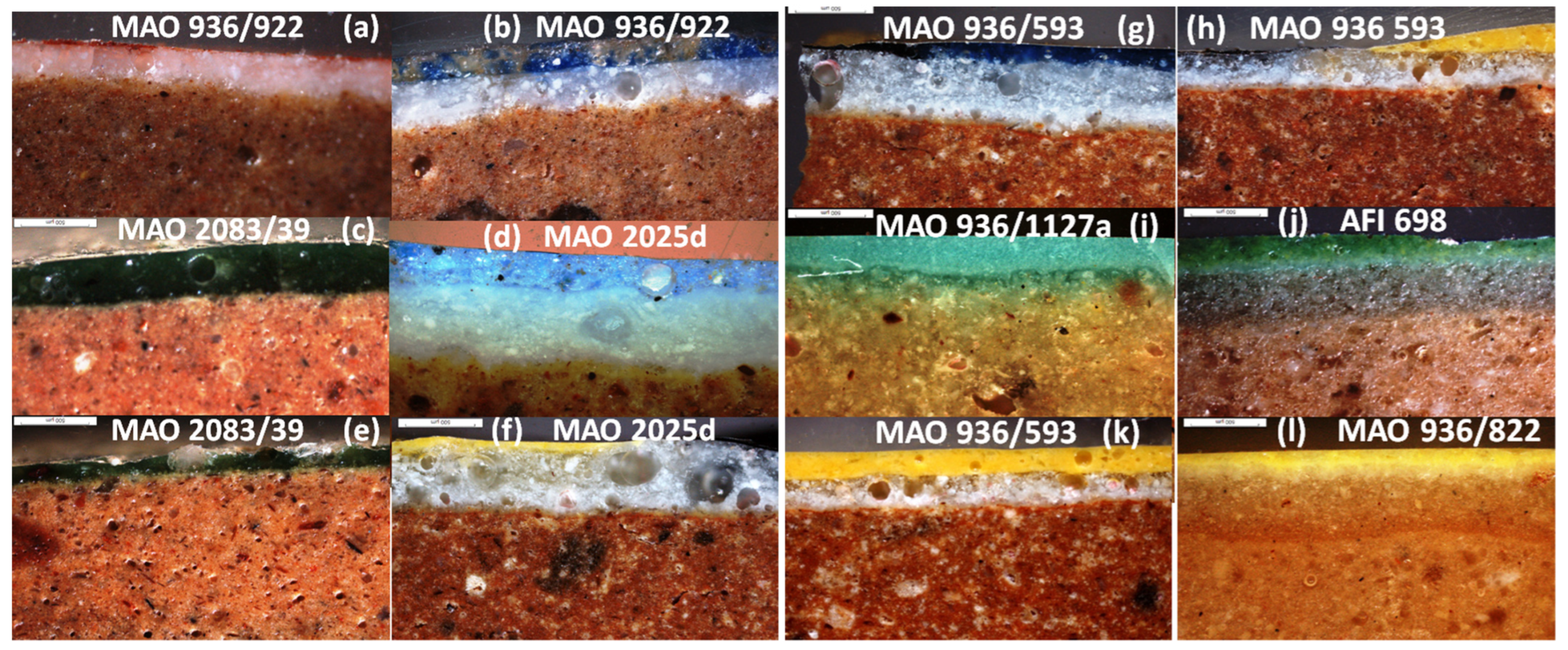
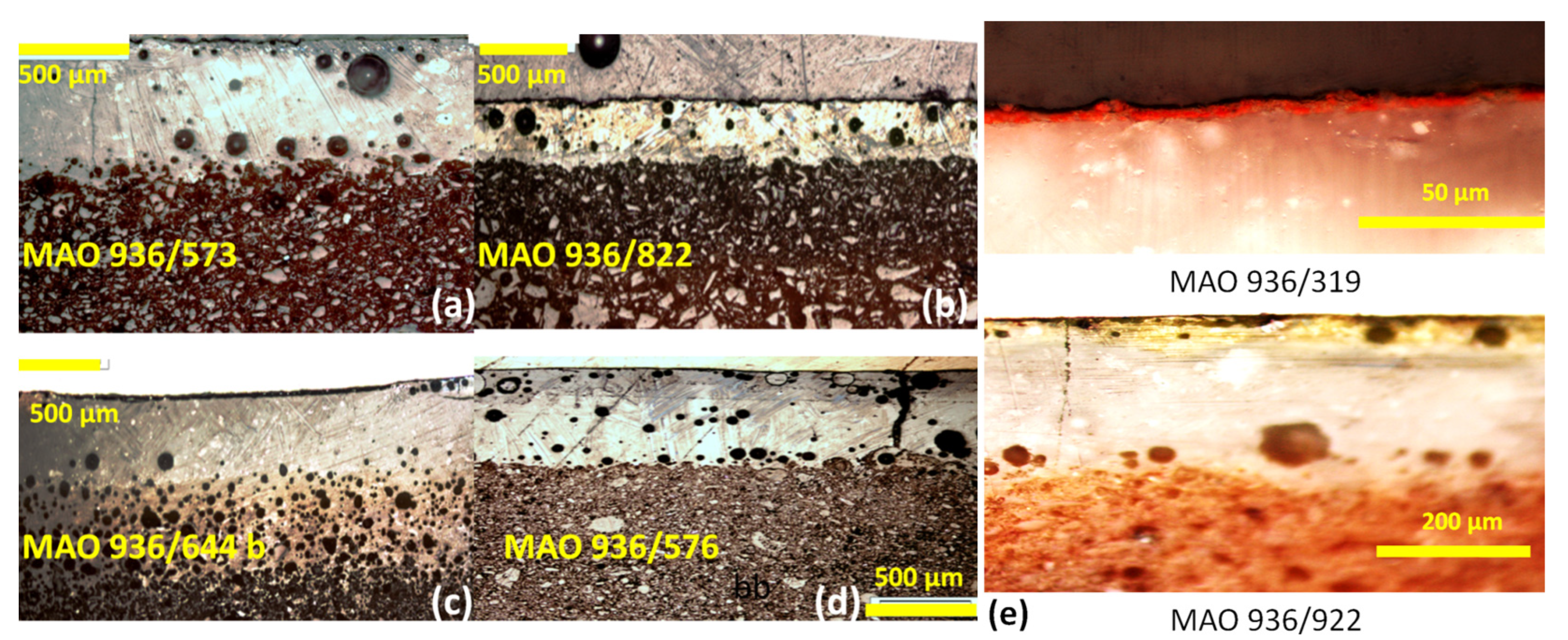
3.1. Microstructures and Enamelling Processes
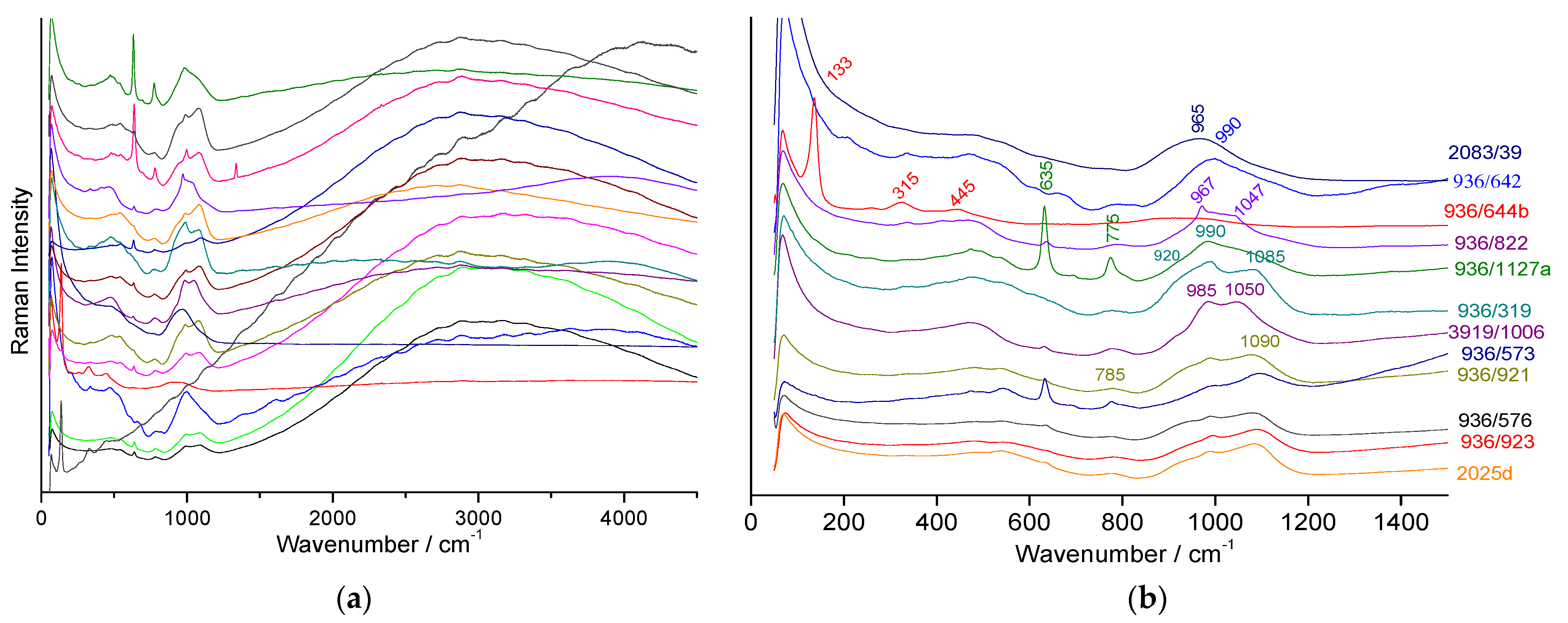
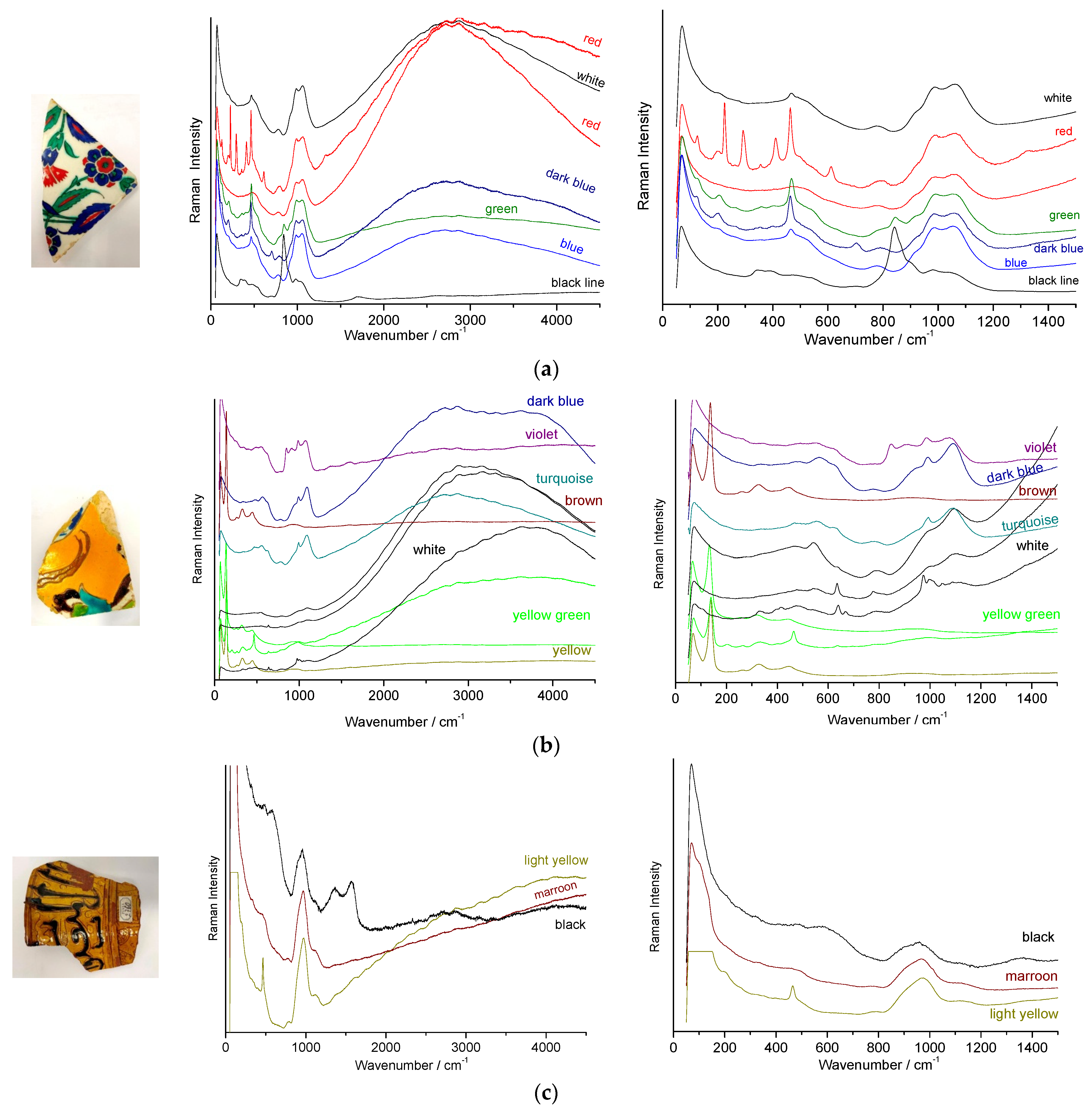
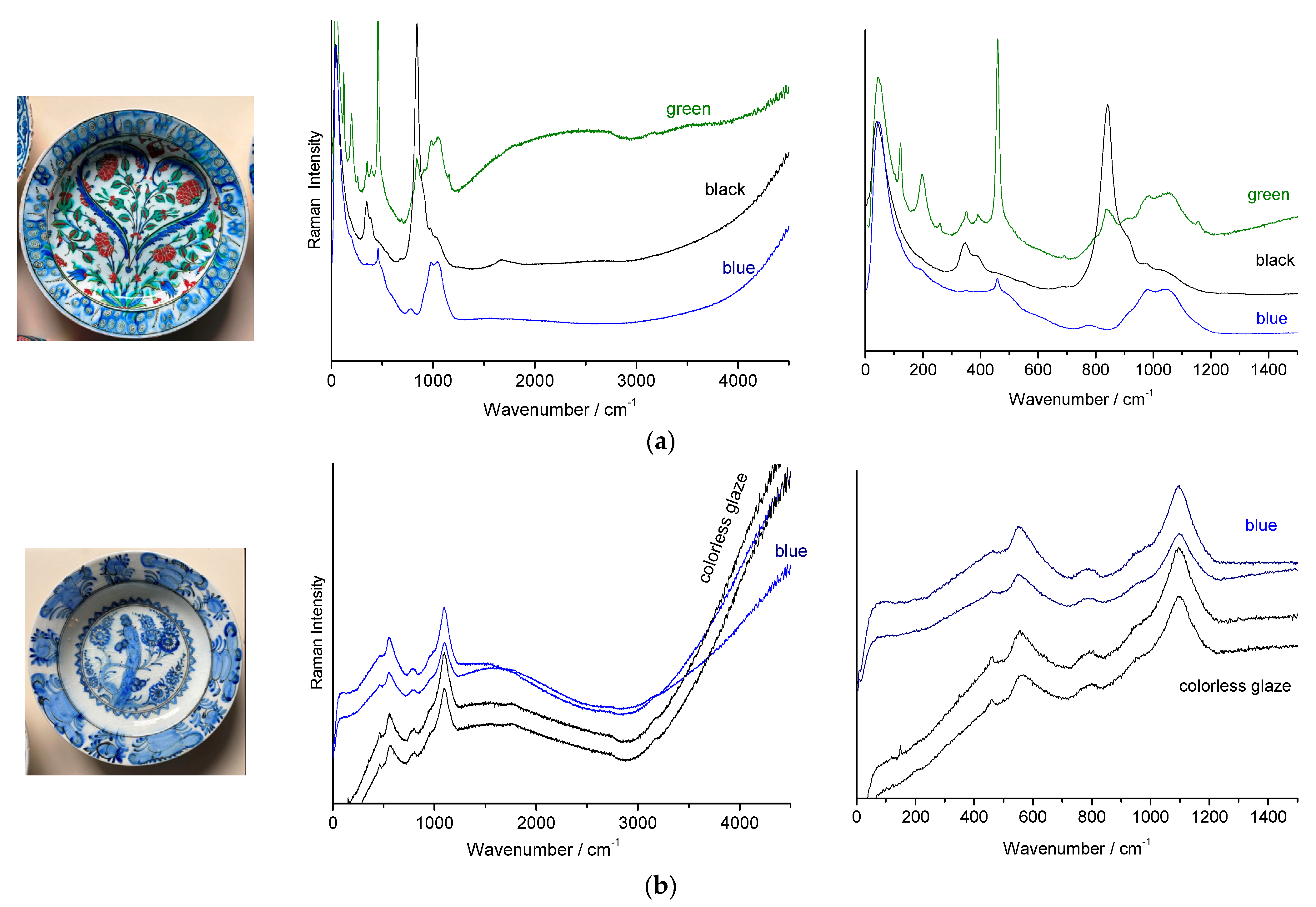
3.2. Raman Classification of Glaze
- (i)
- (ii)
- (iii)
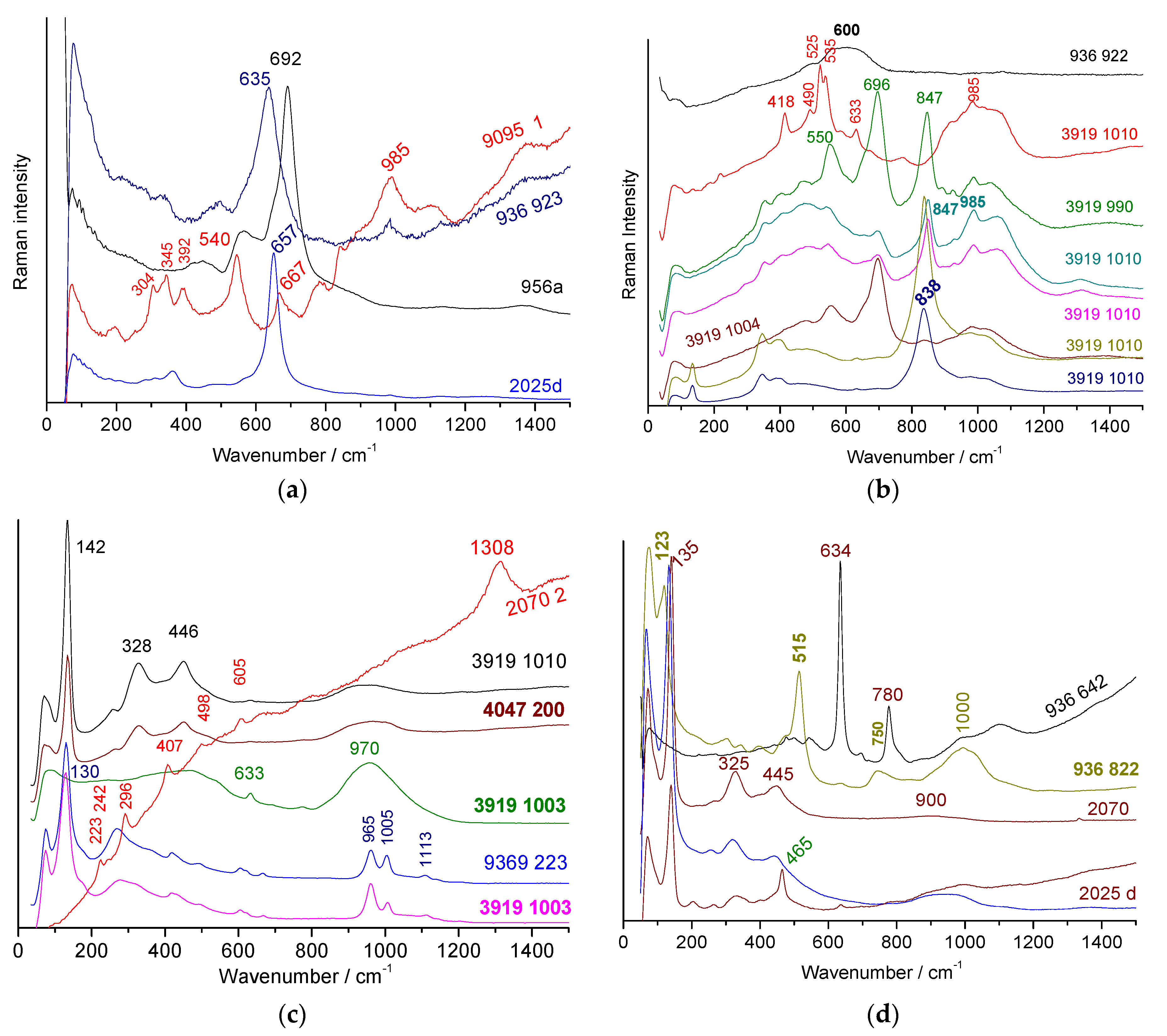
3.3. Identification of Opacifiers
3.4. Black Lines
- (i)
- the first is characterized by an intense peak between ~630 and 695 cm−1 typical of the spinel phase (AB2O4 cubic structure in which A and B atoms are transition metals) rich in iron and/or manganese [84,85,86,87,88,89,90]; the width of the peak, which can be asymmetrical as a function of the site occupancy in the spinel structure [84], can vary by a factor of 2 to 3 (~50 to 150 cm−1) due to ion substitution disorder and non-stoichiometry (oxygen vacancies). Since the main peak can be described as the A1g mode of a B-O stretching mode, its position is a rough indication of the composition: at higher wavenumber for a cobalt-rich (e.g., 690 cm−1), weaker for iron-rich spinel (~670 cm−1), even lower for spinels rich in manganese or nickel (~630 cm−1).
- (ii)
- (iii)
- the third is a very broad peak centred on 600 cm−1 (Figure 8a) which shows similarities with that of the matter rich in manganese oxide.
- (iv)
3.5. Yellow and Red Areas
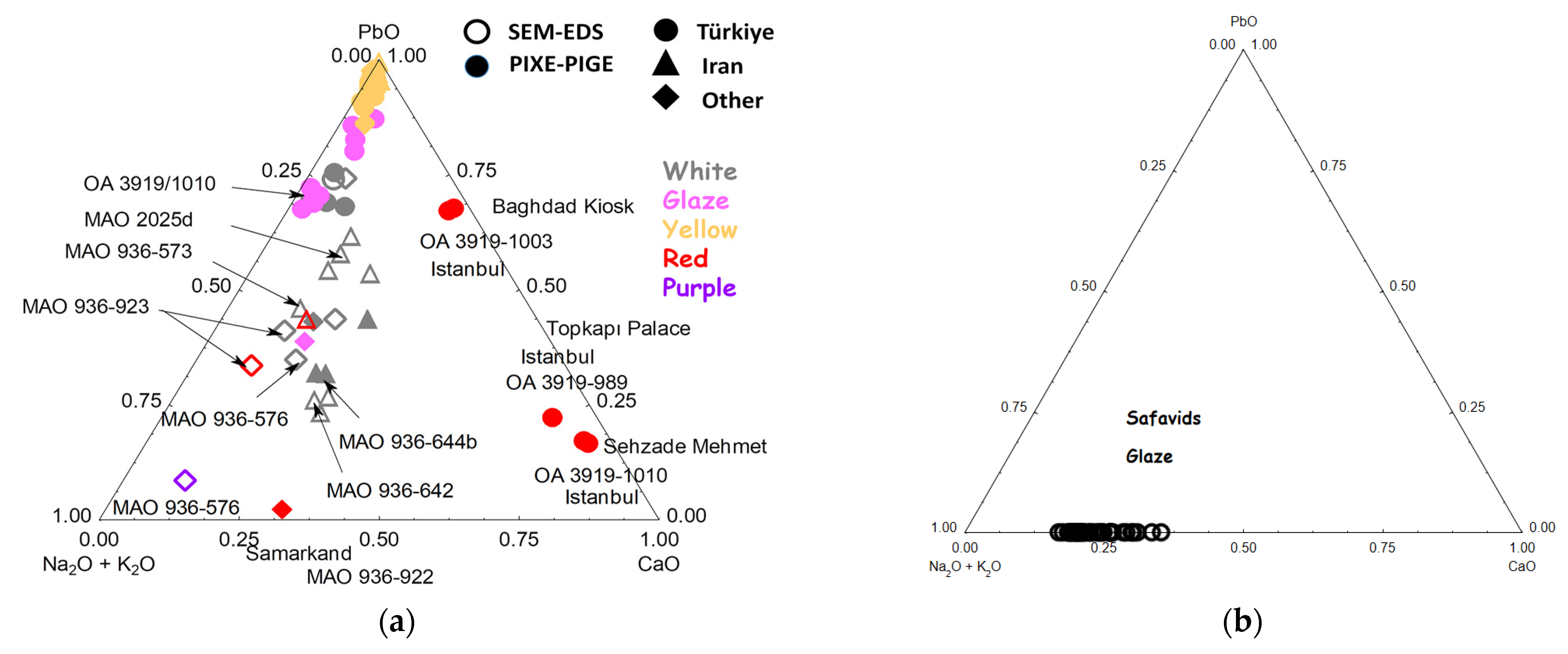
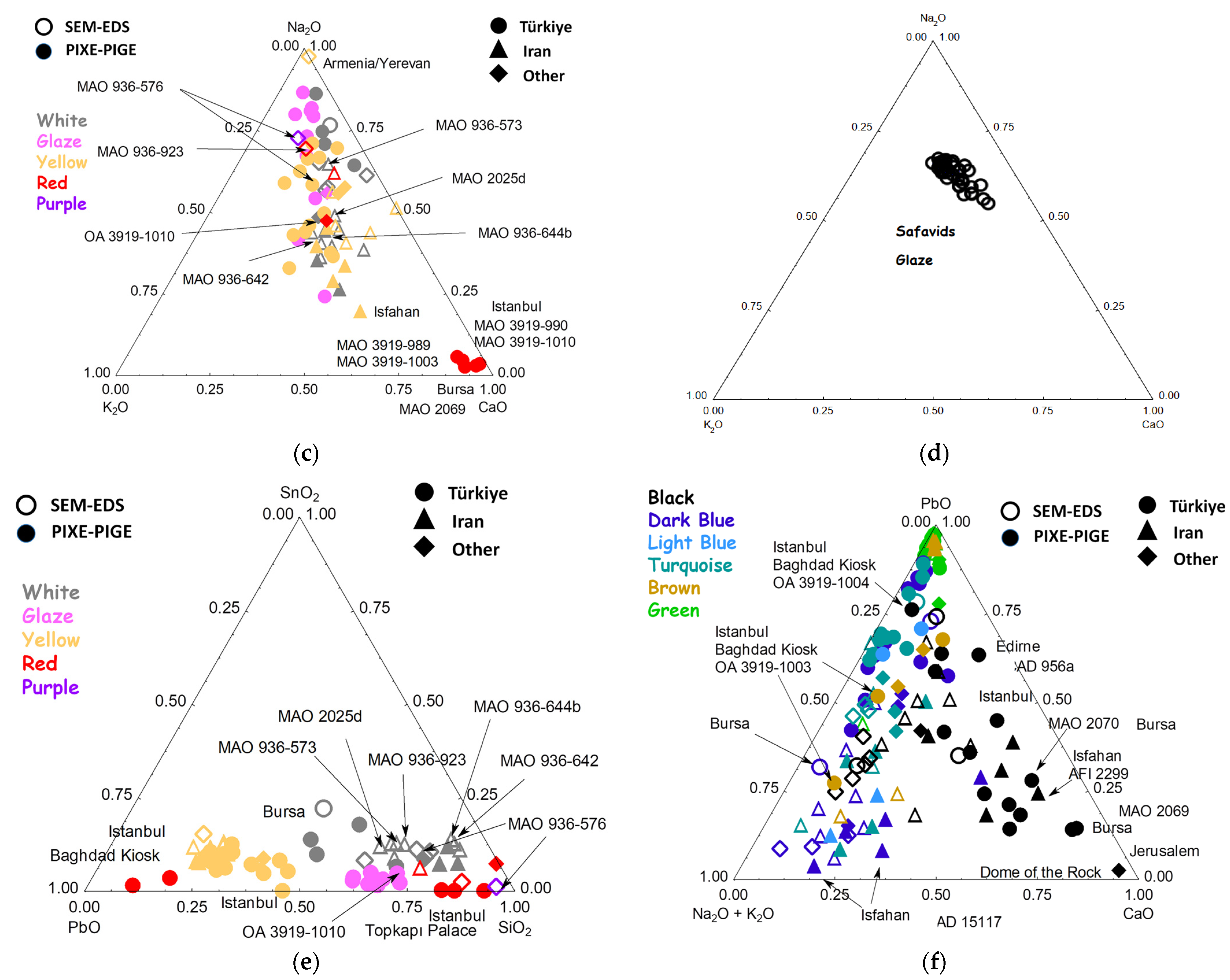
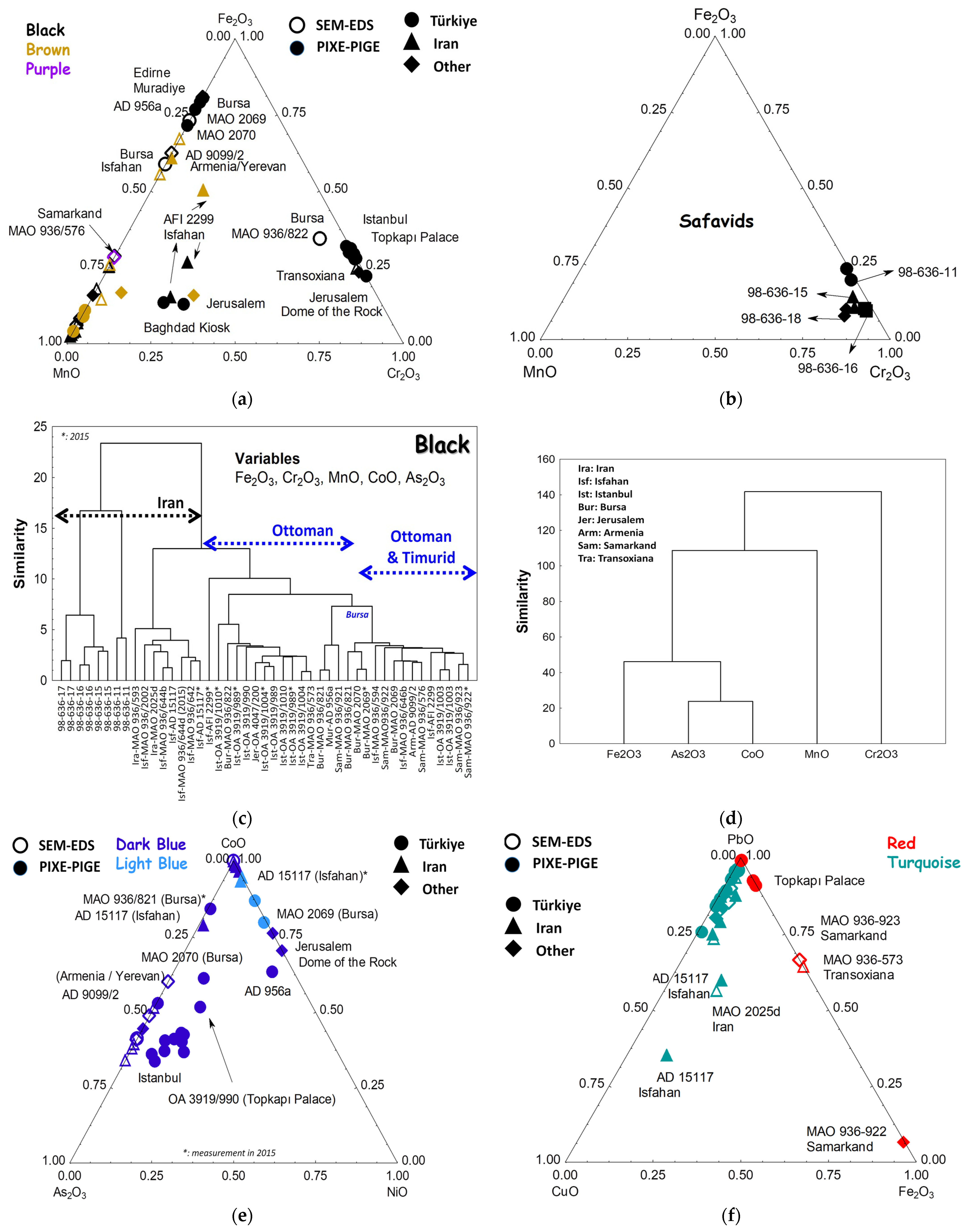
3.6. Elemental Composition Classification and Comparison with the Raman Data
3.6.1. Flux
3.6.2. Opacifiers
3.6.3. Black Lines
3.6.4. Blue
3.6.5. Yellow Pigment
3.7. Correlation between Raman Fingerprint and Raw Materials: Luminescence Contribution—Application to the Identification of Objects
3.8. Application to Tableware
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soustiel, J.; Porter, Y. Tombeaux de Paradis: Le Shâh-e Zende de Samarcande et la Céramique Architecturale d’Asie Centrale; Editions d’Art Monelle Hayot: Saint-Rémy-en-l’Eau, France, 2003. [Google Scholar]
- Soustiel, J. La Céramique Islamique—Le Guide du Connaisseur; Office du Livre; Editions Vilo: Fribourg, Switzerland, 1985. [Google Scholar]
- Yilmaz, G. Edirne’nin erken Osmanli devri yapilarinda çini süsleme. Trak. Üniversitesi Edeb. Fakültesi Derg. 2015, 5, 59–80. [Google Scholar]
- Golombek, L. Timurid potters abroad, N°2 La Civiltà Timuride come fenomeno internazionale vol II (Letteratura-Arte). Oriento Mod. 1996, 15, 577–586. [Google Scholar] [CrossRef]
- Grazhdankina, N.S.; Rakhimov, M.K.; Pletnev, I.E. Architectural Ceramics of Uzbekistan; Bureau de l’UNESCO: Tachkent, Uzbekistan, 2006; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000189335 (accessed on 4 June 2023).
- Aube, S.; Lorain, T.; Bendezu-Sarmiento, J. The Complex of Gawhar Shad in Herat: New Findings about its Architecture and Ceramic Tile Decorations. Iran 2020, 58, 62–83. [Google Scholar] [CrossRef]
- Ben Amara, A.; Schvoerer, M.; Haddad, M.; Akerraz, A. Recherche d’indices sur les techniques de fabrication de Zelliges du XIVe siècle (Chellah, Maroc). Rev. D’archéometrie 2003, 27, 103–113. [Google Scholar] [CrossRef]
- El Amraoui, M.; Azzou, A.; Haddad, M.; Bejjit, L.; Ait Lyazidi, S.; El Amraoui, Y. Zelliges from Dar-El Beïda Palace in Meknes (Morocco): Optical Absorption and Raman Spectrometry. Spectrosc. Lett. 2007, 40, 777–783. [Google Scholar] [CrossRef]
- Henni Ibtissem, M.; Khaldi, M. L’art du Zellige Dans L’architecture Musulmane. Cas des Mosquées Zianides de Tlemcen. 2018. Available online: https://e-biblio.univ-mosta.dz/bitstream/handle/123456789/11974/L%20art?sequence=1 (accessed on 4 May 2023).
- Pérez-Arantegui, J.; Soto, M.; Castillo, J.R. Examination of the ‘Cuerda Seca’ decoration technique on Islamic ceramics from Al-Andalus (Spain). J. Cult. Herit. 1999, 26, 935–941. [Google Scholar] [CrossRef]
- Chapoulié, R.; Delery, C.; Daniel, F.; Venrell-Saz, M. Cuerda Seca ceramics from Al-Andalus, Islamic Spain and Portugal (10th–12th centuries AD): Investigation with SEM-EDX and Cathodoluminescence. Archaeometry 2005, 47, 519–534. [Google Scholar] [CrossRef]
- Colomban, P.; March, G.; Mazerolles, L.; Karmous, T.; Ayed, N.; Ennabli, A.; Slim, H. Raman identification of materials used for jewellery and mosaics in Ifriqiya. J. Raman Spectrosc. 2003, 34, 205–213. [Google Scholar] [CrossRef]
- Ricciardi, P.; Colomban, P.; Tournié, A.; Macchiarola, M.; Ayed, N. A non-invasive study of Roman age mosaic glass tesserae by means of Raman Spectroscopy. J. Archaeol. Sci. 2009, 36, 2551–2559. [Google Scholar] [CrossRef]
- Neri, E.; Morvan, C.; Colomban, P.; Guerra, M.P.; Prigent, V. Late Roman and Byzantine Mosaic opaque “Glass-ceramics” Tesserae (5th–9th century). Ceram. Int. 2016, 42, 18859–18869. [Google Scholar] [CrossRef] [Green Version]
- El Halim, M.; Daoudi, L.; El Alaoui El Fels, A.; Rebbouh, L.; El Ouahabi, M.; Fagel, N. Non-destructive portable X-ray Fluorescence (pXRF) method for the characterization of Islamic architectural ceramic: Example of Saadian tombs and El Badi palace ceramics (Marrakech, Morocco). J. Archaeol. Sci. Rep. 2020, 32, 102422. [Google Scholar] [CrossRef]
- Kiefer, C. Les Céramiques siliceuses d’Anatolie et du Moyen-Orient. Bull. Soc. Fr. Ceram. 1956, 30, 3–24. [Google Scholar]
- Kiefer, C. Les Céramiques siliceuses d’Anatolie et du Moyen-Orient. Bull. Soc. Fr. Ceram. 1956, 31, 17–34. [Google Scholar]
- Kiefer, C. Supplement Materials in La Céramique Islamique—Le Guide du Connaisseur; J. Soustiel Office du Livre; Editions Vilo: Fribourg, Switzerland, 1985; pp. 368–380. [Google Scholar]
- Tite, M.S. Iznik pottery: An investigation of the method of production. Archaeometry 1989, 31, 115–132. [Google Scholar] [CrossRef]
- Henderson, J.; Raby, J. The Technology of Fifteenth Century Turkish Tiles: An Interim Statement on the Origins of the Iznik Industry; World Archaeol. 21 (1) Ceramic Technology; Taylor & Francis, Ltd.: London, UK, 1989; pp. 115–132. [Google Scholar]
- Henderson, J.; Atasoy, N.; Raby, J. Iznik, ceramics: A technical examination. In Iznik: The Pottery of Ottoman Turkey; Petsopoulos, Y., Ed.; Alexandria Press: London, UK, 1989; pp. 65–70. [Google Scholar]
- Atasoy, N.; Raby, J. Part 2: 1480–1560: Development and growth of Iznik pottery. In Iznik: The Pottery of Ottoman Turkey; Petsopoulos, Y., Ed.; Alexandria Press: London, UK, 1989; pp. 50–64. [Google Scholar]
- Necipoglu, S. From International Timurid to Ottoman: A Change of Taste in Sixteenth-Century Ceramic Tiles. Muqarnas 1990, 7, 136–170. Available online: https://www.academia.edu/42017931/From_International_Timurid_to_Ottoman_A_Change_of_Taste_in_Sixteenth–Century_Ceramic_Tiles (accessed on 25 May 2023). [CrossRef]
- Okyar, F. Characterization of Iznik Ceramics. Ph.D. Thesis, Istanbul Technical University, Istanbul, Turkey, 1995. [Google Scholar]
- Paynter, S.; Okyar, F.; Wolf, S.; Tite, M.S. The production of Iznik pottery—A reassessment. Archaeometry 2004, 46, 421–437. [Google Scholar] [CrossRef]
- Denny, W.B. Iznik La Ceramique Turque et l’Art Ottoman; Citadelles and Mazenod: Paris, France, 2004. [Google Scholar]
- Tite, M.S.; Wolf, S.; Mason, R.B. The technological development of stonepaste ceramics from the Islamic Middle East. J. Archaeol. Sci. 2011, 38, 570–580. [Google Scholar] [CrossRef]
- Simsek, G. Characterization of Structure of Alkali and Lead-Alkali Glazes by Raman Spectrometry. Ph.D. Thesis, Istanbul Technical University, Istanbul, Turkey, 2011. [Google Scholar]
- Colomban, P.; Milande, V.; Le Bihan, L. On-site Raman analysis of Iznik pottery glazes and pigments. J. Raman Spectrosc. 2004, 35, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Demirci, E.N.; Caner-Saltik, A.; Turkmenoglu, S.; Ozcilingir-Arkun, O.; Bakirer, O. Raw material characteristics and technological properties of some glazed ceramics and tiles in Anatolia, Euro Ceramics VIII, PTS 1-3. Key Eng. Mater. 2004, 264–268, 2395–2398. [Google Scholar] [CrossRef]
- Colomban, P.; de Laveaucoupet, R.; Milande, V. On Site Raman Analysis of Kütahya fritwares. J. Raman Spectrosc. 2005, 36, 857–863. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Milande, V. Tentative differentiation between Iznik tiles and copies with Raman spectroscopy using both laboratory and portable instrument. J. Raman Spectrosc. 2010, 41, 529–536. [Google Scholar] [CrossRef]
- O’Kane, B. Tiles of many hues: The development of Iranian Cuerda seca Tiles and the Transfer of Tilework Technology-Appendix. In And Diverse Are Their Hues: Color in Islamic Art and Culture; Blair, S.S., Bloom, J.M., Eds.; Yale University Press: New Haven, CT, USA, 2011; pp. 201–203. [Google Scholar]
- Mahi, K. Les «Maitres de Tabriz», Céramistes dans L’empire Ottoman: Une mise au Point sur leur Identification. Eurasian Stud. 2017, 15, 36–79. Available online: https://brill.com/view/journals/eurs/15/1/article-p36_36.xml (accessed on 25 May 2023). [CrossRef]
- Holakkoei, P. Technological Study of the Seventeenth Century Haft Rang Tiles in Iran with a Comparative Views to the Cuerda Seca in Spain. Ph.D. Thesis, Università Degli Studi di Ferrara, Ferrara, Italy, 2012. [Google Scholar]
- Simsek, G.; Geckinli, A.E. An assessment study of tiles from Topkapi Palace Museum with energy-dispersive X-ray and Raman spectrometers. J. Raman Spectrosc. 2012, 43, 917–927. [Google Scholar] [CrossRef]
- Caner Saltık, E.N.; Colomban, P.; Soulet, V.; Demirci, S.; Türkmenoğlu, A.; Özçilingir Akgün, S.; Bakırer, Ö. Analyses of Anatolian medieval ceramic glazes using XRD and non-destructive Raman micro-spectrometry. In Proceedings of the Colloque du GMPCA, Archaeometrie 2013, Bordeaux, France, 16–19 April 2013. [Google Scholar]
- Gulmini, M.; Giannini, R.; Lega, A.M.; Mirti, P. Technology of production of Ghazhanid glazed pottery from Bust and Lashkar-I Bazar (Afghanistan). Archaeometry 2013, 55, 569–590. [Google Scholar] [CrossRef]
- Özçatal, M.; Yaygıngöl, M.; Issi, A.; Kara, A.; Turan, S.; Okyar, F.; Taş, P.; Nastova, I.; Grupče, O.; Minčeva-Šukarova, B. Characterization of lead glazed potteries from Smyrna (Izmir/Turkey) using multiple analytical techniques; Part I: Glaze and engobe. Ceram. Int. 2014, 40, 2143–2151. [Google Scholar] [CrossRef]
- Constantinescu, B.; Cristea-Stan, D.; Kovács, I.; Szökefalvi-Nagy, Z. External milli-beam PIXE analysis of the mineral pigments of glazed Iznik (Turkey) ceramics. Period. Mineral. 2014, 83, 159–169. [Google Scholar]
- Kirmizi, B.; Göktürk, E.H.; Colomban, P. Colouring agents in the pottery glazes of Western Anatolia: New evidence for the use of Naples yellow pigment variations during the late Byzantine period. Archaeometry 2015, 57, 476–496. [Google Scholar] [CrossRef]
- Tite, M.; Watson, O.; Pradell, T.; Matin, M.; Molina, G.; Domoney, K.; Bouquillon, A. Revisiting the beginnings of tin-opacified Islamic glazes. J. Archaeol. Sci. 2015, 57, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Tite, M.S.; Shortland, A.J.; Schibille, N.; Degryse, P. New Data on the Soda Flux Used in the Production of Iznik Glazes and Byzantine Glasses. Archaeometry 2016, 58, 57–67. [Google Scholar] [CrossRef]
- Wen, R.; Pollard, A.M. The pigments applied to Islamic Mina’i wares and the correlation with Chinese blue-and-white porcelain. Archaeometry 2016, 58, 1–16. [Google Scholar] [CrossRef]
- Olcer, S. 15th and 16th centuries blue-white ceramics: Comparison of Ottoman, Safavid and Chinese Samples in the style context. STD XXVII 2018, 27, 265–301. (In Turkish) [Google Scholar]
- Simsek, G.; Unsalan, O.; Bayraktar, K.; Colomban, P. On-site pXRF analysis of glaze composition and colouring agents of “Iznik” tiles at Edirne mosque (15th and 16th-centuries). Ceram. Int. 2019, 45, 595–605. [Google Scholar] [CrossRef]
- Simsek, G.; Demirsar Arli, B.; Kaya, S.; Colomban, P. On-site pXRF analysis of body, glaze and colouring agents of the tiles at excavation site of Iznik kilns. J. Eur. Ceram. Soc. 2019, 39, 2199–2209. [Google Scholar] [CrossRef]
- Burlot, J.; Waksman, S.Y.; MacDonald, B.; Higgins, B. LA-ICP-MS analyses to determine provenances of Na-based fluxes and Co-based pigments used in early Ottoman pottery. In Proceedings of the 43rd Symposium International d’Archéométrie (ISA), Lisbonne, Portugal, 16–20 May 2022. [Google Scholar]
- Edwards, H.G.M.; Vandenabeele, P.; Colomban, P. Raman Spectroscopy of Cultural Heritage Preservation; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Colomban, P.; Simsek Franci, G.; Burlot, J.; Gallet, X.; Zhao, B.; Clais, J.B. Non-Invasive on-Site pXRF Analysis of Coloring Agents, Marks and Enamels of Qing Imperial and Non-Imperial Porcelain. Ceramics 2023, 6, 447–474. [Google Scholar] [CrossRef]
- Colomban, P.; Gironda, M.; Edwards, H.G.M.; Mesqui, V. The enamels of the first (softpaste) European blue-and-white porcelains: Rouen, Saint-Cloud and Paris factories: Complementarity of Raman and X-ray fluorescence analyses with mobile instruments to identify the cobalt ore. J. Raman Spectrosc. 2021, 52, 2246–2261. [Google Scholar] [CrossRef]
- Colomban, P.; Ngo, A.-T.; Fournery, N. Non-invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Technology. Heritage 2022, 5, 233–259. [Google Scholar] [CrossRef]
- Colomban, P. Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis. Materials 2022, 15, 3158. [Google Scholar] [CrossRef]
- Mysen, B.; Virgo, D.; Scarfe, C.M. Relations between the anionic structure and viscosity of silicate melts—A Raman spectroscopic study. Am. Mineral. 1980, 65, 690–710. [Google Scholar]
- Mysen, B.; Finger, L.W.; Virgo, D.; Seifert, F.A. Curve-fitting of Raman-spectra of silicate-glasses. Am. Mineral. 1982, 67, 686–695. [Google Scholar]
- McMillan, P. Structural studies of silicate-glasses and melts—Applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Colomban, P. Polymerisation Degree and Raman Identification of Ancient Glasses used for Jewellery, Ceramics Enamels and Mosaics. J. Non-Crystall. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Colomban, P.; Paulsen, O. Raman Determination of the Structure and Composition of Glazes. J. Am. Ceram. Soc. 2005, 88, 390–395. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramics, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. Non-destructive Raman analysis of ancient glasses and glazes, ch. 4.2. In Modern Methods for Analysing Archaeological and Historical Glass, 1st ed.; Janssens, K., Ed.; John Wiley and Sons Ltd.: London, UK, 2012; pp. 275–300. [Google Scholar]
- Jacquemin, M.; Simon, P.; Canizares, A.; Hennet, L.; Bessada, C.; Skrelic, D.; Gouillart, E.; Burov, E. High-sensitivity Raman imaging of the surface of casted glass plates. J. Raman Spectrosc. 2021, 52, 1048–1054. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Casadio, F.; Bellot-Gurlet, L.; Faber, K.; Zelleke, G.; Milande, V.; Tilliard, L. On-site identification of early Böttger red stonewares using portable XRF/Raman instruments: 2, glaze and gilding analysis. J. Am. Ceram.Soc. 2015, 98, 3006–3013. [Google Scholar] [CrossRef] [Green Version]
- Martinet, L.; Ben Amara, A.; Pacheco, C.; Lemasson, Q.; Moignard, B.; Pichon, L.; Colomban, P. Colored Glaze Tiles during the Ottoman Empire (beginning of the 15th to the/mid 16th century)? In Proceedings of the EMAC 2015 Proceeding 13th European Meeting on Ancient Ceramics, Athens, Greece, 24–26 September 2015. [Google Scholar]
- Martinet, L. “Cloisonné” Colored Glaze Tiles during the Ottoman Era (Fifteenth-Sixteenth Centuries). In Glazed Wares, as Cultural Agents in the Byzantine, Seljuk, and Ottoman Lands, Proceedings of the 13th International Anamed Annual Symposium, 6–7 December 2018; Kontogiannis, N.D., Böhlendorf-Arslan, B., Yenisehirlioglu, F., Eds.; Koç University Press: Istanbul, Turkiye, 2021; pp. 257–267. [Google Scholar]
- Martinet, L. Colored Glaze Tiles during the Ottoman Era (Fifteenth-Sixteenth Centuries, EHESS, Paris, France, in Redaction). Ph.D. Thesis, Universite DE Versailles Saint-Quentin-en-Yvelines, Paris, France, 2015. [Google Scholar]
- Kamura, S.; Tani, T.; Matsuo, H.; Onaka, Y.; Fujisawa, T.; Unno, M. New Probe for Porcelain Glazes by Luminescence at Near-Infrared Excitation. ACS Omega 2021, 6, 7829–7833. [Google Scholar] [CrossRef]
- Available online: https://collections.louvre.fr; (accessed on 20 March 2023).
- Munier, P. Technologie des Faïences; Gauthier-Villars: Paris, France, 1957. [Google Scholar]
- Colomban, P.; Calligaro, T.; Vibert-Guigue, C.; Nguyen, Q.L.; Edwards, H.G.M. Accrochage des dorures sur les céramiques et tesselles anciennes. Rev. D’archéométrie 2006, 29, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Sagon, G.; Louhichi, A.; Binous, H.; Ayed, N. Identification par microscopie Raman des tessons et pigments de glaçures de céramiques de l’Ifriqiya (Dougga, XI-XVIIIe siècles). Rev. D’archéométrie 2001, 25, 101–112. [Google Scholar] [CrossRef]
- Tanevska, V.; Colomban, P.; Minceva-Sukarova, B.; Grupce, O. Study of pottery from the Republic of Macedonia I: Raman analysis of Byzantine glazed pottery excavated from Prilep and Skopje (12th–14th century). J. Raman Spectrosc. 2009, 40, 1240–1248. [Google Scholar] [CrossRef]
- Matin, M.; Gholamnejad, M.; Abkenar, A.N. ‘We must send you a sample’—A Persian–European dialogue: Insights into late nineteenth-century ceramic technology based on chemical analysis of tiles from the Ettehadieh house complex, Tehran, Iran. R. S. Note Rec. 2021, 75, 5–37. [Google Scholar] [CrossRef]
- Crowe, Y. Persia and China Safavid Blue and White Ceramics in the Victoria & Albert Museum 1501–1738; Thames & Hudson Ltd.: London, UK; La Borie, France; Geneva, Switzerland, 2002; pp. 290–304. [Google Scholar]
- Maggetti, M.; d’Albis, A. Phase and compositional analysis of a Sèvres soft paste porcelain plate from 1781, with a review of early porcelain techniques. Eur. J. Mineral. 2017, 29, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Robert, I.; Roche, C.; Sagon, G.; Milande, V. Identification des porcelaines “tendres” du 18ème siècle par spectroscopie Raman: Saint-Cloud, Chantilly, Mennecy et Vincennes/Sèvres. Rev. D’archéométrie 2004, 28, 153–167. [Google Scholar] [CrossRef]
- Liem, N.Q.; Thanh, N.T.; Colomban, P. Reliability of Raman Microspectrometry in Analysis of Ancient Ceramics: The case of Ancient Vietnamese Porcelains and Celadon Glazes. J. Raman Spectrosc. 2002, 33, 287–294. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Neuroth, M.; Geisler, T. In situ Raman imaging of high-temperature solid-state reactions in the CaSO4–SiO2 system. Int. J. Coal Sci. Technol. 2019, 6, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Pereira, M.; de Lacerda-Aroso, T.; Gomes, M.J.M.; Mata, A.; Alves, L.C.; Colomban, P. Ancient Portuguese ceramic wall tiles (“Azulejos”): Characterization of the glaze and ceramic pigments. J. Nano Res. 2009, 8, 79–88. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial Yellow Pigments: Production and Characterization through Spectroscopic Methods of Analysis. E-Preserv. Sci. 2010, 7, 108–115. Available online: http://www.morana-rtd.com/e-preservationscience/2010/Pelosi-10-05-2010.pdf (accessed on 25 May 2023).
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Cvejic, Z.; Rakic, S.; Kremenovic, A.; Antic, B.; Jovalekic, C.; Colomban, P. Nanosize ferrites obtained by ball milling. Crystal structure, cation distribution, size-strain analysis and Raman investigations. Solid State Sci. 2006, 8, 908–915. [Google Scholar] [CrossRef]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Colomban, P.; Jullian, S.; Parlier, M.; Monge-Cadet, P. Identification of the high-temperature impact/friction of aeroengine blades and cases by micro Raman spectroscopy. Aerosp. Sci. Technol. 1999, 3, 447–459. [Google Scholar] [CrossRef]
- Pinto, A.; Sciau, P.; Zhu, T.; Zhao, B.; Groenen, J. Raman study of Ming porcelain dark spots: Probing Mn-rich spinels. J. Raman Spectrosc. 2019, 50, 711–719. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Simsek Franci, G. Cobalt and associated impurities in blue (and green) glass, glaze and enamel: Relationships between raw materials, processing, composition, phases and international trade. Minerals 2021, 11, 633. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Wang, F.; Hole, C.; Cao, J.; Zhu, J.; Zhang, P.; Shi, P.; Sciau, P. Raman study of rusty oil spotted glaze produced in Linfen kilns (Shanxi province, AD 1115–1368). J. Raman Spectrosc. 2020, 53, 582–592. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Burlot, J.; Gallet, X.; Franci, G.S.; Bellot-Gurlet, L.; Colomban, P. Non-Invasive On-Site pXRF Analysis of Coloring Agents of Under- and Over-Glazes: Variability and Representativity of Measurements on Porcelain. Colorants 2023, 2, 42–57. [Google Scholar] [CrossRef]
- Porter, Y. Textes persans sur la céramique. In La Science dans le Monde Iranien; Vesel, Z., Ed.; Institut Français de Recherche en Iran: Teheran, Iran, 1998; pp. 165–189. [Google Scholar]
- Holakooei, P.; Mishmastnehi, M.; Arani, A.M.; Röhrs, S.; Franke, U. Materials and technique of lajvardina ceramics from the thirteenth to fourteenth century Iran. Archaeol. Anthtr. Sci. 2023, 15, 33. [Google Scholar] [CrossRef]
- Porter, Y. Le Cobalt dans le Monde Iranien (IXe-XVIe Siècles). Taoci 2000, 1, 5–14. Available online: https://www.academia.edu/12424228/Le_cobalt_dans_le_monde_iranien_IXe_XVIe_si%C3%A8cles_Notes_sur_son_utilisation_en_c%C3%A9ramique_et_son_commerce_TAOCI_1_2000_pp_5_14 (accessed on 25 May 2023).
- Matin, M.; Pollard, A.M. From ore to pigment: A description of the minerals and experimental study of cobalt ore processing from the Kâshân mine, Iran. Archaeometry 2017, 59, 731–746. [Google Scholar] [CrossRef]
- Matin, M.; Pollard, A.M. Historical accounts of cobalt ore processing from the Kashan mine, Iran. Iran 2015, 53, 171–183. [Google Scholar] [CrossRef]
- Allan, J.W. Abu’l-Qasim’s treatise on ceramics. Iran 1973, 11, 111–120. [Google Scholar] [CrossRef]
- Holakooei, P. A medieval Persian treatise on coloured and enamelled glass: Bayan Al-Sana’at. Iran 2016, 54, 95–106. [Google Scholar] [CrossRef]
- Slodczyk, A.; Colomban, P.; Willemin, S.; Lacroix, O.; Sala, B. Indirect Raman identification of the proton insertion in the high-temperature [Ba/Sr][Zr/Ti]O3-modified perovskite protonic conductors. J. Raman Spectrosc. 2009, 40, 513–521. [Google Scholar] [CrossRef]
- Hagemann, H.; Ayoubipour, S.; Delgado, T.; Schnyder, C.; Gnos, E. Probing luminescence of rare earth ions in natural fluorites using Raman microscopes: Comparison of some rose (pink) fluorites. J. Raman Spectrosc. 2022, 53, 1464–1470. [Google Scholar] [CrossRef]
- El Marraki, A.; Schvoerer, M.; Bechtel, F. Luminescence de cristaux de dévitrivication de wollastonite dans des verres non dopés et dopés en Eu, Dy et Tm. Phys. Status Solidi A 2000, 181, 491–507. [Google Scholar] [CrossRef]
- Colomban, P.; Milande, V.; Lucas, H. On-site Raman analysis of Medici porcelain. J. Raman Spectrosc. 2004, 35, 68–72. [Google Scholar] [CrossRef]
- Colomban, P. Raman µSpectrometry, A unique tool for on-site analysis and identification of ancient ceramics and glasses. In Materials Issues in Art and Archaeology, Vandiver, P., Mass, J., Murray, A., Eds. Mater. Res. Soc. Symp. Proc. 2005, 852, OO8.3. [Google Scholar]
- Ruvalcaba Cornejo, C. Luminescence in Rare Earth Ion-Doped Oxide Compounds. In Luminescence—An Outlook on the Phenomena and Their Applications; Thirumalai, J., Ed.; IntechOpen Book Series: London, UK, 2016; Available online: https://www.intechopen.com/chapters/52672 (accessed on 20 January 2022).
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ions in Crystals; Wiley Interscience: New York, NY, USA, 1968. [Google Scholar]
- Artal-Isbrand, P.; Newman, R. An examination of contemporary Iznik-style Turkish ceramics. Mater. Res. Soc. 1997, 462, 229–238. [Google Scholar] [CrossRef]
- Röhrs, S.; Dumazet, A.; Kuntz, K.; Franke, U. Bodies and Glazes of Architectural Ceramics from the Ilkhanid Period at Takht-e Soleyman (North-Western Iran). Minerals 2022, 12, 158. [Google Scholar] [CrossRef]

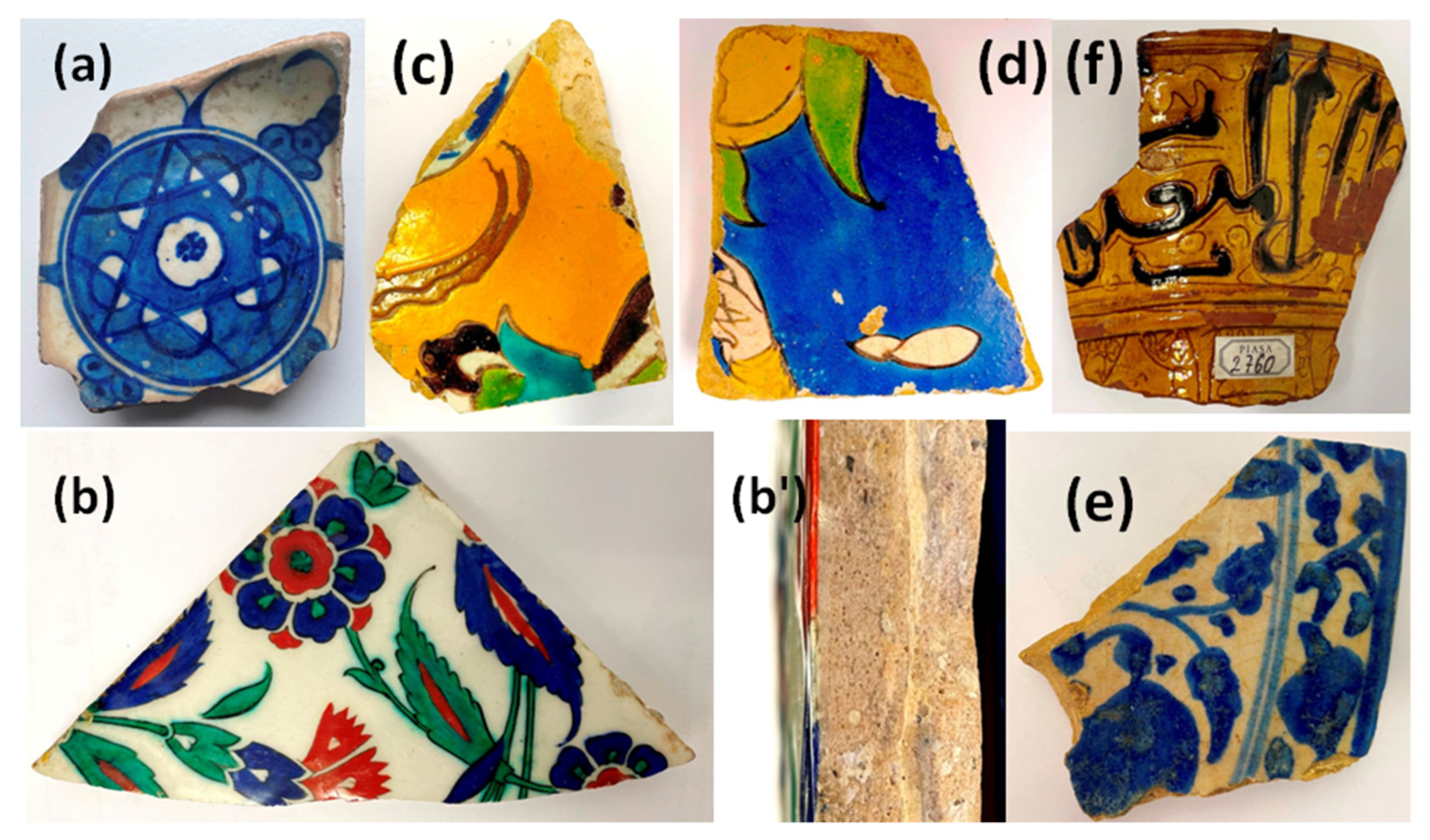





| Country, “Place” (Building) | View | Assumed Period /Century | Inventory Number [Dimensions/cm] | Previous Collection (Date of Entry) |
|---|---|---|---|---|
| al-Andalus (Spain) |  * * | 10th–11th Vessel shard | MAO 2083/39 | Sulzberger, David (2006) |
| Türkiye Bursa (Yeşil Külliyesi/Green Complex, Mausoleum) |  * * | 15th (1419–1424 1421–1425) (Ottoman) | MAO 936/821 [11.5 × 6.7 × 2.7] | Kiefer/Soustiel CK029/CK120 (1995) |
| Türkiye Bursa (Yeşil Külliyesi/Green Complex Mausoleum)? |  * * | 1864 [restoration] Kütahya | MAO 936/822 [12.3 × 12.4 × 1.5] | Kiefer/Soustiel CK0119 (1995) |
| Türkiye Bursa (Yeşil Külliyesi/Green complex, Mausoleum) |  * * | 15th (1419–1424) ca. 142 1(Ottoman) | MAO 2070 [21.5 × 30.5 × 10] | Parvillée (1895) |
| Türkiye Bursa (Yeşil Külliyesi/Green complex, Mausoleum) |  * * | 15th (1419–1424 1421) (Ottoman) | MAO 2069 [21 × 33 × 10] | Parvillée (1895) |
| Türkiye Edirne (Muradiye mosque)? |  * * | 15th (1435,1436) 1400–1500 (Ottoman) | AD 956a [18 × 29.6 × 4.5] | Maciet (1885) |
| Türkiye, Istanbul (Topkapı palace) |  * * | 16th 1543–1548 (Ottoman) | OA 3919/1010 [13.2 × 26 × 1.8] | Sorlin Dorigny (1895) |
| Türkiye Istanbul? Edirne? |  * * | 1514–1555 [ca. 1520] (Ottoman) | OA 3919/990 [26.8 × 27.2 × 2] | Sorlin Dorigny (1895) |
| Türkiye Edirne?, Istanbul? |  * * | 16th (ca. 1520) (Ottoman) | OA 3919/989 [26.3 × 27.5 × 1.9] | Sorlin Dorigny (1895) |
| Türkiye Istanbul? |  | 16th (Ottoman) | OA 3919/1006 | Sorlin Dorigny (1895) |
| Türkiye Istanbul? |  * * | 16th? (Ottoman) | OA 3919/1004 [24 × 26.5 × 2.3] | Sorlin Dorigny (1895) |
| Türkiye Istanbul (Topkapı palace) |  * * | [16th or17th]? Re-use? (Ottoman) | OA 3919/1003 [24 × 26.5 × 2.3] | Sorlin Dorigny (1895) |
| Uzbekistan, Samarkand (Shar-e Sabz, Aq-Saray) |  * * | Turn of 14th–15th (ca. 1380–1404) (Timurid) | MAO 936/921 [8.6 × 12.4 × 2.2] | Soustiel (1995) |
| Uzbekistan Samarkand (Bîbî Khânum) |  * * | ~15th (1399–1405) (Timurid) | MAO 936/923 [11.3 × 15.1 × 3] | Soustiel (1996) |
| Uzbekistan Samarkand (Ulugh Beg madrasa) |  * * | 15th (1417–1420) 1370–1400 (Timurid) | MAO 936/922 [10.4 × 13.9 × 2.7] | Soustiel (1996) |
| Iran Transoxiana Khargerd, (El Ghiyâthiyya madrasa) |  * * | 15th (1438–1445) 1400–1600 (Timurid) | MAO 936/573 [4.9 × 16.2 × 3.5] | Kiefer/Soustiel (1995) CK0429 |
| Uzbekistan Samarkand (Bîbî Khânum) |  * * | ~15th (1399–1405) 1400–1600 (Timurid) | MAO 936/576 [11.5 × 16.6 × 3] | Kiefer/Soustiel (1995) CK0405 |
| Uzbekistan Samarkand (Bîbî Khânum) |  * * | ~15th (1399–1405) (Timurid) | AFI 698 [5.7 × 8.4 × 2.4] | 1996 |
| Armenia, Yerevan (Abbâs Mîrzâ mosque/Serdar Palace?) |  * * | (1807–1827) 1700–1900 (Qajar) | AD 9099/2 [9.5 × 12 × 4] | Krafft, (1899) |
| Armenia Yerevan (Abâs Mîrzâ mosque/Serdar Palace?) |  ° ° | 19th (1807–1827) (Qajar) | AD 9099/1 [18.7 × 18.7 × 3.8] | Krafft (1899) |
| Iran (mina’i) |  * * | Last quarter of 12th–13th | MAO 936/1127a | Kiefer/Soustiel (1995) |
| Iran (mina’i) |  * * | End of 12th–13th | MAO 936/319 | Kiefer/Soustiel (1995) |
| Iran (lajvardina) | Photograph not available | End of 12th–13th | MAO 936/277a | Kiefer/Soustiel (1995) |
| Iran Isfahan? (New Julfâ district?) |  * * | 17th–18th (Safavid) | AFI 2299 [22.2 × 22.8 × 2.5] | ? |
| Iran, Ispahan (Allahverdikan bridge, Pul-e Khwâjû) |  * * | 17th (1654,1655) (Safavid) | MAO 936/2002 [3.3 × 4.7 × 0.7] | Kiefer/Soustiel (1995) |
| Armenia Yerevan? (‘Abbâs Mîrzâ mosque/ Serdar Palace)? |  * * | [19th] 1600–1700 (Qajar) | MAO 936/593 [13.2 × 17.5 × 3.2] | Kiefer/Soustiel (1995) |
| Iran Isfahan (New Julfâ church)? |  * * | (17th–19th) 1600–1700 Safavid/Zand/ Qajar? | MAO 936/594 [10.1 × 10.3 × 2.5] | Kiefer/Soustiel CK0150 |
| Iran Tehran/Qazwin? |  * * | 18th-19th Zand/Qajar? | MAO 2025d | Drouot Auction (2004) |
| Iran Isfahan (New Julfâ district)? |  * * | (17th–19th) 1600–1800 (Safavid) | MAO 936/644b [7.9 × 15.6 × 2.1] | Kiefer/Soustiel (1995) |
| Iran Isfahan (New Julfâ district)? |  * * | (17th-19th) 1700–1800 Safavid-Qajar? | MAO 936/642 [8.2 × 9x2.2] | Kiefer/Soustiel (1995) |
| Iran |  * * | 19th Qajar | MAO 936/646b [9.9 × 12.9 × 2.1] | Kiefer/Soustiel (1995) |
| Iran Isfahan (New Julfâ district)? |  * * | 17th–19th Safavid-Qajar | AD 15117 [15.2 × 23.9 × 2.3] | Filippo (1908) |
| Israel/Palestine Jerusalem Dome of the Rock mosque Haram al-Sharif? |  * * | 16th 1545–1552 | OA 4047/199 [20 × 20.4 × 1.9] | Baudry (1898) |
| Israel/Palestine Jerusalem Dome of the Rock mosque Haram al-Sharif? |  * * | 16th 1545–1552 | OA 4047/200 [20.3 × 20.8 × 2.5] | Baudry (1898) |
| View | Assignment Style | Period | Dimension /cm |
|---|---|---|---|
 | Iznik dish baba Nakkas-style (blue-and-white shard) | 1510–1520 | 8.5 × 8 × 0.6 |
 | Iznik tile (polychrome ware) | ca. 1575 | 13 × 13.6 × 1.35 |
 | Iznik dish (polychrome saz leaves decoration) | ca. 1580 | 34 × 6.5 |
 | Iran, Safavid (blue-and-white shard) | 17th c. | 8 × 8 × 1 |
 | Iran, Safavid tile (blue polychrome tile shard) | 17th c. | 9.5 × 10.4 × 2.8 |
 | Iran, Safavid tile (yellow polychrome with shard) | 17th c. | 10 × 14 × 2.5 |
 | Iran, (mina’i bowl) | middle 12th to early13th | 14 × 5.5 |
 | Iran, Safavid (blue-and-white dish with Chinese landscape decoration) | 17th c. | 32 × 6 |
 | Egypt Mamluk shard | 14th–15th c. | 11 × 11.5 × 0.8 |
| Inventory Number | Colour (Analysed Spot) | Origin (Period /Century) | Paste Colour | Quartz-Rich Layer (Firing) | White Under-Layer | Opacifier | Interfaces | νSi-O (cm−1) | Raman Finger-Print |
|---|---|---|---|---|---|---|---|---|---|
| MAO 2083/39 | cream yellow | al-Andalus 10th–11th? | light red | no | no | no | SI g-paste | 965 | Pb-G |
| MAO 936/573 | black | Transoxiana [1438–1445] 1400–1600 | 900 | ||||||
| MAO 936/1002 | yellow | Iran, Isfahan [beg 17th] 1654–1655 | 950 | ||||||
| MAO 936/593 | yellow green | Iran 19th | red | no | 960 960 | ||||
| MAO 936/645b | yellow | Iran [17th–beg 18th] 1600–1700 | 950 | ||||||
| MAO 936/642 | yellow black | Iran [17th–19th] 1700–1800 | yellow | no | yes | SI g-g | 900 950 | ||
| Priv. coll Table 2 | yellow | Iran | 900–950 | ||||||
| MAO 936/646b | yellow | Iran 19th | light red | yes traces cass | SI g-g | 900–950 | |||
| MAO 936/822 | yellow blue | Türkiye, Bursa 1864 | yellow | yes | Naples Sb yellow woll. | SI g-g yellow & blue | 990 | ||
| MAO 936/922 | yellow | Uzbekistan, Samarkand [1417–1420]/1370–1400 | red | no | SI g-g blue SI g-g red | 890–960 | |||
| AD 3919/1006 | yellow | Türkiye? 16th Ottoman | no | 990 | |||||
| OA 3919/1010 | yellow, green | Türkiye, Istanbul 1543–1548 | quartz | 965 | |||||
| AD 956a | turquoise | Türkiye, Istanbul 1435–1436]/1400–1500 | 920 | ||||||
| MAO 936/821 | turquoise | Türkiye, Bursa [1419–1424/1421–1425 | 960 | ||||||
| OA 3919/990 | yellow green | Türkiye, Istanbul [ca. 1520]/1514–1525 | 960 970 | ||||||
| AD 9099/2 | blue | Armenia, Yerevan 1807–1827/1700–1800 | 1000 | ||||||
| UCAD 9099/1 | yellow | Armenia, Yerevan 1807–1827 | 910 | ||||||
| AFI 2299 | yellow green | Iran, Isfahan 17th–18th | 965 920–970 | ||||||
| MAO 936/644b | yellow | Iran, Isfahan 17th–19th | yes | 940 | |||||
| MAO 936/642 | yellow black | Iran, Isfahan 17th–19th | no | 940 1000 | |||||
| MAO 936/1127a | white turquoise | Iran (mina’i) 12th–13th | yes cass. | 980 | |||||
| MAO 936/277a | turquoise | Iran (lajvardina) 12th–13th | 980 | ||||||
| AD 3919/1006 | glaze | Türkiye? 16th Ottoman | yes traces cass & woll. | 990–1095 | Pb-Na-G | ||||
| MAO 936/319 | blue white | Iran (mina’i) 12th–13th | |||||||
| MAO 936/821 | white turquoise | Türkiye, Bursa [1419–1424]/1421–1425 | yes | 1025 | |||||
| MAO 2070 | white | Türkiye, Bursa [1419–1424]/1421 | yes | 1015 | |||||
| MAO 2069 | white | Türkiye, Bursa [1419–1424]/1421 | yes | 980–1060 | |||||
| AD 956a | white | Türkiye, Istanbul [1435,1436]/1400–1500 | 990–1060 | ||||||
| MAO 936/319 | white | yes, cass. & woll. | 990–1070 | ||||||
| MAO 936/594 (AFI 698) | white blue | yes, cass. | SI g-paste | 990–1085 | |||||
| Pr. Coll | white blue | Türkiye, Iznik Baba Nakkas 1520–1530 | no yes, traces woll. & cass. | 990–1060 | |||||
| MAO 2025d | blue | Iran, Tehran 18th–19th | yellow | yes, cass. | 990–1060 | ||||
| Pr. Coll | blue glaze red turquoise | Türkiye, Iznik, Polychrome ca. 1575 | yes | no no trace cass. | 990–1060 | ||||
| MAO 936/2002 | blue white black | Iran 1654–1655 | yellow | yes | SI g-paste | 1090 | (Pb)Na-K-Ca-G | ||
| MAO 936/922 | white red | Uzbekistan, Samarkand 1417–1420 | yes, cass. | 1090 | |||||
| MAO 936/573 | red turquoise white | Iran 1438–1445 | red | yes | SI g-g yellow SI g-paste | 1090 | |||
| MAO 936/921 | white brown blue | Uzbekistan, Samarkand 1380–1404 | yes traces cass. arsenate? | 1090 1090 1090 | |||||
| AFI 698 | blue white glaze | Uzbekistan, Samarkand (15th) | 1090 | ||||||
| MAO 936/576 | blue white under-g | Uzbekistan, Samarkand 1399–1405 | yes, cass. & woll. idem | 1090 | |||||
| MAO 2025d | white | Iran, Tehran 18th–19th | dark yellow | yes, woll. | SI g-g yellow | ||||
| OA 3919/1010 | white turquoise | Türkiye, Istanbul 1543–1548 | 1078 | ||||||
| OA 3919/990 | white turquoise | Türkiye, Istanbul ca. 1520 | yes | 1070 1070 | |||||
| OA 3919/989 | white turquoise | Türkiye, Istanbul ca. 1520 | yes | 1060 1070 | |||||
| MAO 936/923 | white blue | Uzbekistan, Samarkand 1399–1405 | red | yes, cass. yes, cass. | g-g diff | 1100 1095 | |||
| MAO 936/922 | white red turquoise | Uzbekistan, Samarkand 1417–1420 | yes cass. | 1090 1095 1080 | |||||
| AFI 2299 | turquoise | Iran, Isfahan 17th–18th | yes | 1065 | |||||
| MAO 936/593 | white blue | Iran [19th?]/1600–1700 | yes, woll. | 1065 | |||||
| MAO 936/642 | white | Iran 1700–1800 | yes, cass. & woll. | 1000–1110 | |||||
| MAO 936/644b | white | Iran 1600–1800 | yes, cass. | 1000–1100 | |||||
| Pr. Coll. | white blue | Safavid 17th | no yes, woll. | 1090 | |||||
| MAO 936/2002 | white blue | Uzbekistan, Samarkand | yes, cass. no | g-g diff | 1000–1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Simsek Franci, G. Timurid, Ottoman, Safavid and Qajar Ceramics: Raman and Composition Classification of the Different Types of Glaze and Pigments. Minerals 2023, 13, 977. https://doi.org/10.3390/min13070977
Colomban P, Simsek Franci G. Timurid, Ottoman, Safavid and Qajar Ceramics: Raman and Composition Classification of the Different Types of Glaze and Pigments. Minerals. 2023; 13(7):977. https://doi.org/10.3390/min13070977
Chicago/Turabian StyleColomban, Philippe, and Gulsu Simsek Franci. 2023. "Timurid, Ottoman, Safavid and Qajar Ceramics: Raman and Composition Classification of the Different Types of Glaze and Pigments" Minerals 13, no. 7: 977. https://doi.org/10.3390/min13070977






