A Comparative Study of Malonic and l-Glutamic Acids for Metal Leaching from Spent Lithium-Ion Batteries: Kinetic and Optimization Analysis
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Analytical Methods
2.2.1. Instrumental Methods
2.2.2. Chemical Methods
2.3. Leaching Methods
2.4. Process Optimization
3. Results and Discussion
3.1. Leaching Conditions
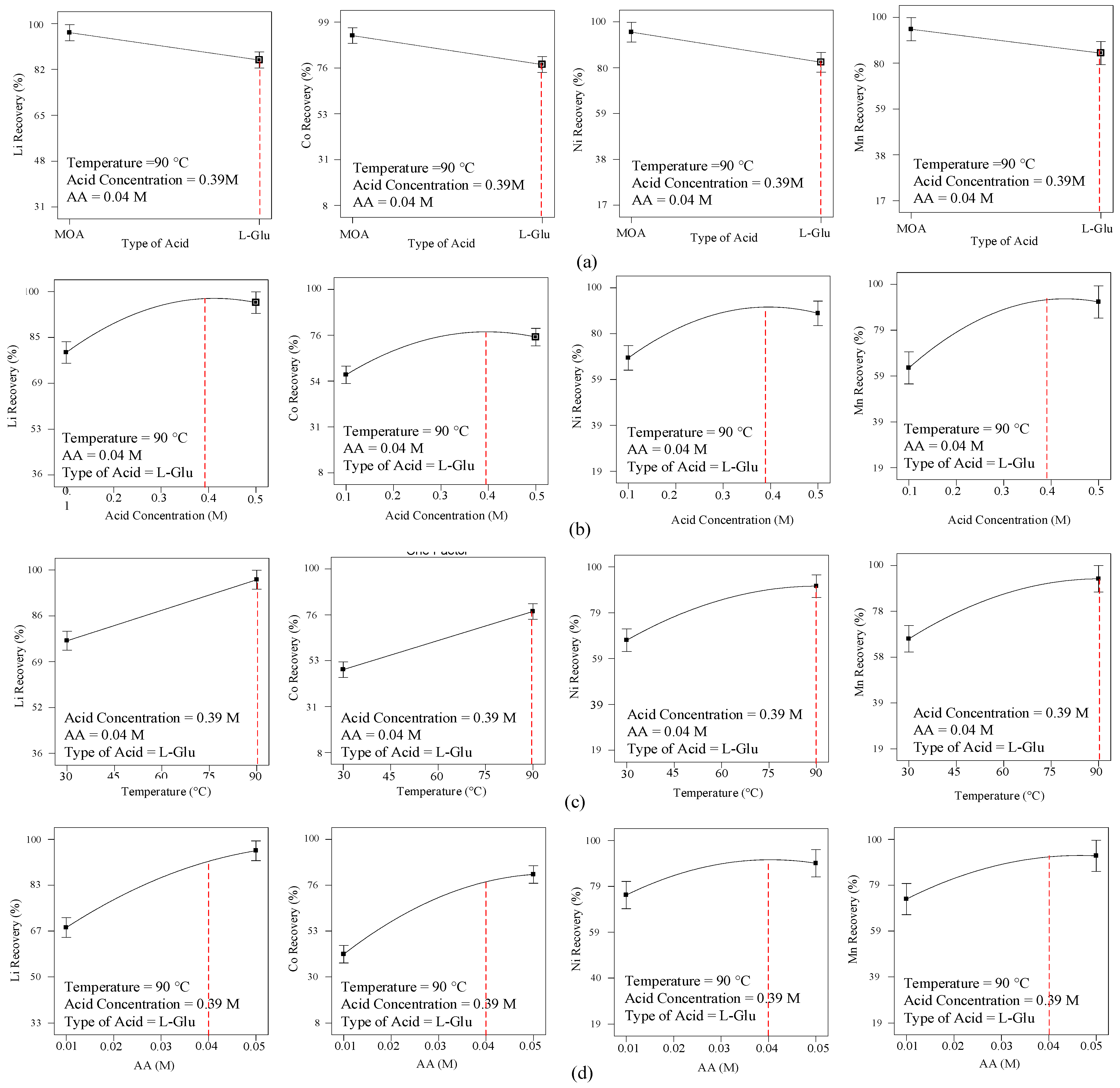
3.1.1. Effect of Organic Acid on the Leaching Process
3.1.2. Effect of the Organic Acid Concentration on the Leaching Process
3.1.3. Effect of the Temperature on the Leaching Process
3.1.4. Effect of the AA Concentration (as a Reducing Agent) on the Leaching Process
3.2. Characterizations
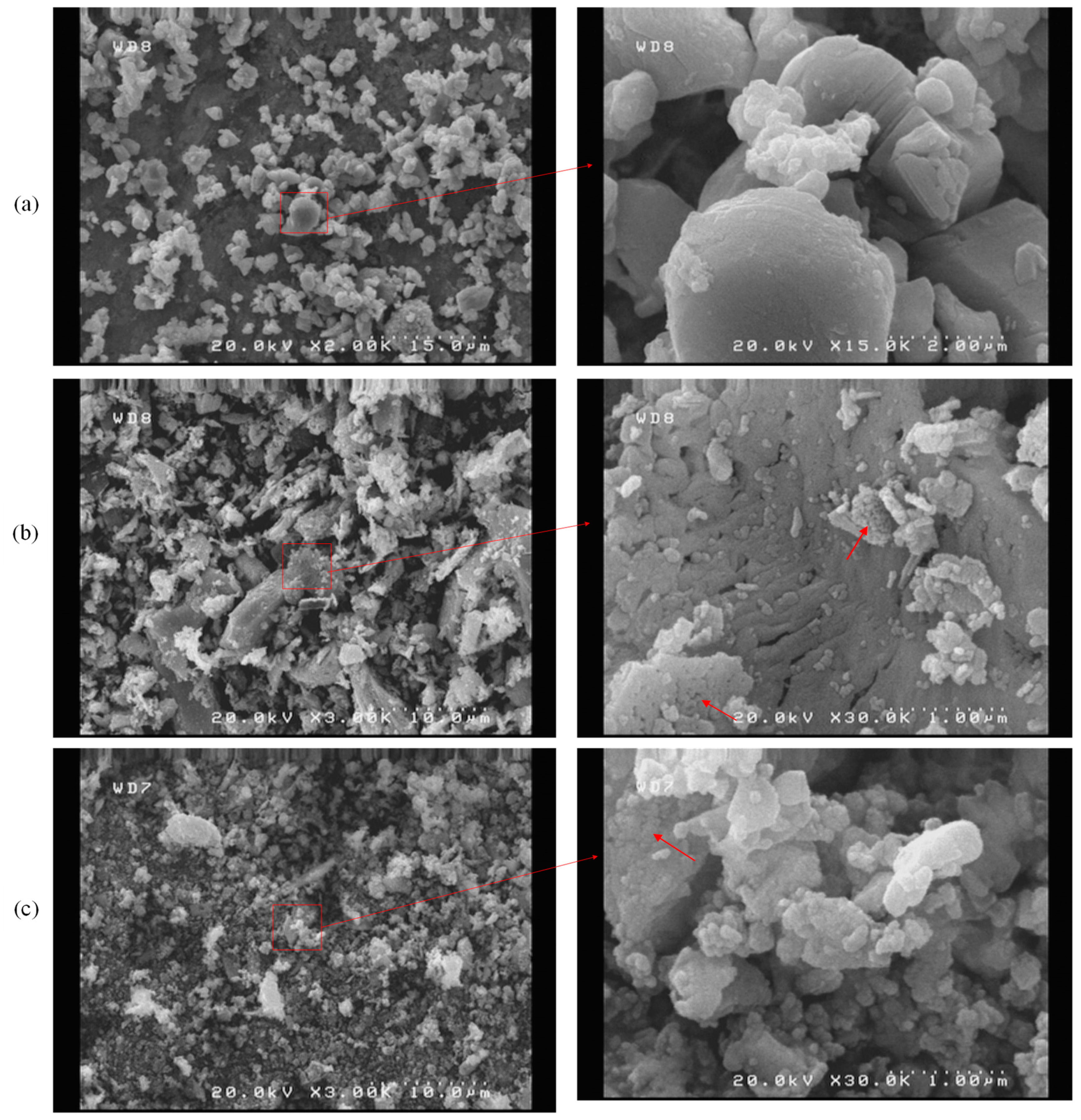
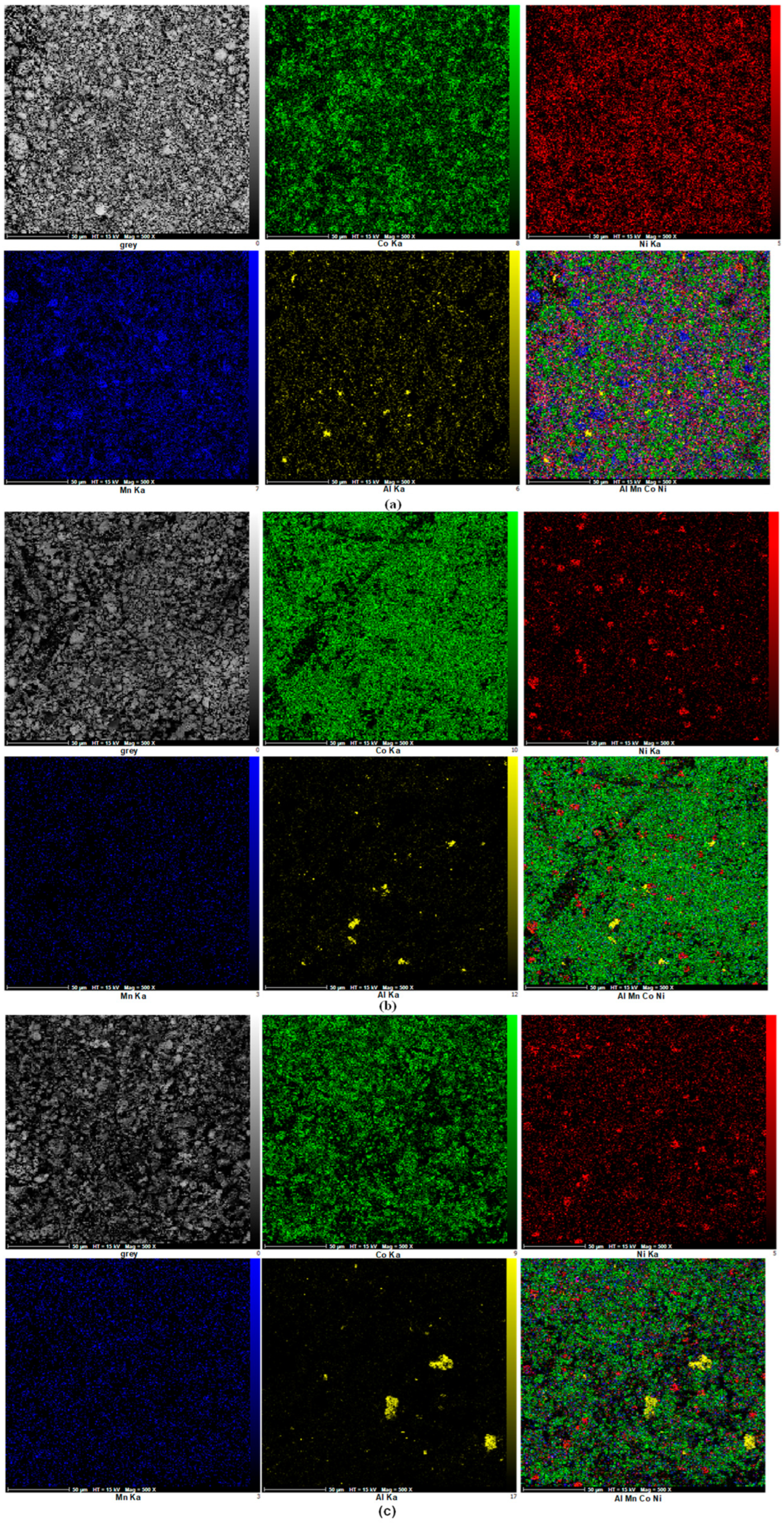
3.2.1. SEM Analysis and EDS Mapping
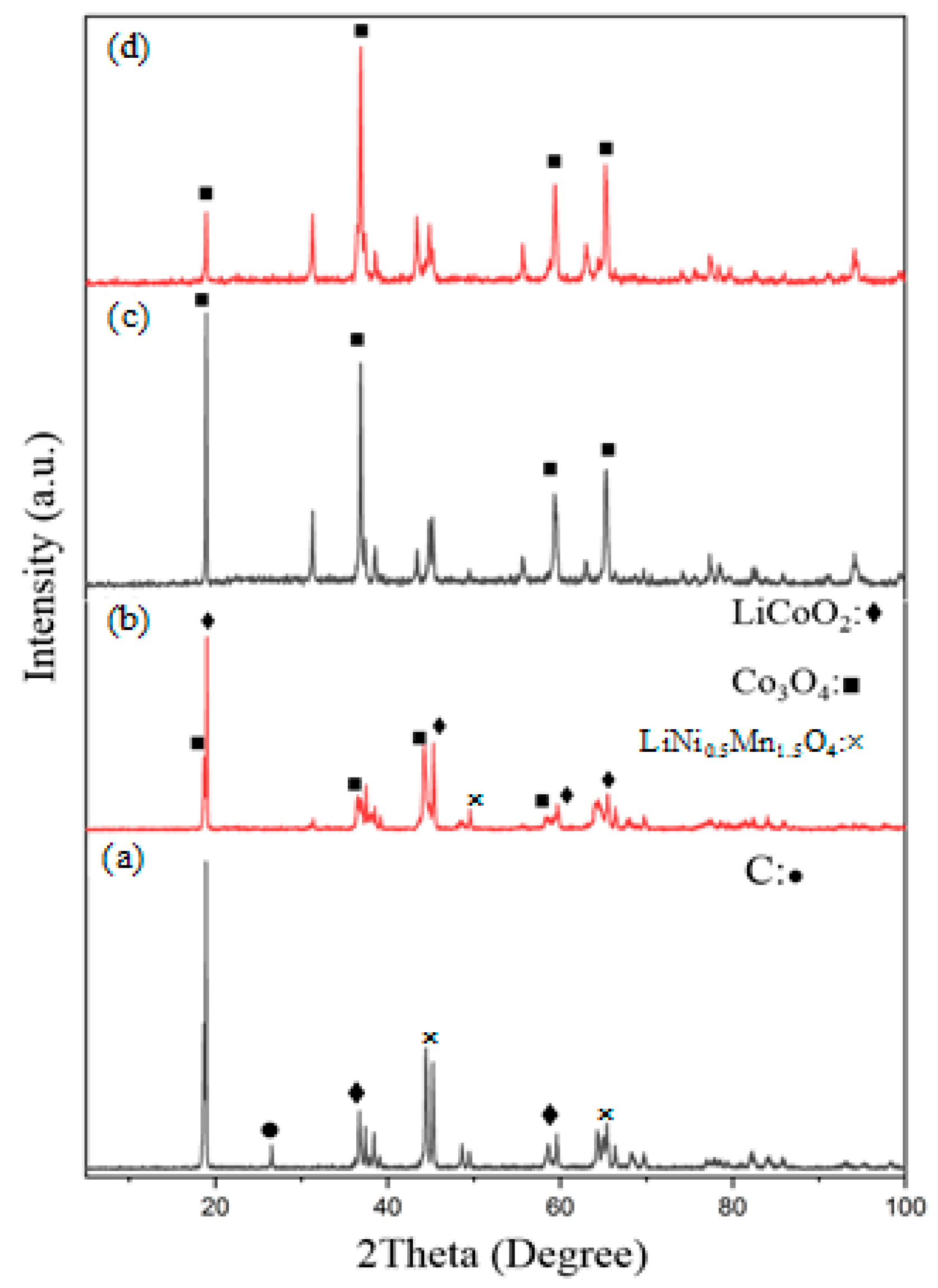
3.2.2. XRD Patterns
3.2.3. UV-Vis Spectrum
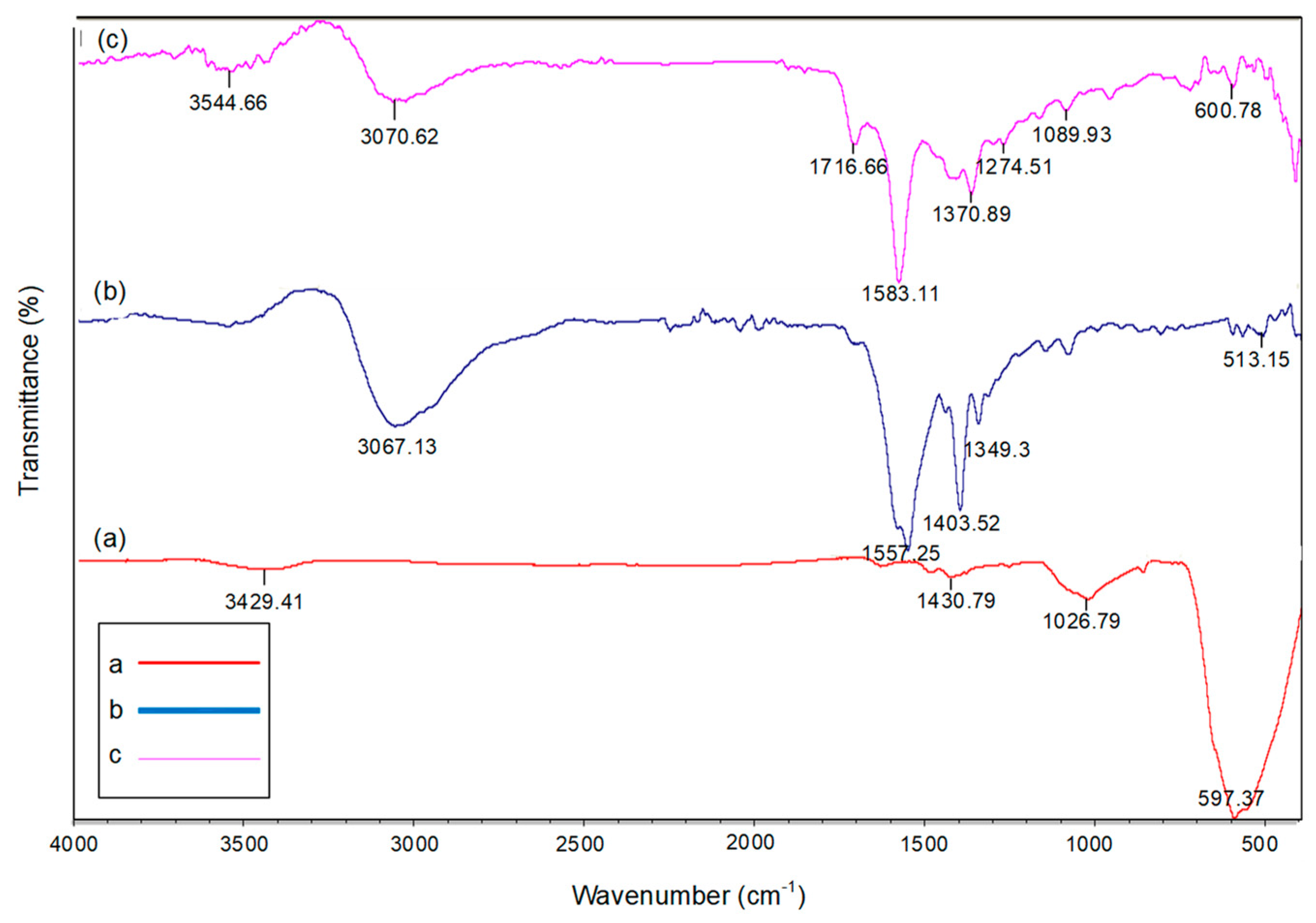
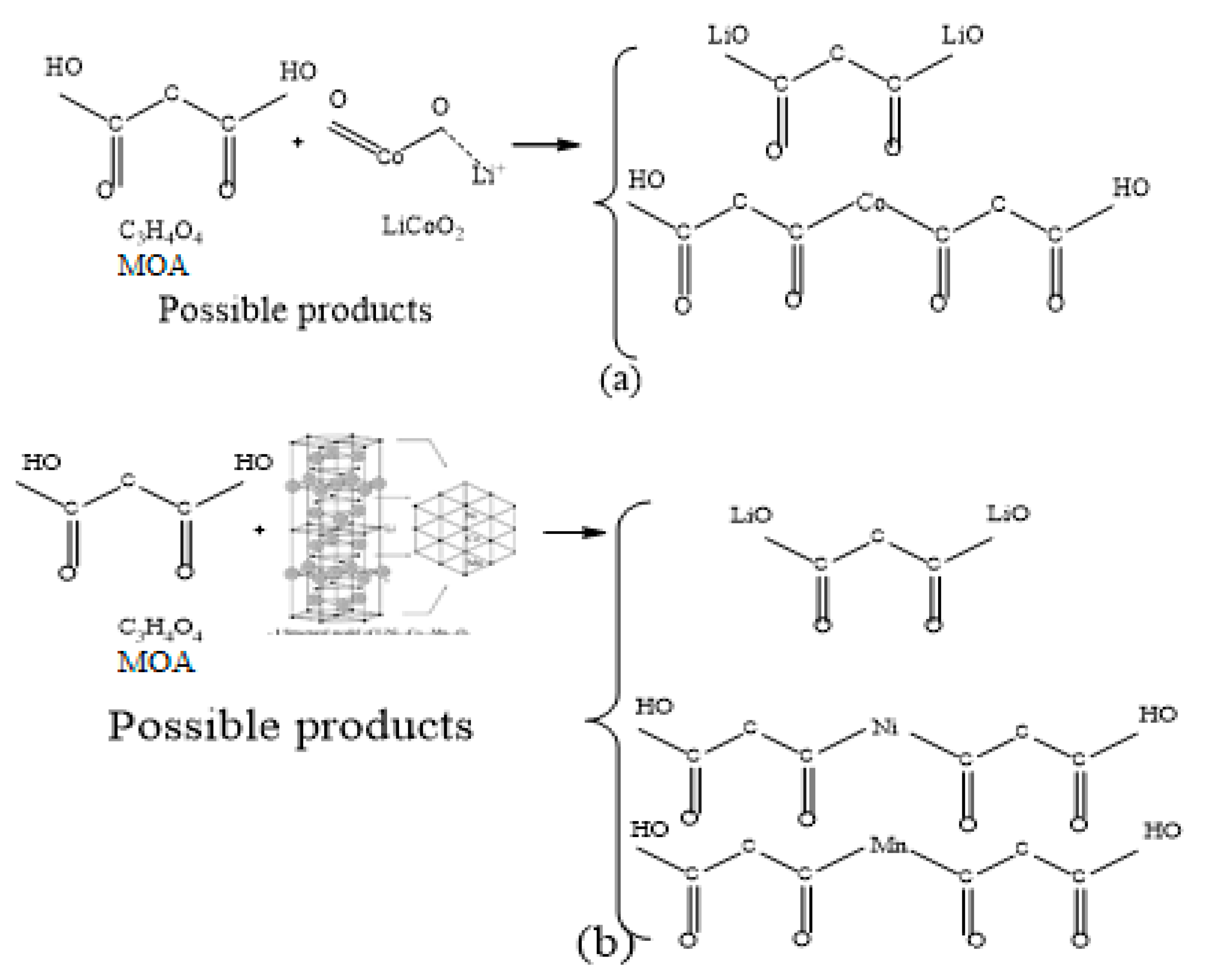
3.2.4. FT-IR Spectrum
3.3. Leaching Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, D.; Wang, T.; Liu, Z.; Zhao, S.; Quan, J.; Li, G.; Zhu, H.; Huang, J.; He, W. Characteristic comparison of leaching valuable metals from spent power Li-ion batteries for vehicles using the inorganic and organic acid system. J. Environ. Chem. Eng. 2022, 10, 107102. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Fan, E.; Lin, J.; Wu, F.; Chen, R.; Li, L. Recovery valuable metals from spent lithium-ion batteries via a low-temperature roasting approach: Thermodynamics and conversion mechanism. J. Hazard. Mater. Adv. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Xia, X.; Li, P. A review of the life cycle assessment of electric vehicles: Considering the influence of batteries. Sci. Total Environ. 2022, 814, 152870. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Tang, X.; Tang, W.; Duan, J.; Yang, W.; Wang, R.; Tang, M.; Li, J. Recovery of valuable metals and modification of cathode materials from spent lithium-ion batteries. J. Alloys Compd. 2021, 874, 159853. [Google Scholar] [CrossRef]
- Weshahy, A.R.; Gouda, A.A.; Atia, B.M.; Sakr, A.K.; Al-Otaibi, J.S.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.; El Sheikh, R.; Radwan, H.A. Efficient Recovery of Rare Earth Elements and Zinc from Spent Ni–Metal Hydride Batteries: Statistical Studies. Nanomaterials 2022, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xia, X.; Guo, J. A review of the life cycle carbon footprint of electric vehicle batteries. Sep. Purif. Technol. 2022, 296, 121389. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M.; Iwatate, Y.; Azuma, D.; Shibazaki, K.; Hiraga, Y.; Kishita, A.; Nakayasu, Y. Hydrothermal leaching of ternary and binary lithium-ion battery cathode materials with citric acid and the kinetic study. J. Supercrit. Fluids 2020, 165, 104990. [Google Scholar] [CrossRef]
- Verma, A.; Corbin, D.R.; Shiflett, M.B. Lithium and cobalt recovery for lithium-ion battery recycle using an improved oxalate process with hydrogen peroxide. Hydrometallurgy 2021, 203, 105694. [Google Scholar] [CrossRef]
- Sommerville, R.; Zhu, P.; Rajaeifar, M.A.; Heidrich, O.; Goodship, V.; Kendrick, E. A qualitative assessment of lithium ion battery recycling processes. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J. Power Sources 2021, 482, 228942. [Google Scholar] [CrossRef]
- Lie, J.; Liu, J.-C. Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid. Sep. Purif. Technol. 2021, 266, 118458. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Meng, Q.; Dong, P.; Fei, Z.; Li, Q. Recycling of LiCoO2 cathode material from spent lithium ion batteries by ultrasonic enhanced leaching and one-step regeneration. J. Environ. Manag. 2021, 277, 111426. [Google Scholar] [CrossRef] [PubMed]
- Chabhadiya, K.; Srivastava, R.R.; Pathak, P. Two-step leaching process and kinetics for an eco-friendly recycling of critical metals from spent Li-ion batteries. J. Environ. Chem. Eng. 2021, 9, 105232. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering valuable metals from LiNixCoyMn1-x-yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, J.; Wanaldi, R.; Zhou, X.; Wang, H.; Zhou, C.; Yang, J. A promising selective recovery process of valuable metals from spent lithium ion batteries via reduction roasting and ammonia leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Xiao, J.; Niu, B.; Song, Q.; Zhan, L.; Xu, Z. Novel targetedly extracting lithium: An environmental-friendly controlled chlorinating technology and mechanism of spent lithium ion batteries recovery. J. Hazard. Mater. 2021, 404, 123947. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Li, J.; Qu, L.; Yang, Y.; Guo, X.; Xie, W. Improved hydrometallurgical extraction of valuable metals from spent lithium-ion batteries via a closed-loop process. J. Alloys Compd. 2020, 847, 156489. [Google Scholar] [CrossRef]
- Weshahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.; Hanfi, M.Y.; Sayyed, M.; El Sheikh, R.; El-Sheikh, E.M.; Radwan, H.A. Selective recovery of cadmium, cobalt, and nickel from spent Ni–Cd batteries using Adogen® 464 and mesoporous silica derivatives. Int. J. Mol. Sci. 2022, 23, 8677. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Peng, C.; Hamuyuni, J.; Wilson, B.P.; Lundström, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0. 5Co0. 2Mn0. 3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Srivastava, R.R.; Kim, H.; Ilyas, S.; Khosa, M.K.; Yameen, B. Leaching of exhausted LNCM cathode batteries in ascorbic acid lixiviant: A green recycling approach, reaction kinetics and process mechanism. J. Chem. Technol. Biotechnol. 2020, 95, 2286–2294. [Google Scholar] [CrossRef]
- Natarajan, S.; Boricha, A.B.; Bajaj, H.C. Recovery of value-added products from cathode and anode material of spent lithium-ion batteries. Waste Manag. 2018, 77, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative application of acid leaching to regenerate Li (Ni1/3Co1/3Mn1/3) O2 cathodes from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- Li, D.; Zhang, B.; Ou, X.; Zhang, J.; Meng, K.; Ji, G.; Li, P.; Xu, J. Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 2333–2337. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sun, C.; Zhou, T.; Gao, R.; Xie, H. Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep. Purif. Technol. 2021, 257, 117930. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.; Mankhand, T. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Ma, H.; Li, J.; Zhou, T.; Cao, L.; Kang, D. Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. Waste Manag. 2018, 75, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, Q.; Dong, P.; Duan, J.; Lin, Y. Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries. J. Ind. Eng. Chem. 2018, 66, 86–93. [Google Scholar] [CrossRef]
- Sahu, S.; Pati, S.; Devi, N. A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid. Metals 2023, 13, 947. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Acid leaching of LiCoO2 enhanced by reducing agent. Model formulation and validation. Chemosphere 2022, 287, 132020. [Google Scholar] [CrossRef]
- Xiao, X.; Hoogendoorn, B.W.; Ma, Y.; Sahadevan, S.A.; Gardner, J.M.; Forsberg, K.; Olsson, R.T. Ultrasound-assisted extraction of metals from Lithium-ion batteries using natural organic acids. Green Chem. 2021, 23, 8519–8532. [Google Scholar] [CrossRef]
- Islam, A.; Roy, S.; Khan, M.A.; Mondal, P.; Teo, S.H.; Taufiq-Yap, Y.H.; Ahmed, M.T.; Choudhury, T.R.; Abdulkreem-Alsultan, G.; Khandaker, S. Improving valuable metal ions capturing from spent Li-ion batteries with novel materials and approaches. J. Mol. Liq. 2021, 338, 116703. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Sung, M.H.; Park, C.; Kim, C.J.; Poo, H.; Soda, K.; Ashiuchi, M. Natural and edible biopolymer poly-γ-glutamic acid: Synthesis, production, and applications. Chem. Rec. 2005, 5, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Kettler, R.M.; Wesolowski, D.J.; Palmer, D.A. Dissociation quotients of malonic acid in aqueous sodium chloride media to 100 C. J. Solut. Chem. 1992, 21, 883–900. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, W.; Mo, W.-T.; Zhou, L.; Liu, J.-W. Lithium fluoride recovery from cathode material of spent lithium-ion battery. RSC Adv. 2018, 8, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Y.; Zou, Y.; Yang, Z.; Zhang, B.; Zhao, Y.; Zhang, M.; Meng, Q.; Dong, P. Leaching kinetics and interface reaction of LiNi0. 6Co0. 2Mn0. 2O2 materials from spent LIBs using GKB as reductant. J. Environ. Manag. 2021, 300, 113710. [Google Scholar] [CrossRef]
- Gao, W.; Liu, C.; Cao, H.; Zheng, X.; Lin, X.; Wang, H.; Zhang, Y.; Sun, Z. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Sohbatzadeh, L.; Shafaei Tonkaboni, S.Z.; Noaparast, M. An Environmentally Friendly Method for Recovery of Metals from Cathode Material of Lithium-Ion Batteries using l-Glutamic, Malonic, and Ascorbic acid. J. Min. Environ. 2022, 13, 1171–1188. [Google Scholar]
- Hamborg, E.S.; Niederer, J.P.; Versteeg, G.F. Dissociation constants and thermodynamic properties of amino acids used in CO2 absorption from (293 to 353) K. J. Chem. Eng. Data 2007, 52, 2491–2502. [Google Scholar] [CrossRef]
- DeRuiter, J. Carboxylic acid structure and chemistry: Part 1. Princ. Drug Action 2005, 1, 1–10. [Google Scholar]
- Su, F.; Meng, Q.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Cao, P.; Li, Z.; Wang, H. One-step leaching mechanism for total elemental recovery from spent lithium-ion batteries utilizing ethylene diamine tetraacetic acid. J. Environ. Chem. Eng. 2023, 11, 110275. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; He, T.; Yuan, X.; Li, X.; Shi, H.; Yang, L.; Shao, P.; Wang, C.; Luo, X. Rapid extraction of valuable metals from spent LiNixCoyMn1-x-yO2 cathodes based on synergistic effects between organic acids. Waste Manag. 2023, 165, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.; Pai, K.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cho, C.-W.; Yun, Y.-S. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J. Hazard. Mater. 2022, 423, 127214. [Google Scholar] [CrossRef]



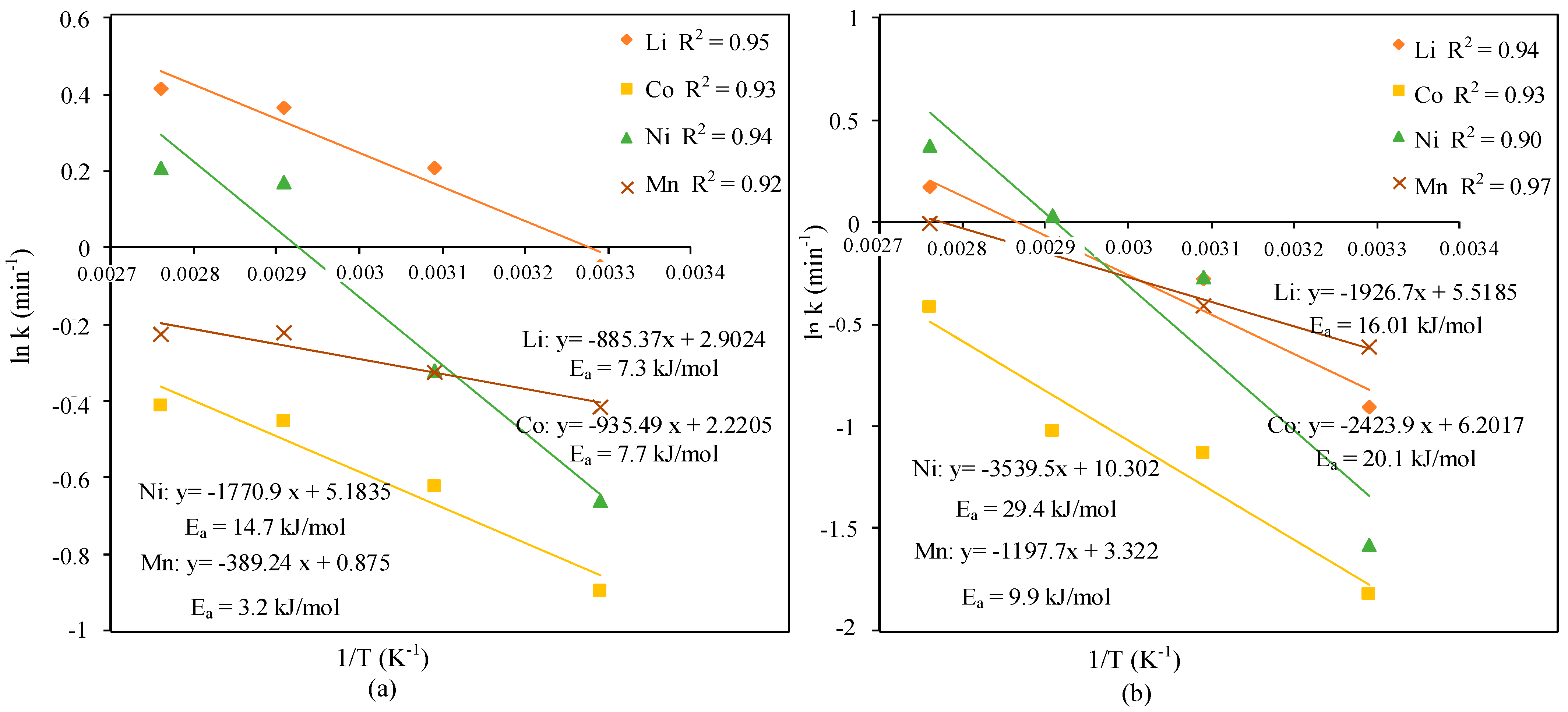

| Leaching Reagents | Conditions | Sample | Leaching Efficiency (%) |
|---|---|---|---|
| 0.4 M tartaric acid + 0.02 M ascorbic acid | 5 h; 80 °C | LiCoO2 | Li: 99.5%; Co: 95% |
| 0.5 M glycine + 0.02 M ascorbic acid | 6 h; 80 °C | LiCoO2 | Co: 95% |
| 0.1 M nitrilotriacetic acid + 0.02 M ascorbic acid | 6 h; 80 °C | LiCoO2 | Li: 96%; Co: 75% |
| 0.1 M adipic acid + 0.02 M ascorbic acid | 6 h; 80 °C | LiCoO2 | Li: 92%; Co: 85% |
| 0.2 M phosphate acid + 0.4 M citric acid | 0.5 h; 90 °C | LiNi0.5Co0.2Mn0.3O2 | Li: 100%; Ni: 93.4%; Co: 91.6%; Mn: 92% |
| The Chemical Elements in Cathode Material of the LIB | Retained Particles | Passed Particles | 2 h Furnace |
|---|---|---|---|
| Al (wt%) | 23.73 | 0.59 | 0.58 |
| K (wt%) | 0.07 | - | 0.07 |
| Ni (wt%) | 8.35 | 14.30 | 15.01 |
| Si (wt%) | 0.14 | 0.09 | 0.11 |
| Ca (wt%) | 0.09 | - | - |
| P (wt%) | 0.10 | 0.20 | 0.19 |
| Mn (wt%) | 5.97 | 8.10 | 10.51 |
| S (wt%) | 0.02 | 0.07 | 0.06 |
| Co (wt%) | 15.80 | 34.66 | 36.27 |
| Mg (wt%) | - | - | 0.11 |
| L.O.I. | 8.18 | 9.35 | 1.90 |
| MOA + AA | ||||||||||||||||||||
| T (°C) | Li | Co | Ni | Mn | ||||||||||||||||
| M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | |
| 30 | 0.85 | 0.85 | 0.85 | 0.95 | 0.87 | 0.75 | 0.78 | 0.75 | 0.90 | 0.76 | 0.89 | 0.91 | 0.90 | 0.98 | 0.94 | 0.91 | 0.91 | 0.91 | 0.95 | 0.92 |
| 50 | 0.95 | 0.95 | 0.95 | 0.90 | 0.95 | 0.88 | 0.86 | 0.88 | 0.94 | 0.88 | 0.74 | 0.75 | 0.76 | 0.91 | 0.82 | 0.72 | 0.73 | 0.74 | 0.90 | 0.78 |
| 70 | 0.88 | 0.88 | 0.88 | 0.95 | 0.89 | 0.91 | 0.92 | 0.92 | 0.94 | 0.92 | 0.93 | 0.93 | 0.94 | 0.99 | 0.96 | 0.92 | 0.92 | 0.93 | 0.94 | 0.93 |
| 88 | 0.93 | 0.97 | 0.97 | 0.94 | 0.97 | 0.98 | 0.98 | 0.98 | 0.96 | 0.99 | 0.85 | 0.85 | 0.89 | 0.97 | 0.93 | 0.91 | 0.91 | 0.93 | 0.96 | 0.96 |
| l-Glu + AA | ||||||||||||||||||||
| T (°C) | Li | Co | Ni | Mn | ||||||||||||||||
| M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | |
| 30 | 0.88 | 0.87 | 0.87 | 0.90 | 0.85 | 0.91 | 0.91 | 0.91 | 0.92 | 0.91 | 0.89 | 0.90 | 0.89 | 0.94 | 0.86 | 0.84 | 0.84 | 0.84 | 0.95 | 0.84 |
| 50 | 0.87 | 0.88 | 0.89 | 0.97 | 0.92 | 0.74 | 0.77 | 0.75 | 0.91 | 0.79 | 0.88 | 0.90 | 0.91 | 0.94 | 0.92 | 0.87 | 0.87 | 0.87 | 0.92 | 0.88 |
| 70 | 0.95 | 0.95 | 0.95 | 0.93 | 0.95 | 0.91 | 0.92 | 0.92 | 0.99 | 0.94 | 0.55 | 0.57 | 0.81 | 0.96 | 0.83 | 0.78 | 0.78 | 0.79 | 0.94 | 0.80 |
| 90 | 0.89 | 0.86 | 0.95 | 0.98 | 0.96 | 0.87 | 0.88 | 0.89 | 0.98 | 0.92 | 0.87 | 0.86 | 0.88 | 0.94 | 0.89 | 0.91 | 0.92 | 0.92 | 0.96 | 0.94 |
| MOA + AA | ||||||||
| T (°C) | Li | Co | Ni | Mn | ||||
| n | lnk | n | lnk | n | lnk | n | lnk | |
| 30 | 0.098 | −0.047 | 0.109 | −0.898 | 0.202 | −0.660 | 0.116 | −0.417 |
| 50 | 0.094 | 0.208 | 0.097 | −0.625 | 0.201 | −0.323 | 0.174 | −0.324 |
| 70 | 0.110 | 0.362 | 0.129 | −0.452 | 0.155 | 0.169 | 0.194 | −0.223 |
| 88 | 0.169 | 0.455 | 0.195 | −0.480 | 0.189 | 0.208 | 0.281 | −0.225 |
| l-Glu + AA | ||||||||
| T (°C) | Li | Co | Ni | Mn | ||||
| n | lnk | n | lnk | n | lnk | n | lnk | |
| 30 | 0.252 | −0.906 | 0.335 | −1.820 | 0.368 | −1.578 | 0.096 | −0.612 |
| 50 | 0.169 | −0.251 | 0.193 | −1.125 | 0.157 | −0.269 | 0.111 | −0.411 |
| 70 | 0.258 | −0.129 | 0.273 | −1.026 | 0.135 | 0.0327 | 0.130 | −0.109 |
| 90 | 0.269 | 0.171 | 0.196 | −0.422 | 0.097 | 0.374 | 0.170 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohbatzadeh, L.; Shafaei Tonkaboni, S.Z.; Noaparast, M.; Entezari-Zarandi, A. A Comparative Study of Malonic and l-Glutamic Acids for Metal Leaching from Spent Lithium-Ion Batteries: Kinetic and Optimization Analysis. Minerals 2023, 13, 1104. https://doi.org/10.3390/min13081104
Sohbatzadeh L, Shafaei Tonkaboni SZ, Noaparast M, Entezari-Zarandi A. A Comparative Study of Malonic and l-Glutamic Acids for Metal Leaching from Spent Lithium-Ion Batteries: Kinetic and Optimization Analysis. Minerals. 2023; 13(8):1104. https://doi.org/10.3390/min13081104
Chicago/Turabian StyleSohbatzadeh, Laleh, Sied Ziaedin Shafaei Tonkaboni, Mohammad Noaparast, and Ali Entezari-Zarandi. 2023. "A Comparative Study of Malonic and l-Glutamic Acids for Metal Leaching from Spent Lithium-Ion Batteries: Kinetic and Optimization Analysis" Minerals 13, no. 8: 1104. https://doi.org/10.3390/min13081104






