Rheology, Setting, Heat of Reaction, and Compressive Strength of a Geopolymer Radioactive Waste Form
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Activator Solution
2.3. Geopolymer Samples Preparation
2.4. Rheology Measurements
2.5. Vicat Needle Test
2.6. Heat of Reaction
2.7. Compressive Strength
2.8. Scanning Electron Microscopy
3. Results
3.1. Rheology
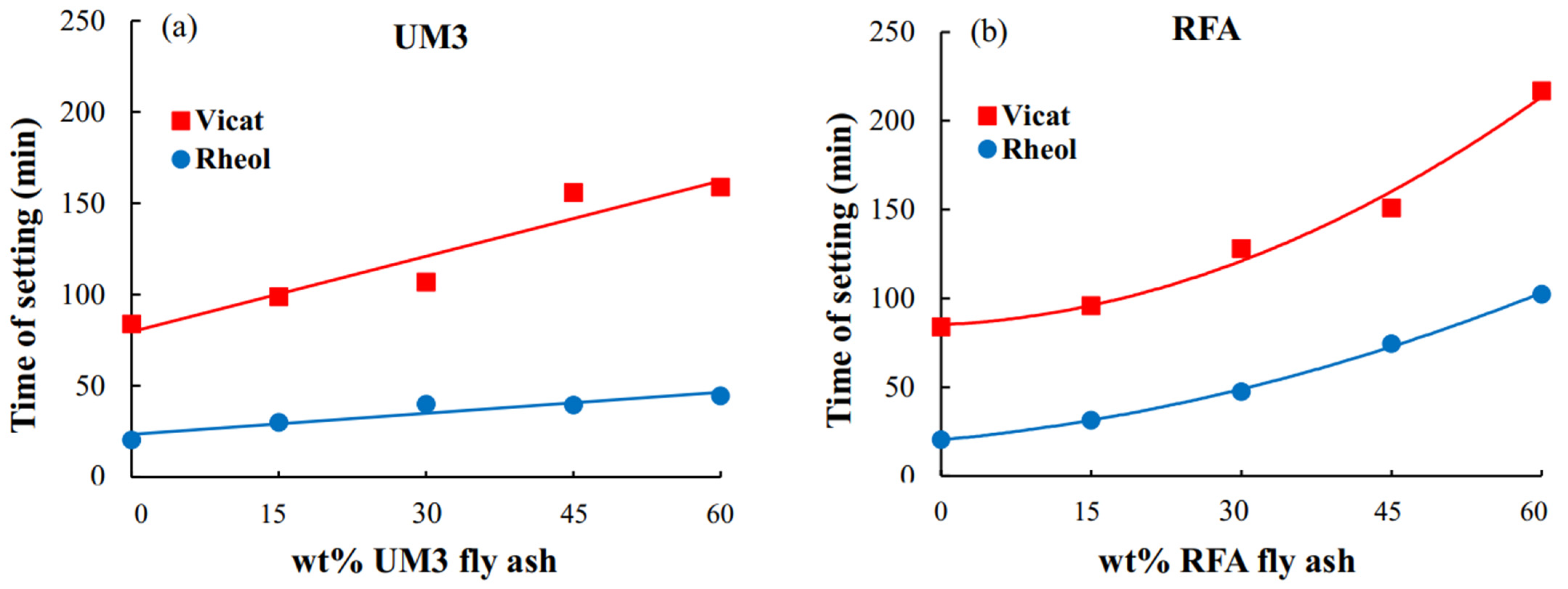
3.2. Vicat Needle Test
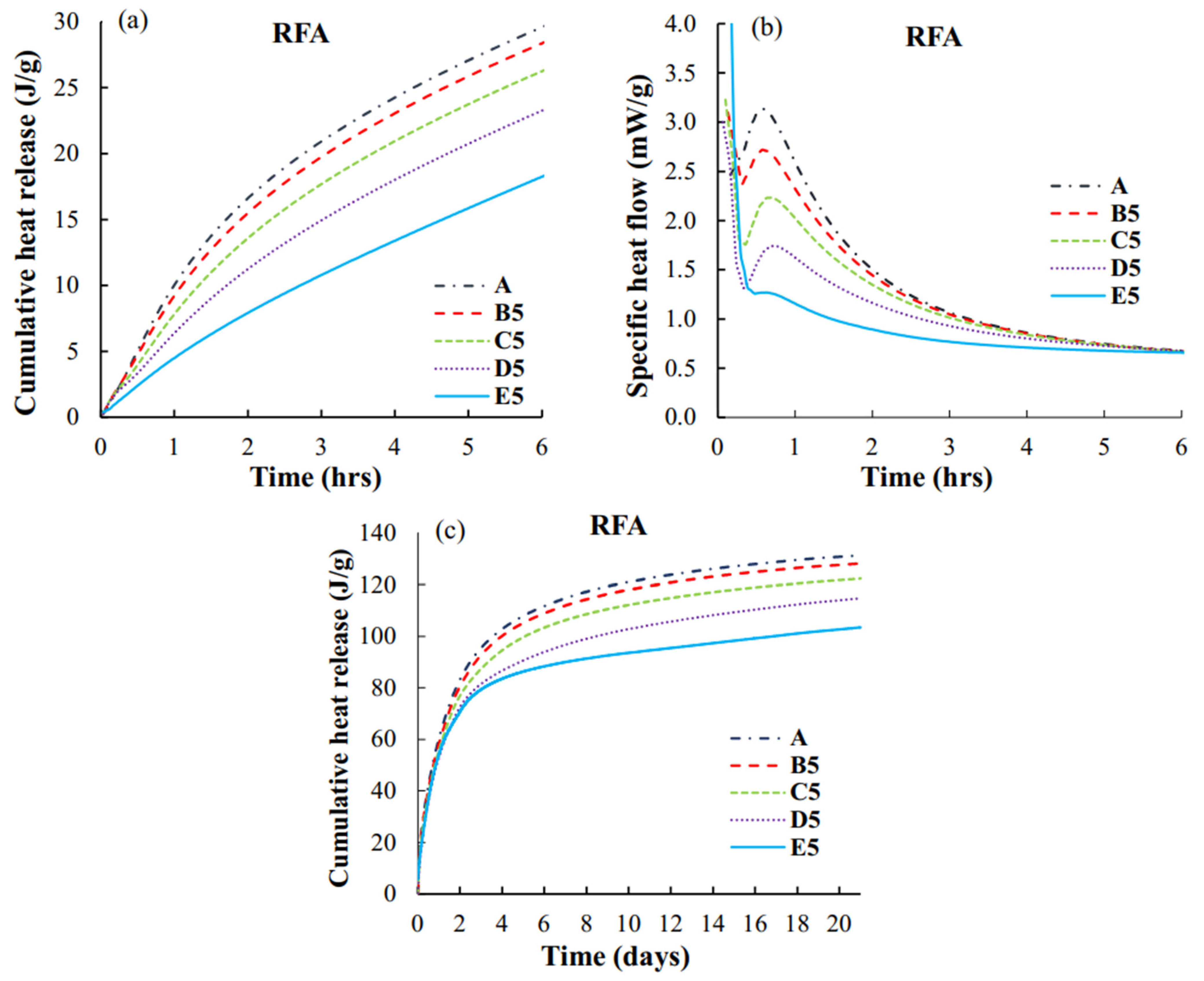
3.3. Heat of Reaction
3.4. Compressive Strength
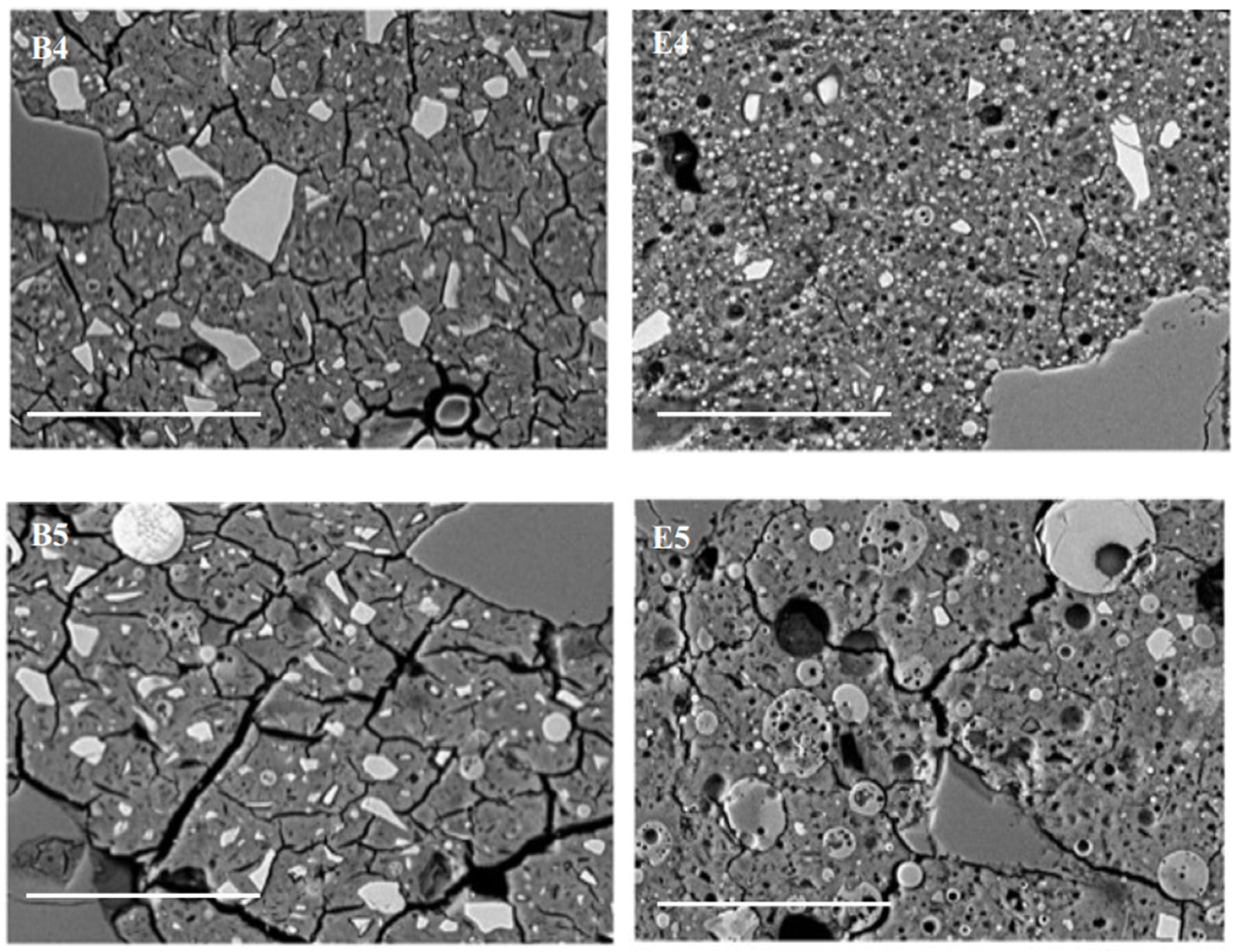
3.5. Scanning Electron Microscopy
4. Discussion
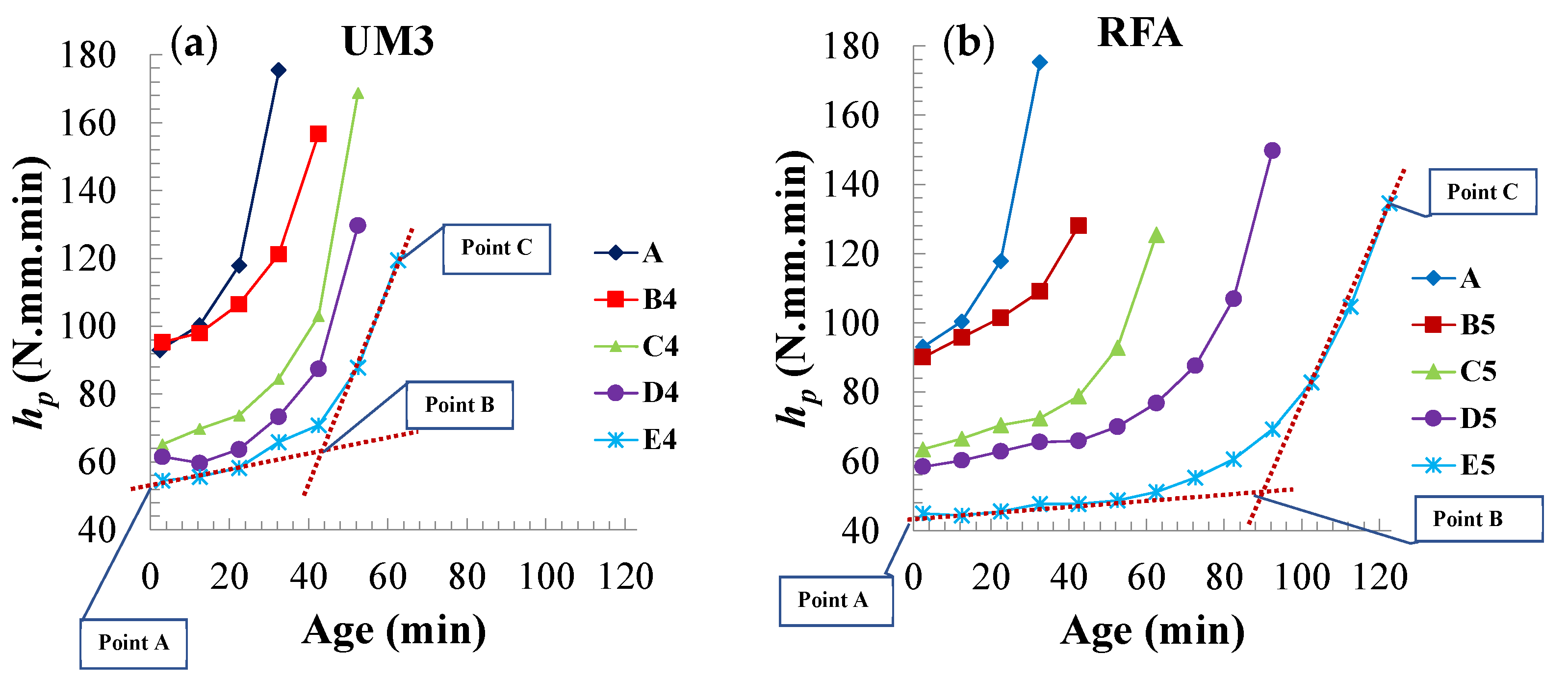
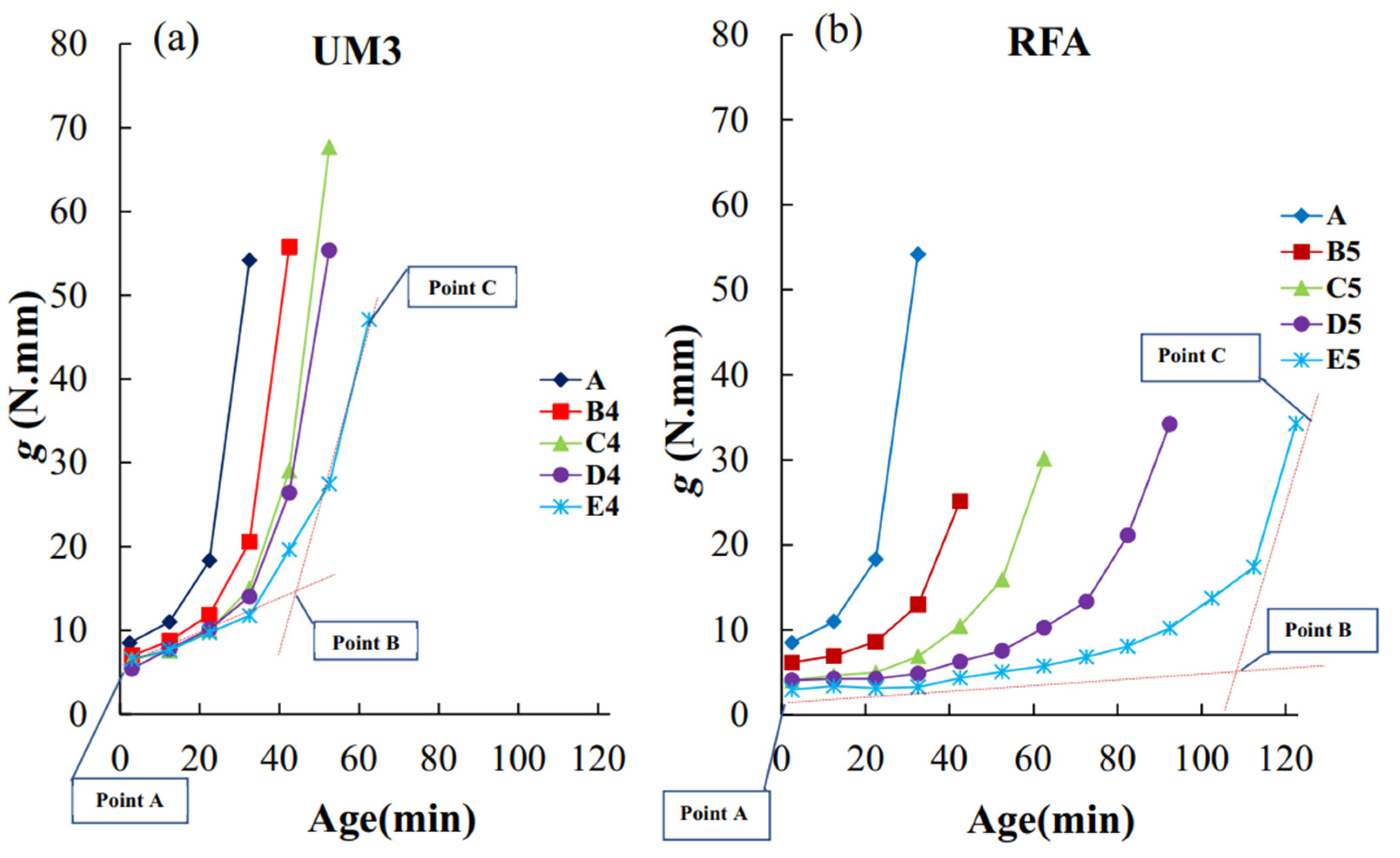
4.1. Rheological Time of Setting, Yield Stress, Plastic Viscosity
4.2. Rheological and Vicat Times of Setting
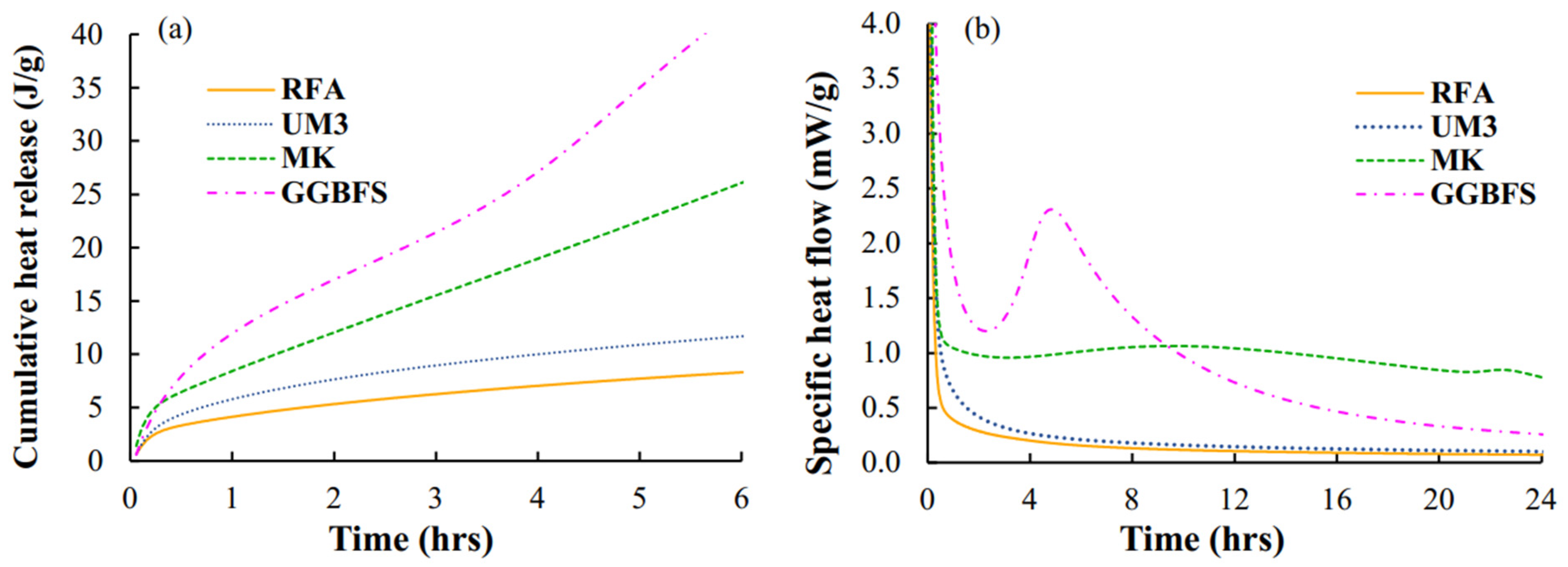
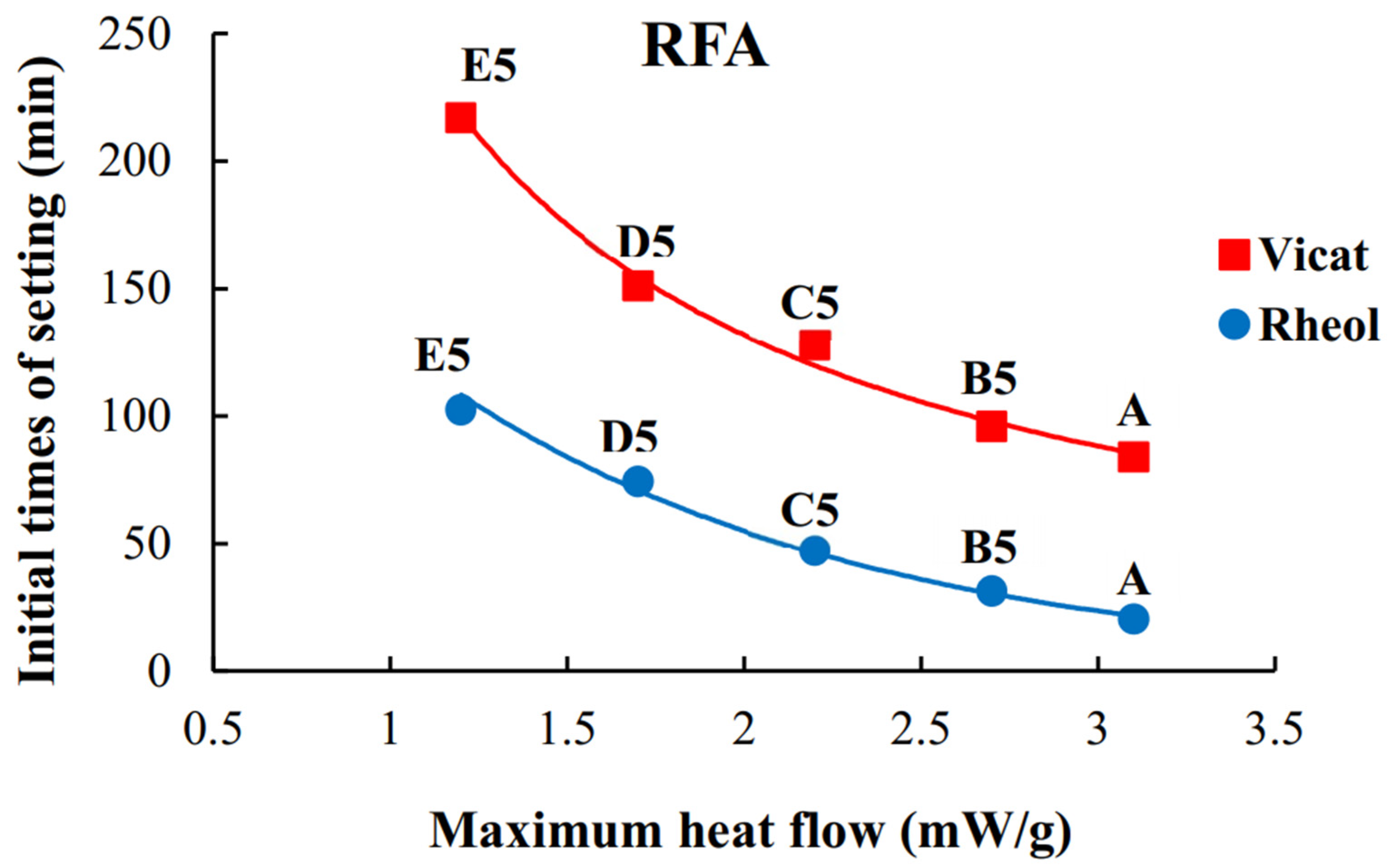
4.3. Heat Flow, Cumulative Heat Release, and Times of Setting
4.4. Heat of Reaction and Mechanical Strength
4.5. Effects of Fly Ash Particle Size
5. Conclusions
- We showed quantitatively that using three instead of two pozzolans significantly widens the property space. This makes it easier to adapt the geopolymer’s composition, its workability (stirring, pumping, pouring), and the workable time to a given large-scale production process;
- Based on plastic viscosity measurements, we reported a rheological time of setting for geopolymers (initial time of setting);
- The rheological time of setting was always shorter than the Vicat initial times of setting;
- Knowledge of both rheological and Vicat setting for a given composition provides sufficient information for how long a paste can be stirred, pumped, or poured. The range of time below and above the rheological setting curve can be considered risk-free and risky, respectively, in terms of workability;
- The higher the maximum heat flow during geopolymerization, the shorter the Vicat- and rheological times of setting;
- The amounts of heat released at rheological and Vicat times of setting are different but independent of the chemical composition of the geopolymers, suggesting that the same mass of gel must always form to initiate setting;
- Cumulative heat release and compressive strength are linearly correlated with the maximum heat flow of the reaction. The confidence of these relationships is high (0.97 < R2 < 0.99). This finding was also seen when the respective literature data for geopolymers were correlated;
- Knowledge of heat generation during waste form development can help us avoid deleterious effects, e.g., excessive water loss during curing, particularly in larger geopolymer waste form units;
- Varying the fly ash particle size affects all materials’ properties significantly.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koťátková, J.; Zatloukal, J.; Reiterman, P.; Kolář, K. Concrete and cement composites used for radioactive waste. J. Environ. Radioact. 2017, 178–179, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rahman, R.O.; Ojovan, M.I. Toward Sustainable Cementitious Radioactive Waste Forms: Immobilization of Problematic Operational Wastes. Sustainability 2021, 13, 11992. [Google Scholar] [CrossRef]
- Staub, A.V.; Reigel, M.M. Development of a performance and processing property acceptance region for cementitious low-level waste forms at Savannah River Site. In Proceedings of the WM Conference, Phoenix, AZ, USA, 24–28 February 2013. [Google Scholar]
- Chung, C.W.; Chun, J.; Um, W.; Sundaram, S.K.; Westsik, J.H., Jr. Setting and stiffening of cementitious components in Cast Stone waste form for disposal of secondary wastes from Hanford waste treatment and immobilization plant. Cem. Conc. Res. 2013, 48, 14–22. [Google Scholar] [CrossRef]
- Lukens, W.W.; Bucher, J.J.; Shuh, D.K.; Edelstein, N.M. Evolution of Technetium Speciation in Reducing Grout. Environ. Sci. Technol. 2005, 39, 8064–8070. [Google Scholar] [CrossRef] [Green Version]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing series in Civil and Structural Engineering; Woodhead Publishing Limited: Oxford, UK; Cambridge, UK; New Delhi, India, 2009. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials; RILEM State-of-Art Reports, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014. [Google Scholar]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. J. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M.; Gao, X.X. Formulation and characterization of blended alkali-activated materials based on flash-calcined metakaolin, fly ash and GGBS. J. Constr. Build. Mater. 2017, 144, 50–64. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag—A guide for Civil Engineer. J. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Gong, W.; Lutze, W.; Pegg, I.L. DuraLith Alkali-Aluminosilicate Geopolymer Waste Form Testing for Hanford Low Activity Waste and LAW Off-Gas Recycles; VSL-11R2360-1/PNNL-20565, PNL Report; Pacific Northwest National Laboratory: Richland, WA, USA, 2011. [Google Scholar]
- Lutze, W.; Gong, W.L.; Xu, H.; Pegg, I.L. A geopolymer waste form for technetium, iodine, and hazardous metals. ATW-Int. J. Nucl. Power 2020, 65, 8–9. [Google Scholar]
- Mattigod, S.V.; Westsik, J.H.; Chung, C.; Lindberg, M.J.; Parker, K.E. Waste Acceptance Testing of Secondary Waste Forms: Cast Stone, Ceramicrete and DuraLith; PNNL-20632; PNL Report; Pacific Northwest National Laboratory: Richland, WA, USA, 2011. [Google Scholar]
- Xu, H.; Gong, W.; Syltebo, L.; Lutze, W.; Pegg, I.L. Geopolymer waste form for Hanford secondary waste: Correlating setting behavior to hydration heat evolution. J. Hazard. Mater. 2014, 278, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Josephson, G.B.; Westsik, J.H.; Pires, R.P.; Bickford, J.; Foote, M.W. Engineering-Scale Demonstration of DuraLith and Ceramicrete Waste Forms; PNNL-20751; PNL Report; Pacific Northwest National Laboratory: Richland, WA, USA, 2011. [Google Scholar]
- Crawford, G.; Martin, R. Portable and Modular Cementation Systems for Stabilization of Nuclear Wastes. In Proceedings of the WM2011 Conference, Phoenix, AZ, USA, 27 February–3 March 2011. [Google Scholar]
- Banfill, P.F.G. The Rheology of fresh cement and concrete: A review. In Proceedings of the 11th International Cement Chemistry Congress, Durban, South Africa, 11–16 May 2003; pp. 50–62. [Google Scholar]
- Bentz, D.P.; Ferraris, C.F.; Galler, M.A.; Hansen, A.S.; Guynn, J.M. Influence of particle size distributions on yield stress and viscosity of cement–fly ash pastes. J. Cem. Concr. Res. 2012, 42, 404–409. [Google Scholar] [CrossRef]
- Wallevik, J.E. Rheological properties of cement paste: Thixotropic behavior and structural breakdown. J. Cem. Concr. Res. 2009, 39, 14–29. [Google Scholar] [CrossRef]
- Bentz, D.P.; Ferraris, C.F. Rheology and setting of high volume fly ash mixtures. J. Cem. Concr. Compos. 2010, 32, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Struble, J.L.; Lei, W.G. Rheological changes associated with setting of cement paste. J. Adv. Cem. Mater. 1995, 2, 224–230. [Google Scholar] [CrossRef]
- Sant, G.; Ferraris, C.F.; Weiss, J. Rheological properties of cement pastes: A discussion of structure formation and mechanical property development. J. Cem. Concr. Res. 2008, 38, 1286–1296. [Google Scholar] [CrossRef]
- Subramaniam, K.V.; Wang, X.J. An investigation of microstructure evolution in cement paste through setting using ultrasonic and rheological measurements. Cem. Concr. Res. 2010, 40, 33–44. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A.; Banfill, P.F.G. Alkali activated fly ash: Effect of admixtures on paste rheology. J. Rheol. Acta 2009, 48, 447–455. [Google Scholar] [CrossRef]
- Palacios, M.; Banfill, P.F.G.; Puertas, F. Rheology and setting of alkali-activated slag pastes and mortars: Effect of organic ad-mixture. J. ACI Mater. 2008, 105, 140–148. [Google Scholar]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; van Deventer, J.S. The interrelationship between surface chemistry and rheology in alkali activated slag paste. J. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; El Idrissi, A.C.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. J. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Kashani, A.; Provis, J.L.; van Deventer, B.B.G.; Qiao, G.G.; van Deventer, J.S.J. Time-resolved yield stress measurement of evolving materials using a creeping sphere. J. Rheol. Acta 2015, 54, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Esteves, L.P.; Cachim, P.; Ferreira, V.M. Effect of fine aggregate on the rheology properties of high performance cement-silica system. J. Constr. Build. Mater. 2010, 24, 640–649. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. J. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Jimenez, A.M.F.; Puertas, F.; Arteaga, A. Determination of Kinetic Equations of Alkaline Activation of Blast Furnace Slag by Means of Calorimetric Data. J. Therm. Anal. Calorim. 1998, 52, 945–955. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Provis, J.L.; Wang, H.; Bullen, F.; Reid, A. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochim. Acta 2013, 565, 163–171. [Google Scholar] [CrossRef]
- Grzeszczyk, S.; Lipowski, G. Effect of content and particle size distribution of high-calcium fly ash on the rheological properties of cement pastes. J. Cem. Concr. Res. 1997, 27, 907–916. [Google Scholar] [CrossRef]
- Amziane, S.; Ferraris, C.F. Cementation paste setting using rheological and pressure measurements. J. ACI Mater. 2007, 104, 137–145. [Google Scholar]
- Sant, G.; Dehadrai, M.; Bentz, D.; Lura, P.; Ferraris, C.F.; Bullard, J.W.; Weiss, W. Detecting the fluid-to-solid transition in cement pastes. Concr. Int. 2009, 31, 53–58. [Google Scholar]
- Gong, W.; Fauvin, M.; Lutze, W.; Akhbarifar, S.; Pegg, I.L. Dissolution and geopolymerization of synthetic fly ash glass. J. Mater. Sci. 2022, 57, 14848–14860. [Google Scholar] [CrossRef]
- Bentz, D.P.; Barrett, T.; De la Varga, I.; Weiss, W. Relating Compressive Strength to Heat Release in Mortars. Adv. Civ. Eng. Mater. 2012, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Aughenbaugh, K.L.; Williamson, T.; Juenger, M.C.G. Critical evaluation of strength prediction methods for alkali-activated fly ash. J. Mater. Struct. 2015, 48, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Frølich, L.; Wadsö, L.; Sandberg, P. Using isothermal calorimetry to predict one day mortar strengths. J. Cem. Concr. Res. 2016, 88, 108–113. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Gong, W.L.; Lutze, W.; Pegg, I.L. Geopolymer Composite for Ultra-High-Performance Concrete Applications. U.S. Patent 909050, 28 July 2015. [Google Scholar]
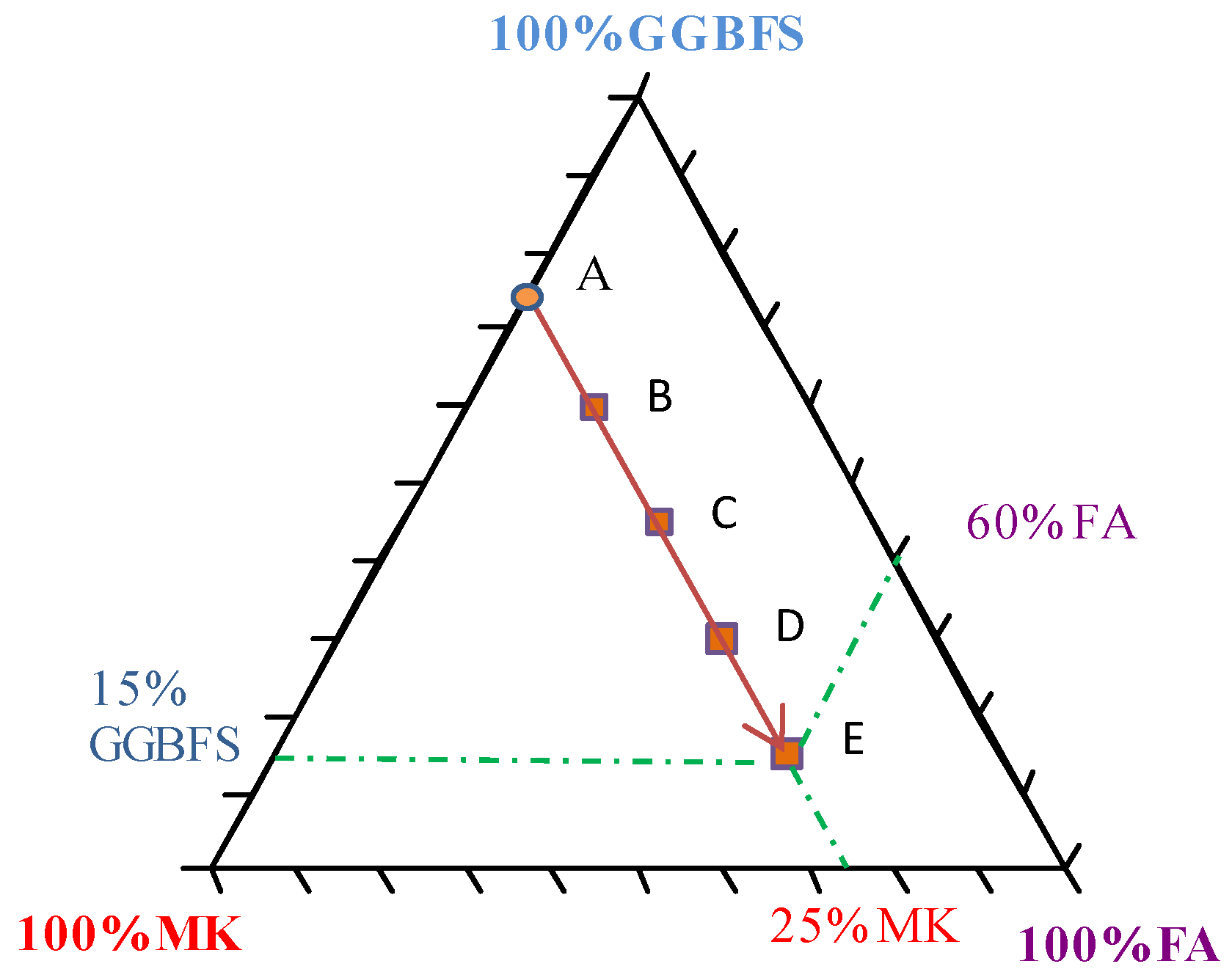

| Chemicals | Mass (g) |
|---|---|
| HSW simulant 6 M Na | 666 |
| Solid KOH (91%) | 143 |
| Solid NaOH (99%) | 12 |
| Silica fume (96%) | 179 |
| %FA | FA | Slag | MK | Sand | SF | Activator | Total | |
|---|---|---|---|---|---|---|---|---|
| A | 0 | 0 | 296 | 103 | 189 | 10 | 402 | 1000 |
| B | 15 | 59 | 237 | 103 | 189 | 10 | 402 | 1000 |
| C | 30 | 118 | 178 | 103 | 189 | 10 | 402 | 1000 |
| D | 45 | 178 | 118 | 103 | 189 | 10 | 402 | 1000 |
| E | 60 | 237 | 59 | 103 | 189 | 10 | 402 | 1000 |
| Raw Materials | Sample ID | Initial Setting (min) | Final Setting (min) |
|---|---|---|---|
| One | GGBFS | 151 | 186 |
| MK | 513 | 780 | |
| UM3 | 207 | 318 | |
| RFA | 1645 | >1980 | |
| Two (GGBFS + MK) | A * | 84 | 177 |
| Three (GGBFS + MK + UM3) | B4 * | 99 | 180 |
| C4 | 107 | 186 | |
| D4 | 156 | 219 | |
| E4 | 159 | 240 | |
| Three (GGBFS + MK + RFA) | B5 * | 96 | 187 |
| C5 | 128 | 211 | |
| D5 | 151 | 240 | |
| E5 | 217 | 285 |
| Time (Days) | Compressive Strength (MPa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A * | UM3 | RFA | |||||||
| B4 | C4 | D4 | E4 | B5 | C5 | D5 | E5 | ||
| 1 | 33 | 23 | 18 | 16 | 14 | 30 | 20 | 14 | 14 |
| 3 | 65 | 49 | 39 | 33 | 25 | 43 | 37 | 29 | 27 |
| 7 | 78 | 76 | 57 | 38 | 35 | 75 | 60 | 43 | 33 |
| 14 | 100 | 93 | 79 | 52 | 43 | 93 | 72 | 55 | 37 |
| 21 | 111 | 105 | 82 | 60 | 53 | 103 | 80 | 64 | 40 |
| 28 | 120 | 107 | 88 | 64 | 56 | 112 | 103 | 72 | 43 |
| Sample ID | Maximum Heat Flow (mW/g) | Cumulative Heat at Vicat Initial Setting (J/g) | Vicat Initial Time of Setting (min) | Cumulative Heat at Rheological Time of Setting (J/g) | Rheological Time of Setting (min) |
|---|---|---|---|---|---|
| A | 3.1 | 12.1 | 84 | 4.7 | 21 |
| B5 | 2.7 | 12.7 | 96 | 6.0 | 32 |
| C5 | 2.2 | 13.0 | 128 | 6.0 | 48 |
| D5 | 1.7 | 12.0 | 151 | 7.1 | 75 |
| E5 | 1.2 | 12.6 | 217 | 7.2 | 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhbarifar, S.; Gong, W.; Lutze, W.; Pegg, I.L. Rheology, Setting, Heat of Reaction, and Compressive Strength of a Geopolymer Radioactive Waste Form. Minerals 2023, 13, 999. https://doi.org/10.3390/min13080999
Akhbarifar S, Gong W, Lutze W, Pegg IL. Rheology, Setting, Heat of Reaction, and Compressive Strength of a Geopolymer Radioactive Waste Form. Minerals. 2023; 13(8):999. https://doi.org/10.3390/min13080999
Chicago/Turabian StyleAkhbarifar, Sepideh, Weiliang Gong, Werner Lutze, and Ian L. Pegg. 2023. "Rheology, Setting, Heat of Reaction, and Compressive Strength of a Geopolymer Radioactive Waste Form" Minerals 13, no. 8: 999. https://doi.org/10.3390/min13080999






