The Mineralogy, Geochemistry and Origin of the Supergene Manganese Occurrences in the Southern Minas Gerais, Brazil
Abstract
:1. Introduction
2. Geological Settings
3. Materials and Methods
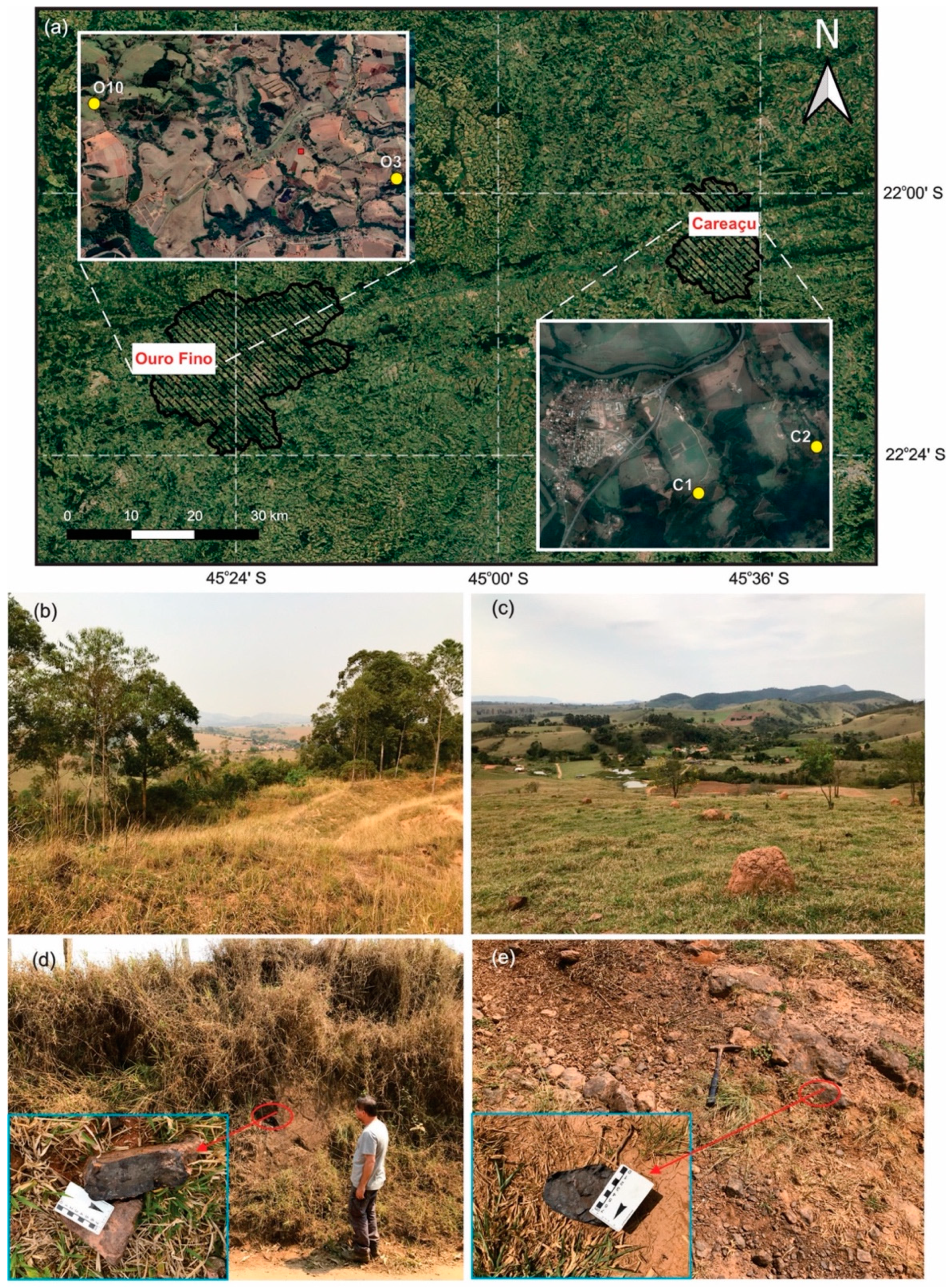
3.1. Study Areas
3.2. Sampling and Analytical Procedures
4. Results
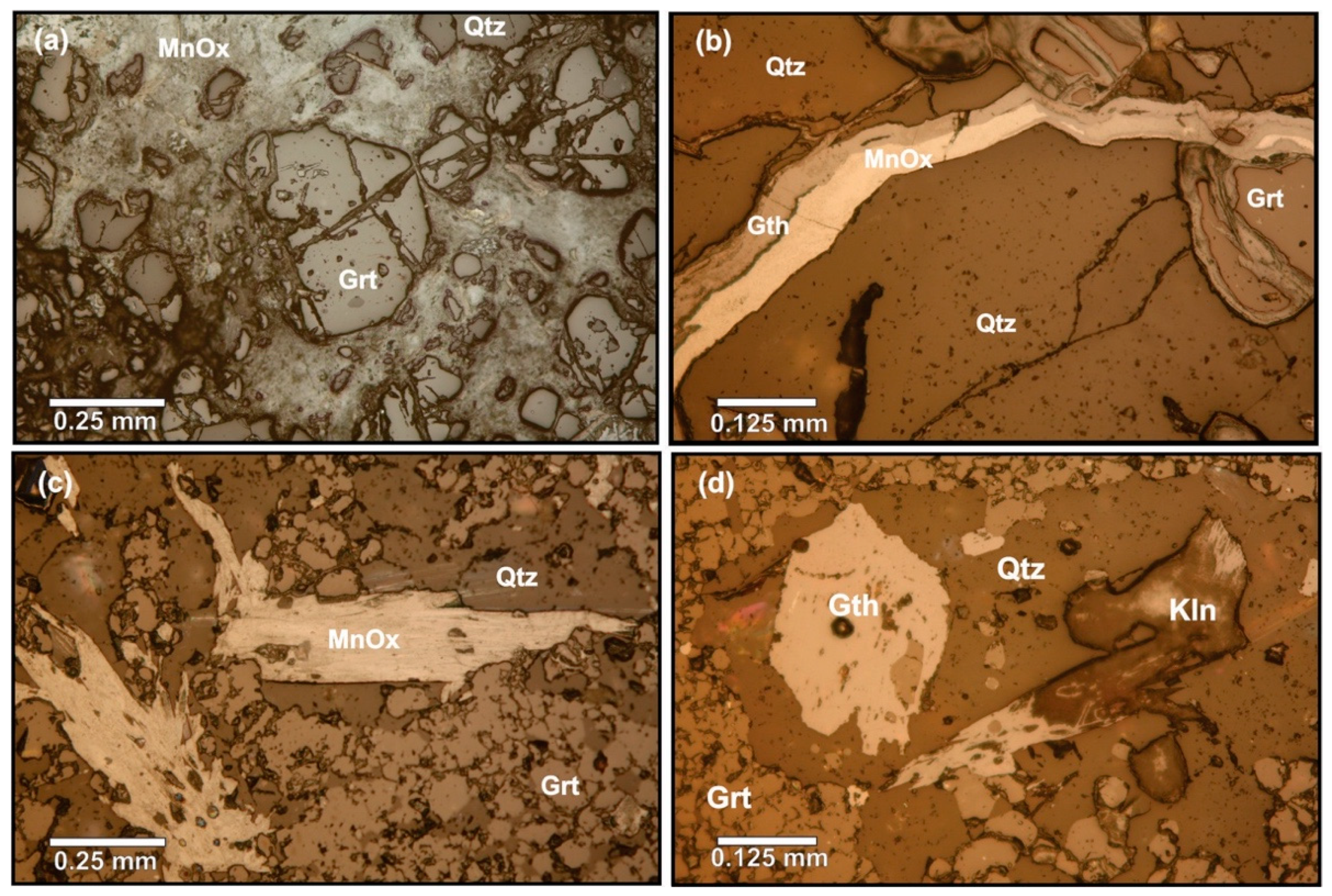
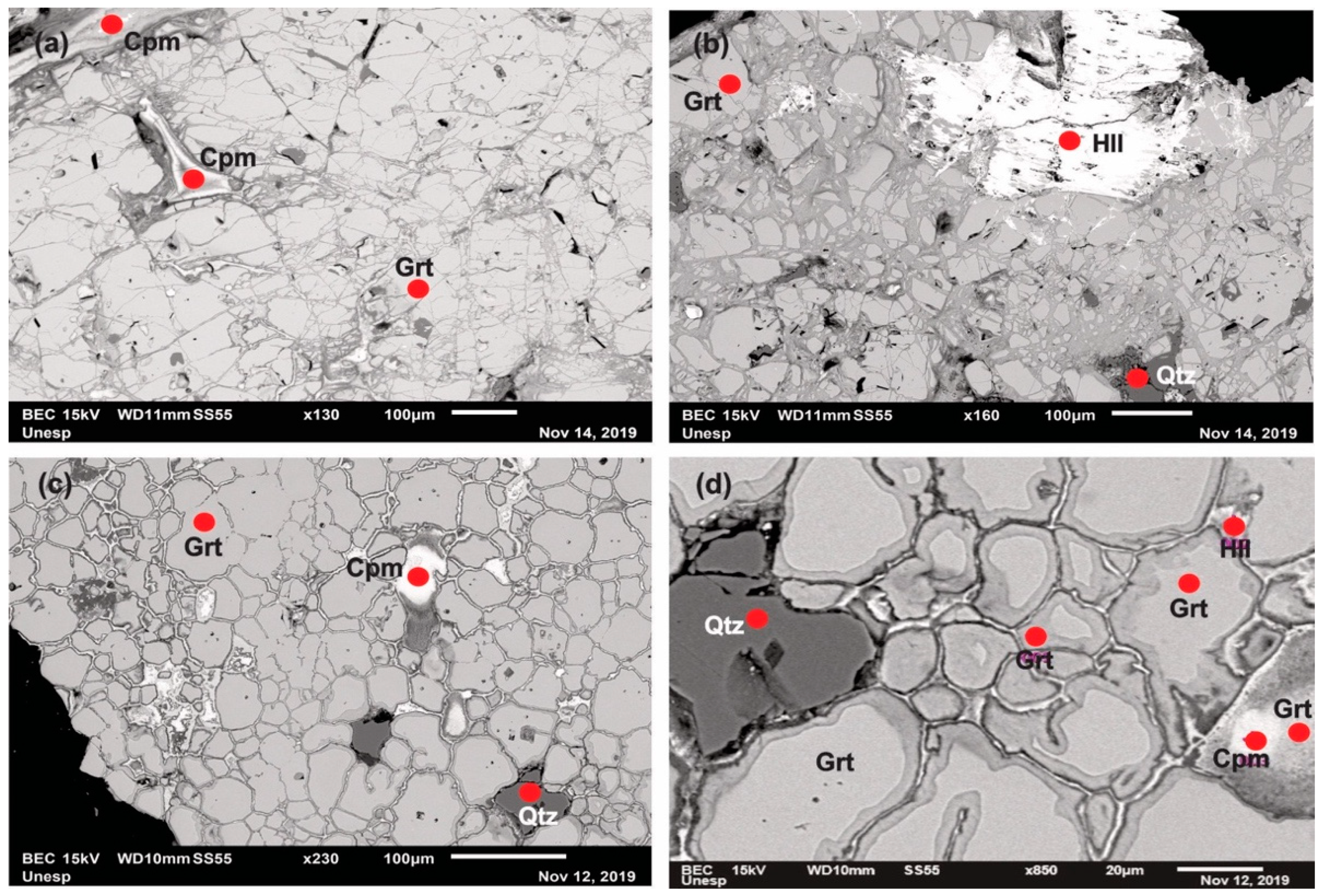
4.1. Petrography
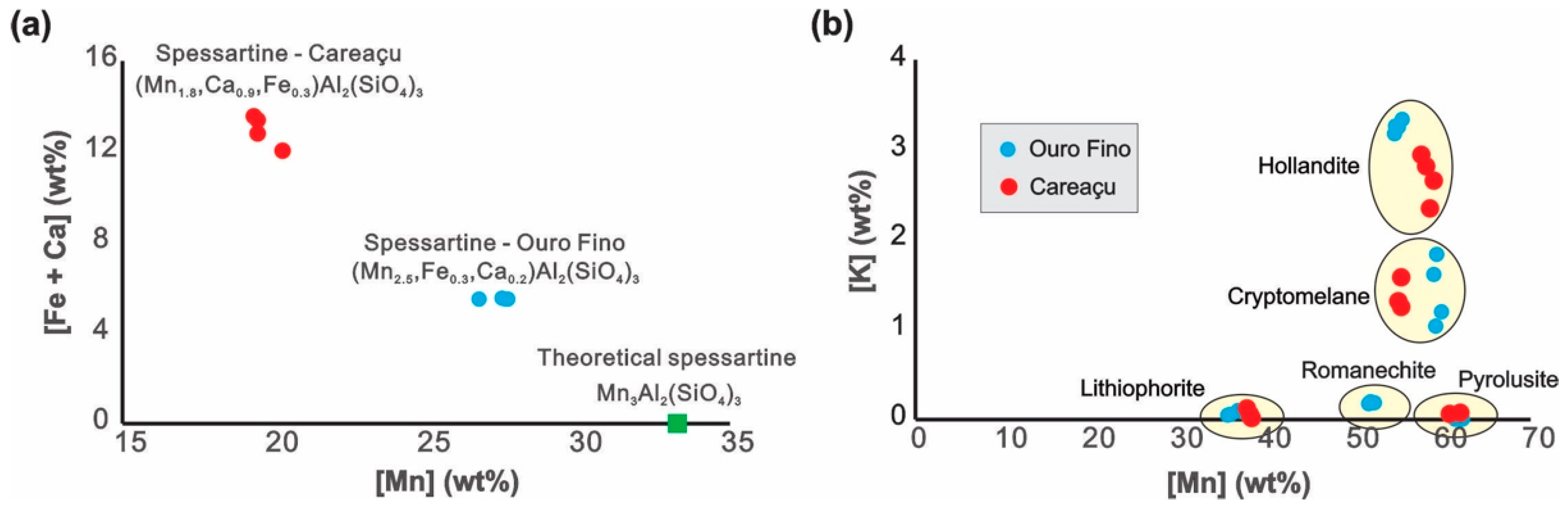
4.2. Chemical Composition
5. Discussion
5.1. Comparison of the Supergene Mn Occurrences in the SBO and Other Brazilian Deposits
5.2. Origin of the Supergene Mn Occurrences
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanton, R.L. Ore Petrology, 1st ed.; McGraw-Hill: New York, NY, USA, 1972; p. 713. [Google Scholar]
- Lima, T.M.; Neves, C.A.R. Sumário Mineral; Departamento Nacional de Produção Mineral: Brasília, Brasil, 2016; Volume 35, pp. 78–79. [Google Scholar]
- Vasconcelos, P.M. K-Ar and 40Ar/39Ar geochronology of weathering processes. Annu. Rev. Earth Planet. Sci. 1999, 27, 183–229. [Google Scholar] [CrossRef]
- Dias, T.G.; Caxito, F. Recursos minerais de Minas Gerais—Manganês. Recur. Min. 2018, 1, 1–18. [Google Scholar]
- IMnL-International Manganese Institute. About Manganese. Available online: https://www.manganese.org/about-manganese/ (accessed on 19 August 2022).
- Roy, S. Mineralogy of the different genetic types of manganese deposits. Econ. Geol. 1968, 63, 760–786. [Google Scholar] [CrossRef]
- Biondi, J.C. Processos Metalogenéticos e os Depósitos Minerais Brasileiros, 1st ed.; Oficina de Textos: Curitiba, Brazil, 2003; pp. 378–423. [Google Scholar]
- Robb, L. Introduction to Ore-Formation Processes, 1st ed.; Blackwell Science Ltd.: Melbourne, Australia, 2005; pp. 219–245. [Google Scholar]
- Kämpf, N.; Curi, N.; Marques, J.J. Óxidos de alumínio, silício, manganês e titânio. In Química e Mineralogia do solo, 1st ed.; Melo, V.F., Alleoni, L.R.F., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brasil, 2009; Volume 1, pp. 573–610. [Google Scholar]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Bernadini, S.; Bellatreccia, F.; Municchia, A.C.; Ventura, G.D.; Sodo, A. Raman spectra of natural manganese oxides. J. Raman Spectrosc. 2019, 50, 873–888. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Tunnel structures. Am. Miner. 2020, 105, 1175–1190. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Layer structures. Am. Miner. 2021, 106, 351–366. [Google Scholar] [CrossRef]
- Costa, M.R.M.; Silva, J.P.A.; Silva, R.D. Sumário Mineral: Manganês; Departamento Nacional de Produção Mineral: Brasília, Brasil, 2018; pp. 1–4. [Google Scholar]
- Medeiros, K.A. Anuário Mineral Brasileiro: Principais Substâncias Metálicas; Agência Nacional de Mineração: Brasília, Brasil, 2022; pp. 1–35. [Google Scholar]
- Pinheiro, W.F.; Filho, O.B.F.; Neves, C.A.R. Anuário Mineral Brasileiro: Principais Substâncias Metálicas; Agência Nacional de Mineração: Brasília, Brasil, 2017; pp. 1–43. [Google Scholar]
- Veríssimo, C.U. Evolução Geológica dos Corpos de Protominério e Mineralizações de Manganês Associadas, Porção Leste de São Paulo e Sul de Minas Gerais. Master’s Thesis, Universidade Estadual Paulista, Rio Claro, Brazil, 1991. [Google Scholar]
- Fermor, L.L. The Manganese Ore Deposits of India. Mem. Geol. Surv. India 1909, 37, 272. [Google Scholar]
- Hasui, Y. A grande colisão pré-cambriana do sudeste brasileiro e a estruturação regional. Geociências 2010, 29, 141–169. [Google Scholar]
- Fuck, R.A.; Jardim de Sá, E.F.; Pimentel, M.M.; Dardenne, M.A.; Pedrosa-Soares, A.C. As faixas de dobramentos marginais do Cráton do São Francisco: Síntese dos conhecimentos. O Cráton São Fr. 1993, 1, 161–186. [Google Scholar]
- Fuck, R.A.; Pimentel, M.M.; D’el-Rey Silva, L.J.H. Compartimentação tectônica na porção oriental da província Tocantins. In Congresso Brasileiro de Geologia; Balneário Camboriú: Santa Catarina, Brasil, 1994; Volume 1, p. 215. [Google Scholar]
- Brito Neves, B.B.; Campos Neto, M.C.; Fuck, R.A. From Rodinia to western Gondwana: An approach to the Brasiliano-pan African cycle and orogenic collage. Episodes 1999, 22, 155–166. [Google Scholar] [CrossRef]
- Campos Neto, M.C. Orogenic systems from southwestern Gondwana, an approach to Brazilian-pan African Cycle and orogenic collage in south-eastern Brazil. In Tectonic Evolution of South America; Cordani, U.G., Milani, E.J., Thomaz Filho, A., Campos, D.A., Eds.; Instituto de Geociências, Universidade de São Paulo: Rio de Janeiro, Brazil, 6–17 August 2000; pp. 335–365. [Google Scholar]
- Trouw, R.A.J.; Nunes, R.P.M.; Castro, E.M.O.; Trouw, C.C.; Matos, G.C. Nota explicativa das Folhas Varginha (SF.23-V-D-VI) e Itajubá (SF.23-Y-B-III). Prog. Geol. Bras. 2008, 1, 1–99. [Google Scholar]
- Pimentel, M.M.; Fuck, R.A. Neoproterozoic crustal accretion in central Brazil. Geology 1992, 20, 375–379. [Google Scholar] [CrossRef]
- Campos Neto, M.C.; Basei, M.A.S. Evolução estrutural brasiliana do nordeste de São Paulo: Dobramentos superpostos e esboço estratigráfico e tectônico. In 4° Simpósio Regional de Geologia; Instituto de Geociencias-Universidade de Sao Paulo: São Paulo, Brasil, 1983; pp. 61–78. [Google Scholar]
- Campos Neto, M.C.; Basei, M.A.S.; Alves, F.R.; Vasconcelos, A.C.B. A nappe de cavalgamento de Socorro (SP-MG). In 33° Congresso Brasileiro de Geologia; Rio de Janeiro, Brasil, 1984; Volume 4, pp. 1809–1822. [Google Scholar]
- Campos Neto, M.C. Evolução do pré-cambriano paulista e regiões adjacentes. In 5° Simpósio Regional de Geologia; São Paulo, Brasil, 1985; Volume 2, pp. 561–576. [Google Scholar]
- Campos Neto, C.M.; Caby, R. Terrane accretion and upward extrusion of high pressure granulites in the Neoproterozoic nappes of Southeast Brazil: Petrological and structural constraints. Tectonics 2000, 19, 669–687. [Google Scholar] [CrossRef]
- Janasi, V.; Alves, A.; Vlach, S.R.F.; Leite, R.J. Granitos peraluminosos da porção central da Faixa Ribeira, Estado de São Paulo: Sucessivos eventos de reciclagem da crosta continental no Neoproterozóico. Geol. USP 2003, 3, 13–24. [Google Scholar] [CrossRef]
- Rocha, B.C.; Moraes, R.; Moller, A.; Cioffi, C.R.; Jercinovic, M.J. Timing of anatexis and melt crystallization in the Socorro–Guaxupé Nappe, SE Brazil: Insights from trace element composition of zircon, monazite and garnet coupled to U-Pb geochronology. Lithos 2017, 277, 337–355. [Google Scholar] [CrossRef]
- Wernick, E. Contribuição à estratigrafia do Pré-Cambriano do leste do estado de São Paulo e áreas vizinhas. Rev. Bras. Geoc. 1978, 8, 206–216. [Google Scholar]
- Zanardo, A. Pesquisa Geológica e de Matérias-Primas Cerâmicas do Centro Nordeste do Estado de São Paulo e Vizinhanças: Sistematização Crítica da Produção Técnico-Científica. Ph.D. Thesis, Universidade Estadual Paulista, Rio Claro, Brazil, 2003. [Google Scholar]
- Ebert, H. Ocorrências de Fácies Granulíticas no Sul de Minas Gerais e áreas adjacentes. Em dependências das estruturas orogênicas: Hipóteses sobre sua origem. Anais Acad. Bras. Ciênc. 1968, 40, 215–229. [Google Scholar]
- Cioffi, C.R.; Neto, M.D.C.C.; Moeller, A.; Rocha, B.C. Paleoproterozoic continental crust generation events at 2.15 and 2.08 Ga in the basement of the southern Brasília Orogen, SE Brazil. Precambrian Res. 2016, 275, 176–196. [Google Scholar] [CrossRef]
- Felicissimo, J. Gondito no estado de São Paulo. Instituto Geográfico e Geológico 1939, 25, 93–125. [Google Scholar]
- Pires, F.R.M.; Leonardos, O.H., Jr.; Parenti Couto, J.G. Gonditos na região de Pouso Alegre, Minas Gerais. Mineração e Metalurgia 1970, 52, 237–239. [Google Scholar]
- Wernick, E.; Fernandes, N.A.; Almeida, N.F., Jr. Gonditos de Socorro e Itapira, SP. Mineração e Metalurgia 1976, 39, 16–21. [Google Scholar]
- Angeli, N.; Khan, H.; Ito, G.M.; De Carvalho, S.G.; Jimenez-Rueda, J.R.; Penha, U.C. Geologia e caracterização tecnológica do minério de manganês da mina Córrego do Cocho, Itapira (SP). Geologia USP 2011, 11, 107–130. [Google Scholar] [CrossRef]
- Ross, J.L.S. Relevo Brasileiro: Uma nova proposta de Classificação. R. Depto. Geogr. 1985, 4, 25–39. [Google Scholar] [CrossRef]
- Brasil. Projeto RADAMBRASIL—Folha SF-23 Vitória/Rio de Janeiro; Ministério das Minas e Energia: Rio de Janeiro, Brazil, 1983. [Google Scholar]
- Köeppen, W. Climatologia: Con un estudio de los climas de la tierra. Fondo Cult. Econ. 1948, 1, 478. [Google Scholar]
- Reboita, M.S.; Rodrigues, M.; Silva, L.F.; Alves, M.A. Aspectos climáticos do estado de Minas Gerais. Rev. Bras. Climat. 2015, 17, 206–226. [Google Scholar]
- Ávila, L.F.; Mello, C.R.D.; Viola, M.R. Mapeamento da precipitação mínima provável para o sul de Minas Gerais. Bras. Eng. Agríc. Ambient. J. 2009, 13, 906–915. [Google Scholar] [CrossRef]
- Zancopé, M.H.C. Análise Morfodinâmica do rio Mogi Guaçu. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2008. [Google Scholar]
- Bacha, A.L.R.; Sardinha, D.S.; Godoy, L.H.; Ancelmi, M.F. Geoquímica de piroclastos intemperizados da Caldeira Vulcânica de Poços de Caldas, Minas Gerias. Geol. USP 2020, 20, 63–80. [Google Scholar]
- Ramdohr, P. The Ore Minerals and Their Intergrowths, 2nd ed.; Pergamon Press: Berlin, Germany, 1980; Volume 2, pp. 1021–1045. [Google Scholar]
- Costa, M.R.M.; Figueredo, R.C. Balanço Mineral: Manganês; Departamento Nacional de Produção Mineral: Brasília, Brasil, 2001; pp. 426–427. [Google Scholar]
- Faria, G.L.D. Estudo da Intensidade de Crepitação de Minérios Granulados de Manganês do Brasil. Master’s Thesis, Universidade Federal de Ouro Preto (UFOP), Ouro Preto, Brazil, 2008. [Google Scholar]
- Walde, D.H.G.; Gierth, E.; Leonardos, O.H. Stratigraphy and mineralogy of the manganese ores of Urucum, Mato Grosso, Brazil. Geol. Rundsch. 1981, 70, 1077–1085. [Google Scholar] [CrossRef]
- Klein, C.; Ladeira, E.A. Geochemistry and mineralogy of Neoproterozoic banded iron-formations and some selected, siliceous manganese formations from the Urucum District, Mato Grosso do Sul, Brazil. Econ. Geol. 2004, 99, 1233–1244. [Google Scholar] [CrossRef]
- Costa, M.L.D.; Fernandez, O.J.C.; Requelme, M.E.R. O depósito de manganês do Azul, Carajás: Estratigrafia, mineralogia, geoquímica e evolução geológica. In Caracterização de Depósitos Minerais em Distritos Mineiros da Amazônia; 2005; pp. 227–334. [Google Scholar]
- Viana, N.C.S. Caracterização mineralógica dos litotipos de minério de manganês sílico-carbonatado da mina morro da mina. In 67° Congresso da Associação Brasileira de Metalurgia, Materiais e Mineração; Rio de Janeiro, Brasil, 2012; pp. 214–221. [Google Scholar]
- Faria, G.L.; Reis, E.L.; Araújo, F.G.S.; Vieira, C.B.; Júnior, N.J. Caracterização química, física e mineralógica do produto granulado de manganês proveniente da Mina de Urucum. In XXIII Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa; Gramado, Brazil, 2009; pp. 87–92. [Google Scholar]
- Vasconcelos, P.M.; Becker, T.A.; Renne, P.R.; Brimhall, G.H. Direct dating of weathering phenomena by K-Ar and 40Ar/39Ar analysis of K-Mn oxides. Geochim. Cosmochim. Acta 1994, 58, 1635–1665. [Google Scholar] [CrossRef]
- Ruffet, G.; Innocent, C.; Michard, A.; Feraud, G.; Beauvais, A.; Nahon, D.; Hamelin, B. A geochronological 40Ar/39Ar and 87Rb/87Sr study of K-Mn oxides from the weathering sequence of Azul, Brazil. Geochim. Cosmochim. Acta 1966, 60, 2219–2232. [Google Scholar] [CrossRef]
- Spier, C.A.; Vasconcelos, P.M.; Oliveira, S.M.B. 40Ar/39Ar geochronological constraints on the evolution of lateritic iron deposits in the Quadrilatero Ferrifero, Minas Gerais, Brazil. Chem. Geol. 2006, 234, 79–104. [Google Scholar] [CrossRef]
- Piacentini, T.; Vasconcelos, P.M.; Farley, K.A. 40Ar/39Ar constraints on the age and thermal history of the Urucum Neoproterozoic banded iron-formation, Brazil. Precambrian Res. 2013, 228, 48–62. [Google Scholar] [CrossRef]
- Vasconcelos, P.M.; Farley, K.A.; Stone, J.; Piacentini, T.; Fifield, L.K. Stranded landscapes in the humid tropics: Earth’s oldest land surface. Earth Planet. Sci. Lett. 2019, 519, 152–164. [Google Scholar] [CrossRef]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved load. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Martini, I.P.; Chesworth, W. Weathering, Soils and Paleosols, 1st ed.; Elsevier Science Publications: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Pedro, G. Essai sur la caracterisation geochimique des differents processus zonaux resultant de l’alteration des roches superficielles (cycle aluminosilicique). Comptes Rendus Hebd. Seances Acad. Sci. Ser. 1966, 262, 1828–1831. [Google Scholar]
- Tardy, Y. Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. Chem. Geol. 1971, 7, 253–271. [Google Scholar] [CrossRef]
- White, A.F.; Blum, A.E. Effects of climate on chemical weathering in watersheds. Geochim. Cosmochim. Acta 1995, 59, 1729–1747. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Oliva, P.; Viers, J.; Dupré, B. Chemical weathering in granitic environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- West, A.J.; Galy, A.; Bickle, M. Tectonic and climatic controls on silicate weathering. Earth Planet. Sci. Lett. 2005, 235, 211–228. [Google Scholar] [CrossRef]
- Dessert, C.; Dupré, B.; François, L.M.; Schott, J.; Gaillardet, J.; Chakrapani, G.; Bajpai, S. Erosion of Deccan Traps determined by river geochemistry: Impact on the global cli- mate and the 87Sr/86Sr ratio of seawater. Earth Planet. Sci. Lett. 2001, 188, 459–474. [Google Scholar] [CrossRef]
- Dessert, C.; Dupré, B.; Gaillardet, J.; François, L.M.; Allègre, C.J. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 2003, 202, 257–273. [Google Scholar] [CrossRef]
- Rimstidt, J.D. Quartz solubility at low temperatures. Geochim. Cosmochim. Acta 1997, 61, 2552–2558. [Google Scholar] [CrossRef]
- Velbel, M.A. Natural weathering mechanisms of almandine garnet. Geology 1984, 12, 631–634. [Google Scholar] [CrossRef]
- Hem, J.D. Chemical Equilibria and Rates of Manganese Oxidation; US Government Printing Office: Washington, DC, USA, 1963. [Google Scholar]
- Vasconcelos, P.M.; Carmo, I.O. Calibrating denudation chronology through 40Ar/39Ar weathering geochronology. Earth-Sci. R. 2018, 179, 411–435. [Google Scholar] [CrossRef]
- Carmo, I.O.; Vasconcelos, P.M. 40Ar/39Ar geochronology constraints on Late Miocene weathering rates in Minas Gerais, Brazil. Earth Planet. Sci. Lett. 2006, 241, 80–94. [Google Scholar] [CrossRef]
- Hénoque, O.; Ruffet, G.; Colin, F.; Feraud, G. 40Ar/39Ar dating of West African lateritic cryptomelanes. Geochim. Cosmochim. Acta 1998, 62, 2739–2756. [Google Scholar] [CrossRef]
- Dammer, D.; McDougall, I.; Chivas, A.R. Timing of weathering-induced alteration of manganese deposits in Western Australia: Evidence from K/Ar and 40Ar/39Ar dating. Econ. Geol 1999, 94, 87–108. [Google Scholar] [CrossRef]
- Bonnet, N.J.; Beauvais, A.; Arnaud, N.; Chardon, D.; Jayanada, J. Cenozoic lateritic weathering and erosion history of Peninsular India from 40Ar/39Ar dating of supergene K-Mn oxides. Chem. Geol. 2016, 446, 33–53. [Google Scholar] [CrossRef]
- De Putter, T.; Ruffet, G.; Yans, J.; Mees, F. The age of supergene manganese deposits in Katanga and its implications for the Neogene evolution of the African Great Lakes Region. Ore Geol. Rev. 2015, 71, 350–362. [Google Scholar] [CrossRef]
- De Putter, T.; Liégeois, J.; Dewaele, S.; Caiteux, J.; Mees, F. Paleoproterozoic manganese and base deposits at Kisenge-Kamata (Katanga, D.R. Congo). Ore Geol. Rev. 2018, 96, 181–200. [Google Scholar] [CrossRef]
- Dekoninck, A.; Monié, P.; Blockmans, S.; Hatert, F.; Rochez, G.; Yans, J. Genesis and 40Ar/39Ar dating of K-Mn oxides from Stavelot Massif (Ardenne, Belgium): Insights into Oligocene to Pliocene weathering periods in Western Europe. Ore Geol. Rev. 2019, 115, 103191. [Google Scholar] [CrossRef]
- De Putter, T.; Ruffet, G. Supergene manganese ore records 75 Myr-long Campanian to Pleistocene geodynamic evolution and weathering history of the Central African Greta Lakes Region—Tectonics drives, climate assists. Gondwana Res. 2020, 83, 96–117. [Google Scholar] [CrossRef]
- Pharoe, B.K.; Evdokimov, A.N., Gembitskaya; Gembitskaya, I.M.; Bushuyev, Y.Y. Mineralogy, geochemistry and genesis of the post-Gondwana supergene manganese deposit of the Carletonville-Ventersdorp area, North West Province, South Africa. Ore Geol. Rev. 2020, 120, 103372. [Google Scholar] [CrossRef]
- Conceição, F.T.; Bonotto, D.M. Use of U-isotopes disequilibrium to evaluate the weathering rates and fertilizer derived uranium at São Paulo State, Brazil. Environ. Geol. 2003, 44, 408–418. [Google Scholar] [CrossRef]
- Conceição, F.T.; Bonotto, D.M. Weathering rates and anthropogenic influences in a sedimentary basin, São Paulo State, Brazil. Appl. Geochem. 2004, 19, 575–591. [Google Scholar] [CrossRef]
- Conceição, F.T.; Sardinha, D.S.; Souza, A.D.G.; Bonito, D.M. Hydrochemical relationship at Meio Stream watershed (Leme city), São Paulo State, Brazil. Rev. Bras. Geoc. 2007, 37, 389–400. [Google Scholar]
- Sardinha, D.S.; Conceição, F.T.; Bonotto, D.M.; Salles, M.H.F.; Angelucci, V.A. Avaliação do balanço anual de cátions e ânions na bacia do Alto Sorocaba (SP). Rev. Bras. Geoc. 2008, 38, 730–740. [Google Scholar] [CrossRef]
- Conceição, F.T.; Sardinha, D.S.; Souza, A.D.G.; Navarro, G.R.B. Anthropogenic influences on annual flux of cations and anions at Meio Stream basin, São Paulo State, Brazil. Water Air Soil Pollut. 2010, 205, 79–91. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Bonotto, D.M.; Conceição, F.T. Weathering rates at Alto Sorocaba basin, Brazil, using U-isotopes and major cations. Environ. Earth Sci. 2010, 61, 1025–1036. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Bonotto, D.M.; Godoy, L.H.; Conceição, F.T.; Moreno, M.M.T. Denudação química e implicações na qualidade das águas superficiais da bacia do Rio Jaú (SP). Rev. Bras. Geoc. 2012, 13, 337–349. [Google Scholar]
- Conceição, F.T.; Santos, C.M.; Sardinha, D.S.; Navarro, G.R.B.; Godoy, L.H. Chemical weathering rate, denudation rate, and atmospheric and soil CO2 consumption of Paraná flood basalts in São Paulo state, Brazil. Geomorphology 2015, 233, 41–51. [Google Scholar] [CrossRef]
- Couto Júnior, A.A.; Conceição, F.T.; Fernandes, A.M.; Cunha, C.M.L.; Spatti Júnior, E.P. Geoquímica fluvial aplicada à avaliação das taxas de intemperismo químico e remoção de solo da Formação Rio Claro. Ver. Bras. Geomorfol. 2016, 17, 451–464. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Conceição, F.T.; Spatti Junior, E.P.; Sardinha, D.S.; Mortatti, J. Chemical weathering rates and atmospheric/soil CO2 consumption of igneous and metamorphic rocks under tropical climate in southeastern Brazil. Chem. Geol. 2016, 443, 54–66. [Google Scholar] [CrossRef]
- Sardinha, D.S.; Godoy, L.H.; Conceição, C.T. Taxa de intemperismo e consumo de CO2 em relevo cuestiforme com substrato basáltico e arenítico no estado de São Paulo. Geol. USP 2019, 19, 117–134. [Google Scholar] [CrossRef]
- Spatti Júnior, E.P.; Conceição, F.T.; Fernandes, A.M.; Sardinha, D.S.; Moruzzi, R.B. Chemical weathering rates of clastic sedimentary rocks from the Paraná Basin in the Paulista Peripheral Depression, Brazil. J. South Am. Earth Sci. 2019, 96, 102–369. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Conceição, F.T.; Spatti Júnior, E.P.; Couto Júnior, A.A.; Hissler, C.; Mortatti, J. Human influences on the present denudation rates of the Paulista Peripheral Depression, Brazil. Geomorphology 2020, 351, 106–955. [Google Scholar] [CrossRef]
- Conceição, F.T.; Vasconcelos, P.M.; Godoy, L.H.; Navarro, G.R.B.; Montibeller, C.C.; Sardinha, D.S. Water/rock interactions, chemical weathering and erosion, and supergene enrichment in the Tapira and Catalão I alkaline-carbonatite complexes, Brazil. J. Geochem. Explor. 2022, 237, 106–999. [Google Scholar] [CrossRef]
- Meneguel, E.C.; Conceição, F.T.; Navarro, G.R.B.; Christofoletti, S.R.; Mota, J.F.M. Chemical weathering processes generating the raw material used in porcelain stoneware industry in the São Paulo State, Brazil. J. South Am. Earth Sci. 2022, 118, 103–926. [Google Scholar] [CrossRef]
- Ling, F.T.; Post, J.E.; Heaney, P.J.; Santelli, C.M.; Ilton, E.S.; Burgos, W.D.; Rose, A.W. A multi-method characterization of natural terrestrial birnessites. Am. Miner. 2020, 105, 833–847. [Google Scholar] [CrossRef]



| Sample | Na2O | K2O | MgO | CaO | SiO2 | TiO2 | Al2O3 | Fe2O3 | P2O5 | MnO | BaO | LOI 1 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouro Fino | |||||||||||||
| O3a | <0.01 | 0.21 | 0.22 | 0.26 | 36.10 | 0.53 | 13.60 | 5.41 | 0.10 | 28.40 | 0.39 | 13.49 | 98.71 |
| O3b | <0.01 | 0.67 | 0.16 | 0.76 | 35.70 | 0.47 | 14.90 | 6.14 | 0.19 | 27.40 | 0.12 | 12.03 | 98.54 |
| O10a | <0.01 | 0.11 | 0.29 | 1.14 | 39.10 | 0.28 | 13.20 | 6.87 | 0.13 | 25.50 | 0.32 | 11.42 | 98.36 |
| O10b | <0.01 | 0.25 | 0.45 | 1.61 | 38.10 | 0.61 | 12.20 | 7.53 | 0.15 | 26.90 | 0.17 | 10.89 | 98.86 |
| Careaçu | |||||||||||||
| C1a | <0.01 | 0.07 | 1.26 | 5.93 | 33.20 | 0.15 | 18.60 | 10.40 | 0.08 | 19.50 | 0.04 | 7.43 | 97.66 |
| C1b | <0.01 | 0.19 | 1.50 | 3.24 | 32.50 | 0.32 | 19.90 | 10.21 | 0.08 | 21.20 | 0.13 | 8.68 | 97.95 |
| C2a | <0.01 | 0.08 | 1.16 | 4.92 | 39.60 | 0.15 | 16.80 | 12.20 | 0.08 | 16.80 | 0.12 | 6.28 | 98.19 |
| C2b | <0.01 | 0.06 | 1.14 | 4.99 | 38.30 | 0.30 | 16.30 | 13.19 | 0.18 | 17.30 | 0.07 | 6.81 | 98.64 |
| Sample | Mineral | O | Al | Si | Na | Mg | Ca | Ba | Ti | K | P | Mn | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouro Fino | ||||||||||||||
| O3 | Spessartine | 40.85 | 10.95 | 14.58 | <0.01 | 0.55 | 2.13 | <0.01 | 0.09 | 0.01 | 0.01 | 26.74 | 3.35 | 99.26 |
| O3 | Spessartine | 39.23 | 10.91 | 15.95 | 0.01 | 0.51 | 2.17 | <0.01 | 0.11 | <0.01 | <0.01 | 27.65 | 3.30 | 99.83 |
| O10 | Spessartine | 40.70 | 10.50 | 14.02 | 0.01 | 0.52 | 2.41 | <0.01 | 0.14 | <0.01 | <0.01 | 27.63 | 3.32 | 99.24 |
| O10 | Spessartine | 39.90 | 10.99 | 15.15 | <0.01 | 0.51 | 2.13 | <0.01 | 0.15 | <0.01 | <0.01 | 27.51 | 3.39 | 99.74 |
| O3 | Cryptomelane | 36.29 | 0.02 | 0.58 | <0.01 | 0.05 | <0.01 | <0.01 | <0.01 | 1.61 | 0.08 | 58.98 | 0.81 | 98.41 |
| O3 | Cryptomelane | 35.90 | 0.35 | 0.34 | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 | 1.20 | 0.09 | 59.85 | 0.85 | 98.63 |
| O10 | Cryptomelane | 35.72 | 0.25 | 0.26 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 1.84 | 0.08 | 59.30 | 0.82 | 98.30 |
| O10 | Cryptomelane | 36.74 | 0.62 | 0.39 | <0.01 | 0.03 | <0.01 | <0.01 | <0.01 | 1.04 | 0.11 | 59.26 | 0.86 | 99.05 |
| O3 | Romanechite | 34.78 | 0.11 | 0.08 | 0.02 | 0.02 | 0.05 | 11.09 | 0.02 | 0.18 | 0.38 | 51.89 | 0.11 | 98.72 |
| O3 | Romanechite | 35.19 | 0.08 | 0.08 | 0.02 | 0.02 | 0.06 | 11.26 | 0.02 | 0.17 | 0.40 | 51.57 | 0.07 | 98.93 |
| O10 | Romanechite | 35.45 | 0.09 | 0.08 | 0.02 | 0.01 | 0.06 | 11.09 | 0.02 | 0.18 | 0.41 | 52.24 | 0.10 | 99.75 |
| O10 | Romanechite | 35.42 | 0.14 | 0.07 | 0.02 | 0.02 | 0.05 | 10.65 | 0.03 | 0.20 | 0.36 | 51.83 | 0.13 | 98.91 |
| O3 | Hollandite | 37.32 | 0.72 | 0.05 | 0.18 | 0.06 | <0.01 | 3.29 | <0.01 | 3.26 | 0.11 | 54.71 | 0.11 | 99.81 |
| O3 | Hollandite | 36.56 | 0.77 | 0.06 | 0.20 | 0.05 | <0.01 | 3.23 | <0.01 | 3.25 | 0.11 | 54.95 | 0.10 | 99.29 |
| O10 | Hollandite | 36.82 | 0.83 | 0.06 | 0.20 | 0.06 | <0.01 | 3.40 | <0.01 | 3.18 | 0.11 | 54.54 | 0.14 | 99.35 |
| O10 | Hollandite | 36.19 | 0.58 | 0.06 | 0.25 | 0.05 | <0.01 | 3.12 | <0.01 | 3.34 | 0.10 | 55.45 | 0.12 | 99.27 |
| O3 | Pyrolusite | 35.83 | 0.25 | 0.80 | 0.01 | 0.02 | 0.09 | 0.17 | <0.01 | <0.01 | <0.01 | 62.24 | 0.42 | 99.83 |
| O3 | Pyrolusite | 36.06 | 0.23 | 0.68 | 0.01 | 0.03 | 0.10 | 0.21 | <0.01 | <0.01 | <0.01 | 61.96 | 0.46 | 99.74 |
| O10 | Pyrolusite | 36.12 | 0.26 | 0.71 | 0.01 | 0.03 | 0.11 | 0.15 | <0.01 | <0.01 | <0.01 | 61.42 | 0.40 | 99.21 |
| O10 | Pyrolusite | 36.23 | 0.27 | 0.73 | 0.01 | 0.03 | 0.08 | 0.20 | <0.01 | <0.01 | <0.01 | 61.37 | 0.51 | 99.43 |
| O3 | Lithiophorite | 43.00 | 13.94 | 0.26 | <0.01 | 0.04 | 0.04 | 0.44 | 0.14 | 0.05 | 0.18 | 35.64 | 4.51 | 98.24 |
| O3 | Lithiophorite | 42.22 | 14.35 | 0.28 | <0.01 | 0.05 | 0.03 | 0.55 | 0.16 | 0.06 | 0.20 | 35.97 | 4.18 | 98.05 |
| O10 | Lithiophorite | 41.95 | 13.40 | 0.22 | 0.02 | 0.04 | 0.04 | 0.40 | 0.01 | 0.09 | 0.05 | 37.04 | 4.95 | 98.21 |
| O10 | Lithiophorite | 42.55 | 13.64 | 0.12 | 0.01 | 0.03 | 0.01 | 0.51 | 0.00 | 0.10 | 0.03 | 36.84 | 4.83 | 98.67 |
| Careaçu | ||||||||||||||
| C1 | Spessartine | 39.02 | 11.13 | 16.45 | 0.01 | 0.87 | 9.67 | <0.01 | 0.04 | <0.01 | <0.01 | 19.46 | 3.12 | 99.76 |
| C1 | Spessartine | 38.95 | 11.06 | 16.43 | 0.01 | 0.85 | 9.10 | 0.02 | 0.04 | <0.01 | <0.01 | 20.28 | 2.92 | 99.64 |
| C2 | Spessartine | 39.16 | 11.04 | 16.05 | 0.01 | <0.01 | 9.32 | 0.01 | 0.02 | <0.01 | 0.01 | 19.31 | 4.21 | 99.14 |
| C2 | Spessartine | 39.23 | 10.76 | 16.10 | 0.03 | 0.03 | 9.15 | 0.01 | <0.01 | 0.01 | 0.01 | 19.46 | 4.24 | 99.03 |
| C1 | Cryptomelane | 35.81 | 0.03 | 0.05 | 0.12 | 0.01 | <0.01 | <0.01 | <0.01 | 2.66 | 0.19 | 58.97 | 0.59 | 98.43 |
| C1 | Cryptomelane | 36.70 | 0.15 | 0.06 | 0.13 | 0.02 | <0.01 | <0.01 | <0.01 | 2.82 | 0.19 | 58.07 | 0.65 | 98.79 |
| C2 | Cryptomelane | 36.91 | 0.14 | 0.08 | 0.17 | 0.02 | <0.01 | <0.01 | <0.01 | 2.95 | 0.24 | 57.56 | 0.63 | 98.70 |
| C2 | Cryptomelane | 36.60 | 0.22 | 0.18 | 0.07 | 0.04 | <0.01 | <0.01 | <0.01 | 2.35 | 0.17 | 58.51 | 0.94 | 99.08 |
| C1 | Hollandite | 36.64 | 0.16 | 0.11 | 0.04 | 0.03 | 0.09 | 4.47 | <0.01 | 1.59 | 0.20 | 55.23 | 0.59 | 99.15 |
| C1 | Hollandite | 36.01 | 0.14 | 0.08 | 0.17 | 0.05 | 0.03 | 4.99 | <0.01 | 2.66 | 0.19 | 54.05 | 0.65 | 99.02 |
| C2 | Hollandite | 36.60 | 0.22 | 0.18 | 0.07 | 0.02 | 0.05 | 4.04 | <0.01 | 2.32 | 0.24 | 54.93 | 0.36 | 99.03 |
| C2 | Hollandite | 36.03 | 0.17 | 0.11 | 0.07 | 0.05 | 0.03 | 4.82 | <0.01 | 2.26 | 0.17 | 55.29 | 0.63 | 99.63 |
| C1 | Pyrolusite | 35.10 | 0.21 | 0.74 | 0.01 | 0.03 | 0.10 | 0.18 | <0.01 | <0.01 | <0.01 | 63.06 | 0.41 | 99.84 |
| C1 | Pyrolusite | 35.22 | 0.24 | 0.69 | 0.01 | 0.02 | 0.11 | 0.20 | <0.01 | <0.01 | <0.01 | 62.88 | 0.50 | 99.87 |
| C2 | Pyrolusite | 34.89 | 0.22 | 0.70 | 0.01 | 0.03 | 0.10 | 0.17 | <0.01 | <0.01 | <0.01 | 62.63 | 0.44 | 99.19 |
| C2 | Pyrolusite | 35.16 | 0.23 | 0.72 | 0.01 | 0.02 | 0.09 | 0.19 | <0.01 | <0.01 | <0.01 | 62.91 | 0.47 | 99.80 |
| C1 | Lithiophorite | 45.09 | 13.21 | 0.02 | 0.02 | 0.01 | 0.02 | 0.06 | <0.01 | 0.02 | <0.01 | 38.21 | 2.07 | 98.73 |
| C1 | Lithiophorite | 44.66 | 12.93 | 0.08 | 0.01 | 0.02 | 0.02 | 0.04 | <0.01 | 0.03 | <0.01 | 38.15 | 2.08 | 98.02 |
| C2 | Lithiophorite | 45.82 | 12.28 | 0.12 | 0.01 | 0.02 | 0.02 | 0.01 | <0.01 | 0.06 | <0.01 | 38.01 | 2.07 | 98.42 |
| C2 | Lithiophorite | 45.88 | 12.42 | 0.02 | 0.02 | 0.02 | 0.03 | 0.05 | <0.01 | 0.03 | <0.01 | 37.77 | 2.10 | 98.34 |
| (Mn,Ca,Fe)3Al2(SiO4)3 (spessartine) + 12 H+ (aq) → Mn2+ (aq) + Ca2+ (aq) + Fe2+ (aq) + 2 Al3+ (aq) + 3 H4SiO4 (aq) |
| 2 Al3+ (aq) + 3 (OH)2- (aq) → 2 Al(OH)3 (aq) |
| 2 Al(OH)3 (aq) + 2 H4SiO4 (aq) → Al2Si2O5(OH)4 (kaolinite) + 5 H2O (liq) |
| 4 Fe2+ (aqs) + O2 (aq) + 6 H2O (liq) → 4 FeOOH (goethite) + 8 H+ (aq) |
| Mn2+ (aq) + 0.5 O2 (aq) + H2O (liq) → MnO2 (pyrolusite) + 2 H+ (aq) |
| K+ (aq) + 8 Mn2+ (aq) + 8 H2O (liq) + 4 O2 (aq) + (OH)− (aq) → KMn8O16(OH) (cryptomelane) +16 H+ (aq) |
| K+ (aq) + Ba2+ (aq) + 8 Mn2+ (aq) + 8 H2O (liq) + 3.5 O2 (aq) + 3 (OH)− (aq) → (Ba,K)Mn8O16(OH) (hollandite) + 16 H+ (aq) + H2O (liq) |
| Ba2+ (aq) + 5 Mn2+ (aq) + 5 H2O (liq) + 2.5 O2 (aq) + 2 (OH)− (aq) → BaMn5O10(H2O) (romanechite) + 10 H+ (aq) + 0.5 O2 (aq) |
| Al3+ (aq) + Li+1 (aq) + Mn2+ (aq) + 2 H2O (liq) + 2 (OH)- (aq) → (Al,Li)MnO2(OH)2 (lithiophorite) + 4 H+ (aq) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrotti, D.D.; da Conceição, F.T.; Navarro, G.R.B. The Mineralogy, Geochemistry and Origin of the Supergene Manganese Occurrences in the Southern Minas Gerais, Brazil. Minerals 2023, 13, 1216. https://doi.org/10.3390/min13091216
Parrotti DD, da Conceição FT, Navarro GRB. The Mineralogy, Geochemistry and Origin of the Supergene Manganese Occurrences in the Southern Minas Gerais, Brazil. Minerals. 2023; 13(9):1216. https://doi.org/10.3390/min13091216
Chicago/Turabian StyleParrotti, Davi Diorio, Fabiano Tomazini da Conceição, and Guillermo Rafael Beltran Navarro. 2023. "The Mineralogy, Geochemistry and Origin of the Supergene Manganese Occurrences in the Southern Minas Gerais, Brazil" Minerals 13, no. 9: 1216. https://doi.org/10.3390/min13091216





