Recovery of Noble Metals (Au, Pt, Ir, and Ta) from Spent Single-Use Medical–Technological Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Pyrolysis
2.3. Characterization
2.4. Acid Digestion
3. Results and Discussion
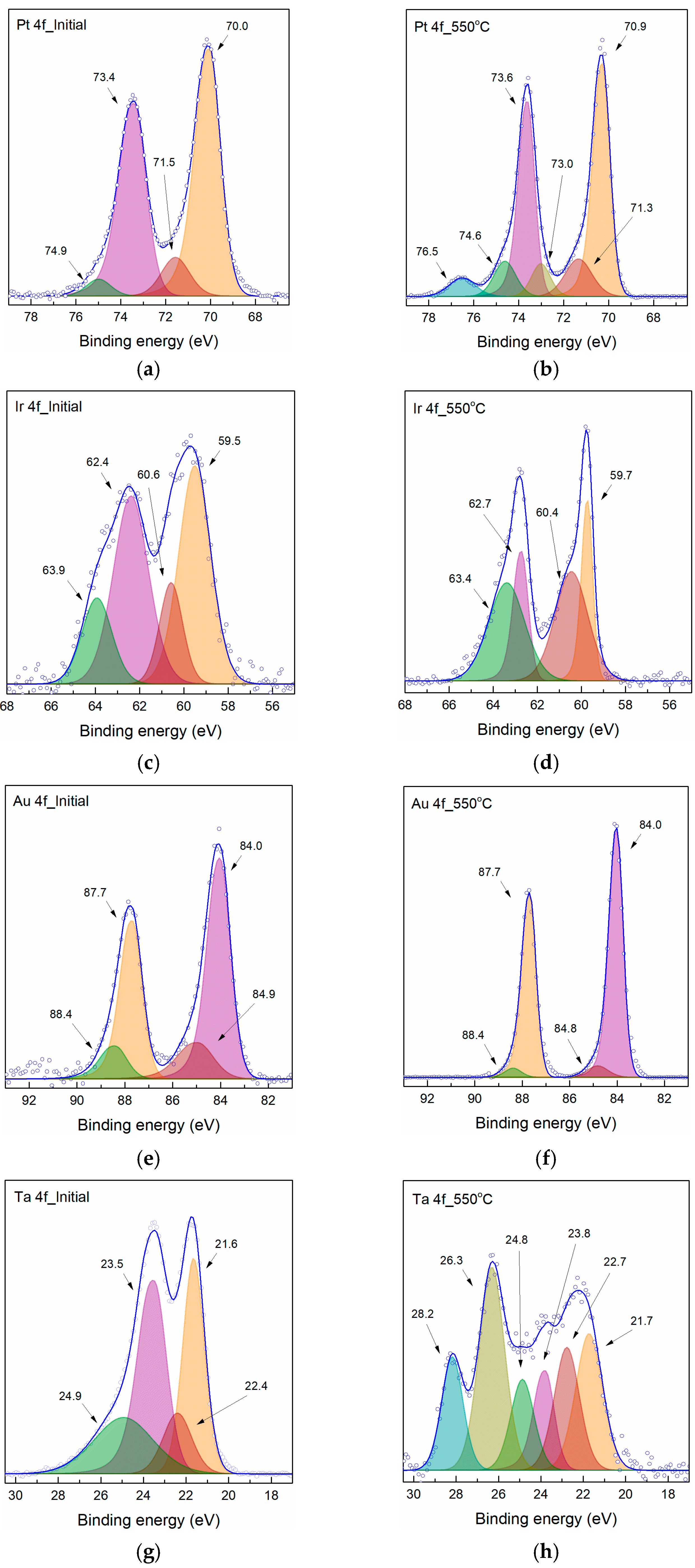
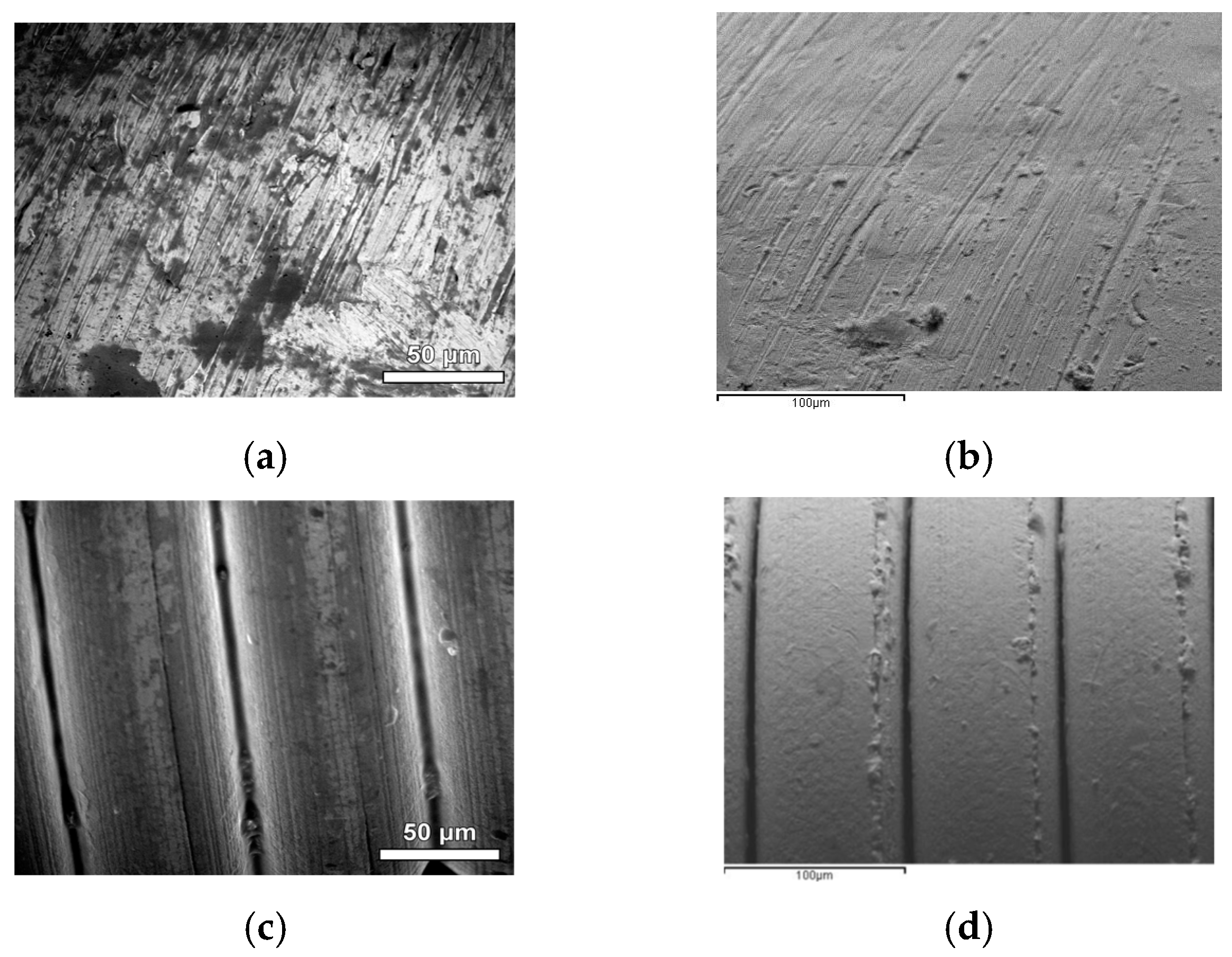
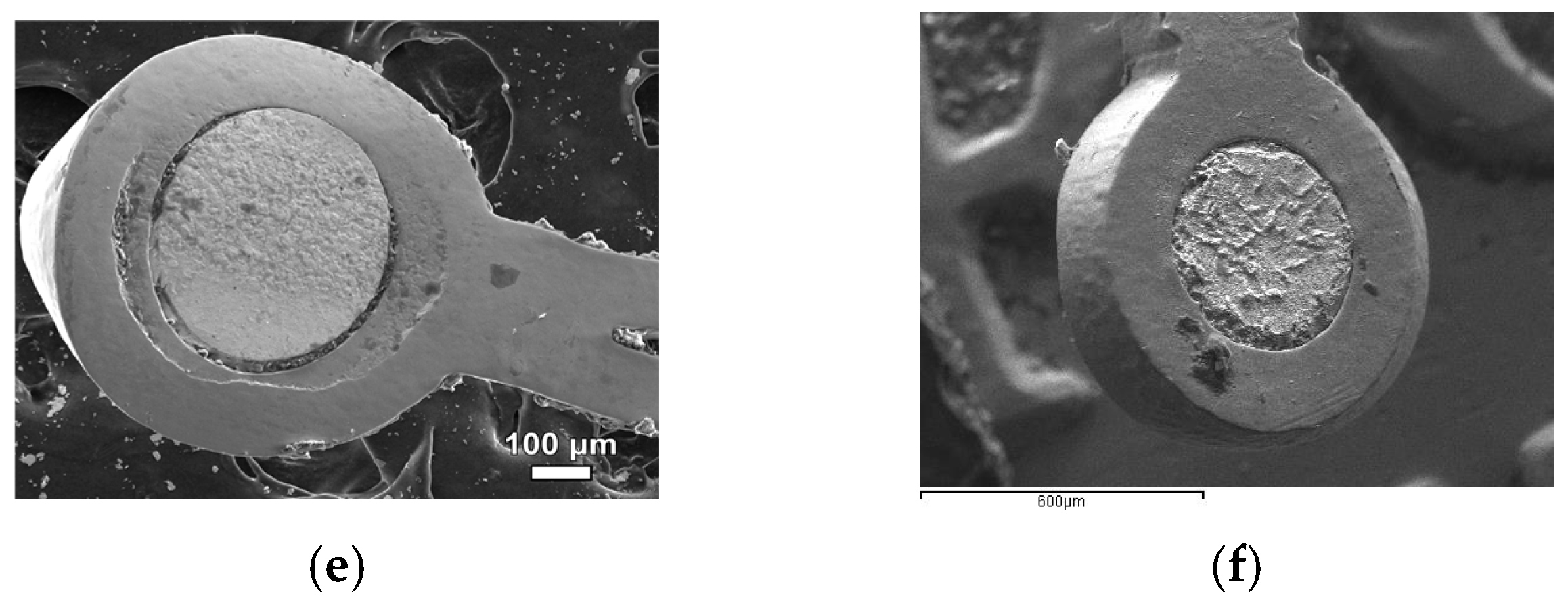
3.1. Characterization of Samples
3.2. Characterization of Pyrolyzed Samples
3.3. Separation of Metals
3.4. Economic and Environmental Aspects
| Waste | Acidic Solution | Metal | % Dissolution | Ref. |
|---|---|---|---|---|
| Electrical and electronic equipment | Aqua regia | Au, Ta | 80–100%, 0% | [22] |
| Anode slime | Aqua regia | Au | 100% | [35] |
| Printed circuit boards | Aqua regia | Au | 100% | [45] |
| Spent automotive catalysts | HCl/AlCl3/ HNO3 | Pt | 97–98% | [46] |
| Epoxy-coated solid electrolyte tantalum capacitors | HF | Ta | 100% | [47] |
| Slags/Mining tailings | HF/H2SO4 | Ta | 87% | [24] |
| Medical–technological products | Aqua regia HF/H2SO4 | Au, Pt, Ir, Ta | >95% in all metals | Present study |
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ranjbari, M.; Shams Esfandabadi, Z.; Shevchenko, T.; Chassagnon-Haned, N.; Peng, W.; Tabatabaei, M.; Aghbashlo, M. Mapping healthcare waste management research: Past evolution, current challenges, and future perspectives towards a circular economy transition. J. Hazard. Mater. 2022, 422, 126724. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Vora, A.; Nataraj, G.; Mishra, S.; Kerkar, P.; Manjunath, C.N. Guidance on reuse of cardio-vascular catheters and devices in India: A consensus document. Indian Heart J. 2017, 69, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Attrah, M.; Elmanadely, A.; Akter, D.; Rene, E.R. A Review on Medical Waste Management: Treatment, Recycling, and Disposal Options. Environments 2022, 9, 146. [Google Scholar] [CrossRef]
- Kane, G.M.; Bakker, C.A.; Balkenende, A.R. Towards design strategies for circular medical products. Resour. Conserv. Recycl. 2018, 135, 38–47. [Google Scholar] [CrossRef]
- Niinomi, M. Metals for Biomedical Devices, 2nd ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 1–188. [Google Scholar]
- Cowley, A.; Woodward, B. A Healthy Future: Platinum in Medical Applications. Platin. Met. Rev. 2011, 55, 98–107. [Google Scholar] [CrossRef]
- Nzulumike, A.N.O.; Thormann, E. Fibrin Adsorption on Cardiovascular Biomaterials and Medical Devices. ACS Appl. Bio Mater. 2022, 6, 2667–2676. [Google Scholar] [CrossRef]
- Park, J.S.; Yim, K.H.; Jeong, S.; Lee, D.H.; Kim, D.G. A novel high-visibility radiopaque tantalum marker for biliary self-expandable metal stents. Gut Liver 2019, 13, 366–372. [Google Scholar] [CrossRef]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and Selective Modern Methods of Noble Metal Recycling. Angew. Chem. —Int. Ed. 2023, 62, e2022144. [Google Scholar] [CrossRef]
- Granados-Fernández, R.; Montiel, M.A.; Díaz-Abad, S.; Rodrigo, M.A.; Lobato, J. Platinum recovery techniques for a circular economy. Catalysts 2021, 11, 937. [Google Scholar] [CrossRef]
- Fan, C.; Quan, K.; Han, Z.; Han, F.; Li, Z.; Liu, J.; Liu, X. Recovery and Purification of Iridium from Secondary Resources: A Review. J. Sustain. Metall. 2023, 9, 909–926. [Google Scholar] [CrossRef]
- Charles, V.; Anumah, A.O.; Adegoke, K.A.; Adesina, M.O.; Ebuka, I.P.; Gaya, N.A.; Ogwuche, S.; Yakubu, M.O. Progress and challenges pertaining to the earthly-abundant electrocatalytic materials for oxygen evolution reaction. Sustain. Mater. Technol. 2021, 28, e00252. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Guo, S. Noble metal-free electrocatalytic materials for water splitting in alkaline electrolyte. EnergyChem 2021, 3, 100053. [Google Scholar] [CrossRef]
- Xing, W.D.; Sohn, S.H.; Lee, M.S. A Review on the Recovery of Noble Metals from Anode Slimes. Miner. Process. Extr. Metall. Rev. 2020, 2, 130–143. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyukhin, D.Y. Recovery of Noble Metals from Spent Catalysts: A Review. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51B, 2413–2435. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, B.; Zheng, H.; Chang, C.C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Guimarães, R.; Carvalho, J.; Leal, V.; Guerner Dias, A.J. Characterization, treatment proposal and metal recovery in waste of active implantable medical devices. Comun. Geol. 2014, 101, 1011–1014. [Google Scholar]
- He, Y.; Hosseinzadeh-Bandbafha, H.; Kiehbadroudinezhad, M.; Peng, W.; Tabatabaei, M.; Aghbashlo, M. Environmental footprint analysis of gold recycling from electronic waste: A comparative life cycle analysis. J. Clean. Prod. 2023, 432, 139675. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Ibrahim, S.; Fattah, I.M.R.; Mofijur, M.; Chong, C.T. Valorisation of medical waste through pyrolysis for a cleaner environment: Progress and challenges. Environ. Pollut. 2021, 279, 116934. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Ahmad, E.; Pant, K.K.; Nigam, K.D.P. Environmentally friendly approach for the recovery of metallic fraction from waste printed circuit boards using pyrolysis and ultrasonication. Waste Manag. 2020, 118, 150–160. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Witt, K.; Urbaniak, W.; Kaczorowska, M.A.; Bożejewicz, D. Simultaneous recovery of precious and heavy metal ions from waste electrical and electronic equipment (WEEE) using polymer films containing cyphos IL 101. Polymers 2021, 13, 1454. [Google Scholar] [CrossRef]
- Jimenez De Aberasturi, D.; Pinedo, R.; Ruiz De Larramendi, I.; Ruiz De Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Rodríguez, O.; Alguacil, F.J.; Baquero, E.E.; García-Díaz, I.; Fernández, P.; Sotillo, B.; López, F.A. Recovery of niobium and tantalum by solvent extraction from Sn-Ta-Nb mining tailings. RSC Adv. 2020, 10, 21406–21412. [Google Scholar] [CrossRef]
- Kokkinos, E.; Merachtsaki, D.; Lampou, A.; Prochaska, C.; Peleka, E.; Simeonidis, K.; Vourlias, G.; Zouboulis, A. Investigating the Recovery of Noble Metals from Single-Use Medical Technology-Specific Waste Streams. Mater. Proc. 2023, 15, 27. [Google Scholar] [CrossRef]
- Romanchenko, A.; Likhatski, M.; Mikhlin, Y. X-ray photoelectron spectroscopy (XPS) study of the products formed on sulfide minerals upon the interaction with aqueous platinum (IV) chloride complexes. Minerals 2018, 8, 578. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velasco Vélez, J.J.; Arrigo, R.; Piccinin, S.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. In situ observation of reactive oxygen species forming on oxygen-evolving iridium surfaces. Chem. Sci. 2017, 8, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Hepperle, P.; Herman, A.; Khanbabaee, B.; Baek, W.Y.; Nettelbeck, H.; Rabus, H. XPS Examination of the Chemical Composition of PEGMUA-Coated Gold Nanoparticles. Part. Part. Syst. Charact. 2022, 39, 2200070. [Google Scholar] [CrossRef]
- Simpson, R.; White, R.G.; Watts, J.F.; Baker, M.A. XPS investigation of monatomic and cluster argon ion sputtering of tantalum pentoxide. Appl. Surf. Sci. 2017, 405, 79–87. [Google Scholar] [CrossRef]
- Peng, W.; Zeng, W.; Zhang, Y.; Shi, C.; Quan, B.; Wu, J. The effect of colored titanium oxides on the color change on the surface of Ti-5Al-5Mo-5V-1Cr-1Fe alloy. J. Mater. Eng. Perform. 2013, 22, 2588–2593. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.; Cho, M.; Lee, Y. A layered hollow sphere architecture of iridium-decorated carbon electrode for oxygen evolution catalysis. Carbon N. Y. 2017, 115, 50–58. [Google Scholar] [CrossRef]
- Kolosov, V.N.; Miroshnichenko, M.N. On the Synthesis of Tantalum-Carbide Powder by the Reaction of Tantalum with Toluene. Nanobiotechnol. Rep. 2022, 17, 507–513. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Iwasaki, S.; Okamura, K. Pure gold dissolution with hydrogen peroxide as the oxidizer in HBr or HI solution. J. Mol. Liq. 2017, 246, 372–378. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst. Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Niemelä, M.; Pitkäaho, S.; Ojala, S.; Keiski, R.L.; Perämäki, P. Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchem. J. 2012, 101, 75–79. [Google Scholar] [CrossRef]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcination. Hydrometallurgy 2003, 68, 69–75. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources-A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Agulyansky, A. Chemistry of Tantalum and Niobium Fluoride Compounds, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780080529028. [Google Scholar]
- Shikika, A.; Sethurajan, M.; Muvundja, F.; Mugumaoderha, M.C.; Gaydardzhiev, S. A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 2020, 198, 105496. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- He, Y.; Kiehbadroudinezhad, M.; Hosseinzadeh-Bandbafha, H.; Gupta, V.K.; Peng, W.; Lam, S.S.; Tabatabaei, M.; Aghbashlo, M. Driving sustainable circular economy in electronics: A comprehensive review on environmental life cycle assessment of e-waste recycling. Environ Pollut. 2023, 342, 123081. [Google Scholar] [CrossRef] [PubMed]
- Worldwide Cardiac Ablation Catheters Industry to 2025—US Is Expected to Experience the Most Rapid Growth. Available online: https://www.globenewswire.com/news-release/2021/07/13/2261690/28124/en/Worldwide-Cardiac-Ablation-Catheters-Industry-to-2025-US-is-Expected-to-Experience-the-Most-Rapid-Growth.html (accessed on 28 December 2023).
- Vlasopoulos, D.; Mendrinou, P.; Oustadakis, P.; Kousi, P.; Stergiou, A.; Karamoutsos, S.D.; Hatzikioseyian, A.; Tsakiridis, P.E.; Remoundaki, E.; Agatzini-Leonardou, S. Hydrometallurgical recovery of silver and gold from waste printed circuit boards and treatment of the wastewater in a biofilm reactor: An integrated pilot application. J. Environ. Manag. 2023, 344, 118334. [Google Scholar] [CrossRef] [PubMed]
- Rzelewska, M.; Regel-Rosocka, M. Wastes generated by automotive industry—Spent automotive catalysts. Phys. Sci. Rev. 2019, 3, 20180021. [Google Scholar] [CrossRef]
- Chen, W.S.; Ho, H.J.; Lin, K.Y. Hydrometallurgical process for tantalum recovery from epoxy-coated solid electrolyte tantalum capacitors. Materials 2019, 12, 1220. [Google Scholar] [CrossRef]

| Reagent | Waste Type | Metals of Interest | Other Metals | Ref. | |||
|---|---|---|---|---|---|---|---|
| Au | Pt | Ir | Ta | ||||
| Aqua regia | Electrical and electronic equipment | ✓ | - | - | - | Ni, Co, Fe | [22] |
| HCl/H2O2 | Anode slimes | ✓ | ✓ | - | - | Pd, Cu | [14] |
| Aqua regia | Spent catalysts | - | ✓ | ✓ | - | Pd, Rh | [23] |
| Aqua regia | Spent catalysts | - | ✓ | ✓ | - | Pd, Rh | [15] |
| HF/H2SO4 | Slags/Mining tailings | - | - | - | ✓ | Nb | [24] |
| Aqua regia and HF/H2SO4 | Medical–technological products | ✓ | ✓ | ✓ | ✓ | Fe, Cr, Ni, Ti | Present study |
| Medical Product | Specific Area | Pt | Ir | Au | Ta | O |
|---|---|---|---|---|---|---|
| wt% | ||||||
| Diagnostic catheter | Pt/Ir marker band | 77.6 ± 4.7 | 8.3 ± 1.6 | - | - | 14.1 ± 3.2 |
| Guide-wire | Au-coated guide wire | - | - | 86.6 ± 5.4 | - | 11.3 ± 2.7 |
| Self-expanding stent | Ta marker dot | - | - | - | 81.9 ± 4.8 | 18.1 ± 3.8 |
| Medical Product | Solid Residue (wt%) | Mass Loss (wt%) |
|---|---|---|
| Guide wire, metal of interest: Au Plastic part/Metallic part | 1.06/100 | 98.9/0 |
| Diagnostic catheter, metals of interest: Pt/Ir | 46.36 | 53.6 |
| Self-expanding stent, metal of interest: Τa | 93.0 | 7.0 |
| Medical Product | Specific Area | Pt | Ir | Au | Ta | O |
|---|---|---|---|---|---|---|
| wt% | ||||||
| Diagnostic catheter | Pt/Ir marker band | 74 ± 4.5 | 7.5 ± 0.4 | - | - | 15.9 ± 2.6 |
| Guide-wire | Au-coated guide wire | - | - | 89.9 ± 1.2 | - | 7.4 ± 1.4 |
| Self-expanding stent | Ta marker dot | - | - | - | 71.2 ± 5.1 | 24.7 ± 4.8 |
| Acidic Solution | Au | Pt | Ir | Ta | Fe | Cr | Ni | Ti |
|---|---|---|---|---|---|---|---|---|
| (50% v/v) | mg/L | |||||||
| HCl | 62 | - | - | - | 114 | 30 | 8 | 3 |
| HNO3 | 42 | - | - | - | 98 | 21 | 5 | 2 |
| HCl/H2O2 (3:1) | 102 | - | - | - | 156 | 42 | 37 | 28 |
| HCl/HNO3 (3:1) | 208 | - | - | - | 270 | 81 | 306 | 150 |
| HCl/HNO3-concentrated | 210 | 0.8 | - | - | 392 | 115 | 431 | 182 |
| Acidic Solution | Material | T | Time | Pt | Ir | Ta | Fe | Cr | Ni | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | h | mg/L | ||||||||
| HCl/HNO3-concentrated | Au digestion residue | 25 | 0.5 | 0.8 | - | - | 392 | 115 | 431 | 182 |
| HCl/HNO3-concentrated | Au digestion residue | 90 | 48 | 449 | 43.6 | 5 | 584 | 202 | 1587 | 1372 |
| HCl/HNO3-concentrated | Au digestion residue | 90 | 72 | 653 | 61 | 8 | 587 | 200 | 1672 | 1411 |
| HF 50% v/v | Pt/Ir digestion residue | 90 | 10 | - | - | 38 | - | - | - | - |
| HF/H2SO4 50% v/v | Pt/Ir digestion residue | 90 | 10 | - | - | 175 | - | - | - | - |
| HF/H2SO4 50% v/v | Pt/Ir marker band | 90 | 72 | 16 | 30 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, E.; Prochaska, C.; Lampou, A.; Peleka, E.; Simeonidis, K.; Vourlias, G.; Zouboulis, A. Recovery of Noble Metals (Au, Pt, Ir, and Ta) from Spent Single-Use Medical–Technological Products. Minerals 2024, 14, 90. https://doi.org/10.3390/min14010090
Kokkinos E, Prochaska C, Lampou A, Peleka E, Simeonidis K, Vourlias G, Zouboulis A. Recovery of Noble Metals (Au, Pt, Ir, and Ta) from Spent Single-Use Medical–Technological Products. Minerals. 2024; 14(1):90. https://doi.org/10.3390/min14010090
Chicago/Turabian StyleKokkinos, Evgenios, Charikleia Prochaska, Angeliki Lampou, Effrosyni Peleka, Konstantinos Simeonidis, Georgios Vourlias, and Anastasios Zouboulis. 2024. "Recovery of Noble Metals (Au, Pt, Ir, and Ta) from Spent Single-Use Medical–Technological Products" Minerals 14, no. 1: 90. https://doi.org/10.3390/min14010090







