Eddavidite, Cu12Pb2O15Br2, a New Mineral Species, and Its Solid Solution with Murdochite, Cu12Pb2O15Cl2
Abstract
1. Introduction
2. Materials and Methods
3. Results
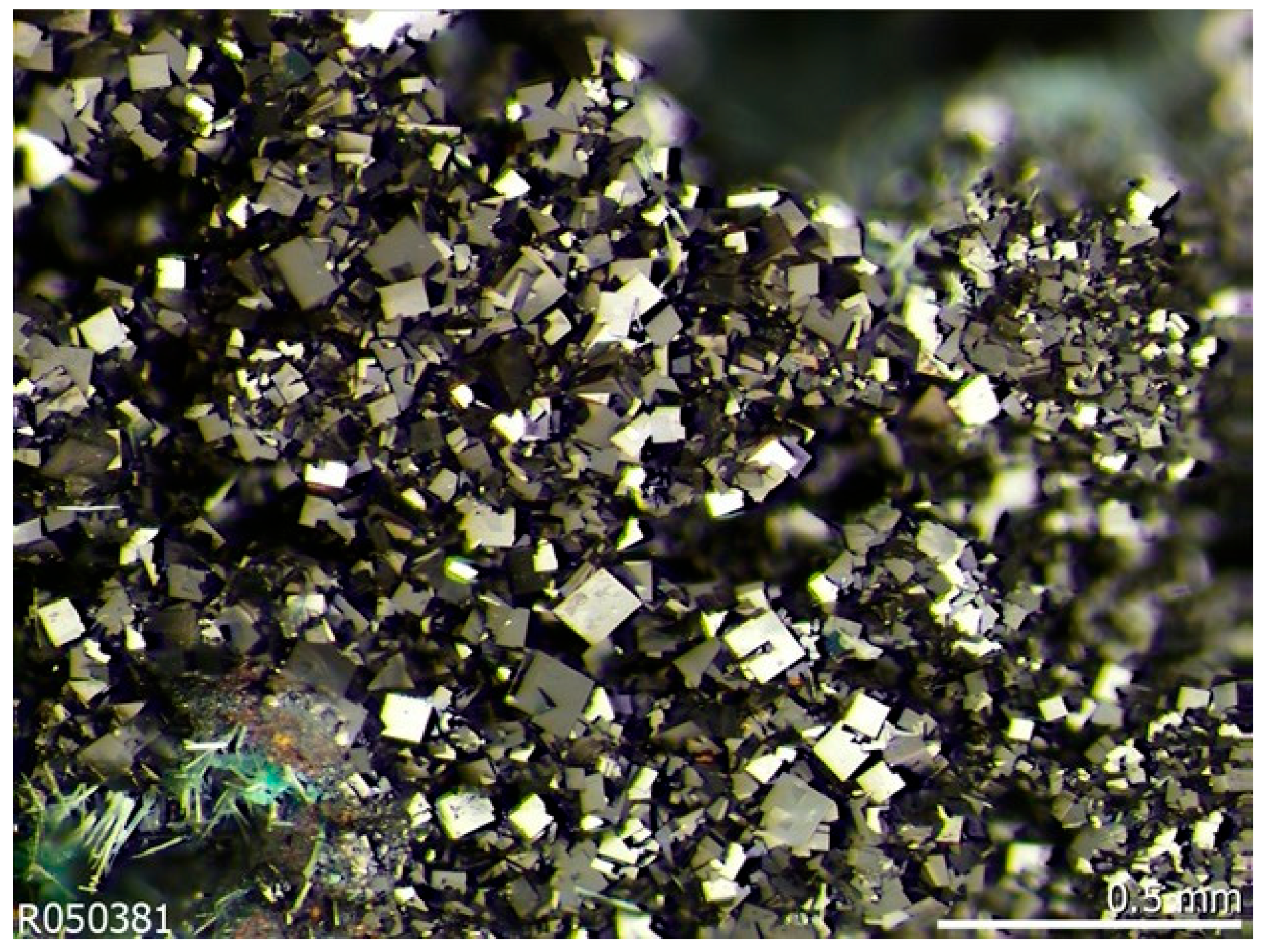
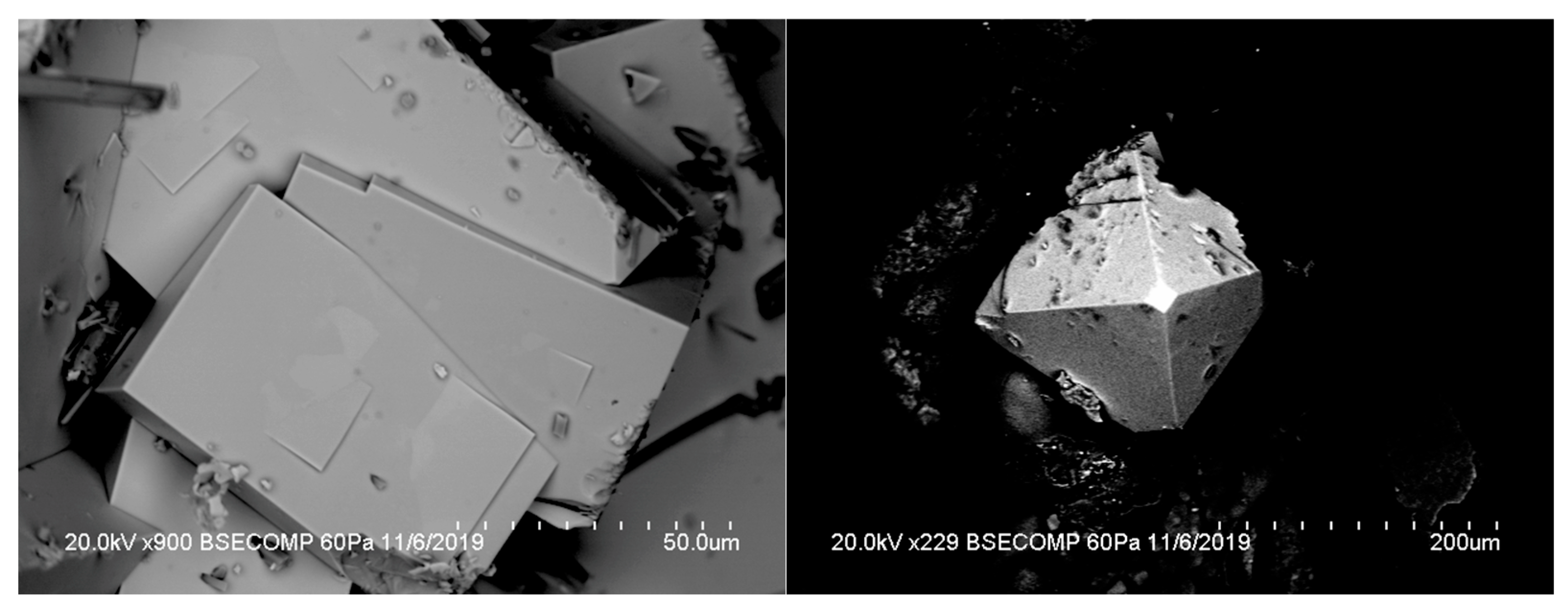
3.1. Description
3.2. Occurrence and Paragenesis of Eddavidite–Murdochite
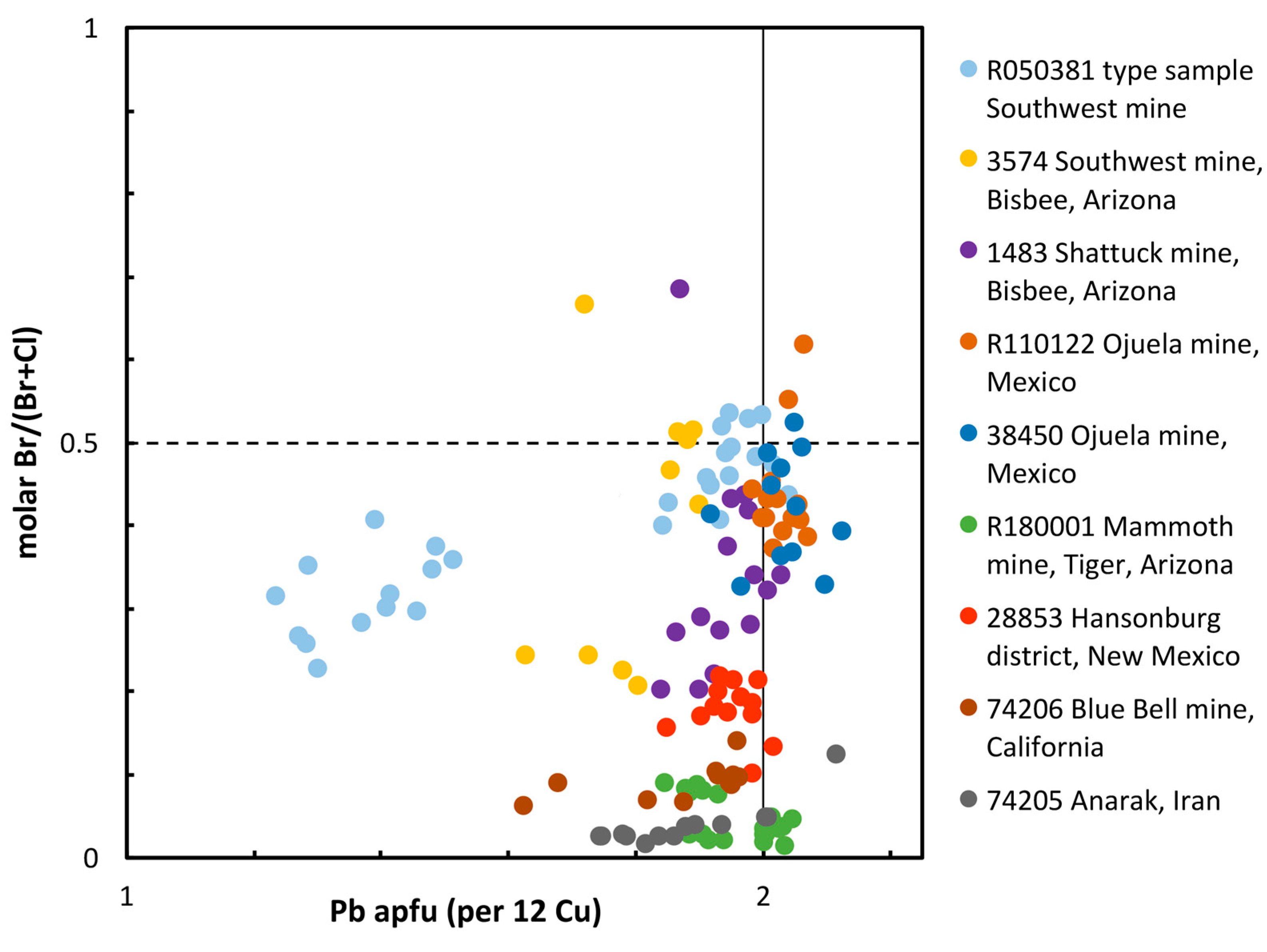
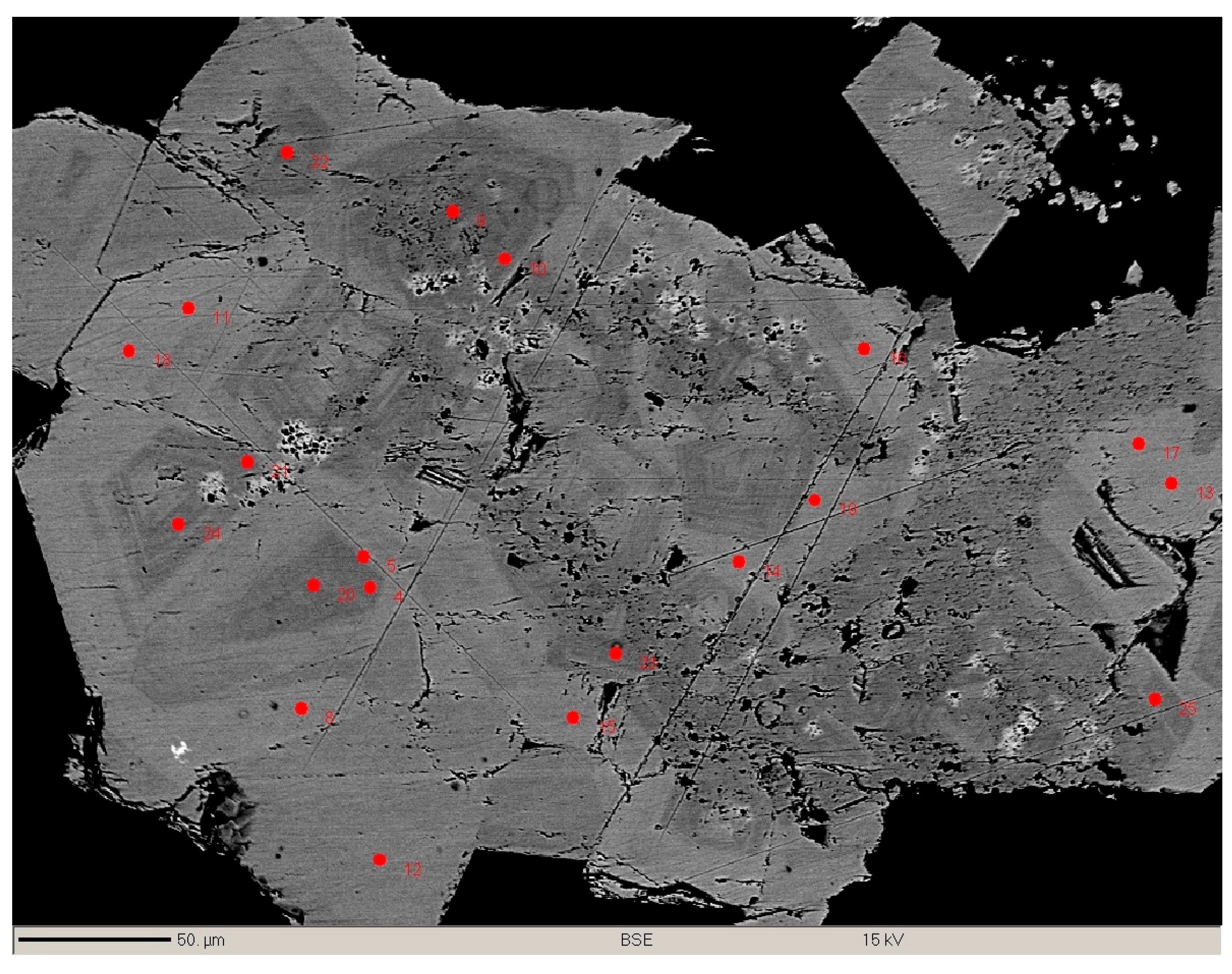
3.3. Eddavidite–Murdochite Chemistry
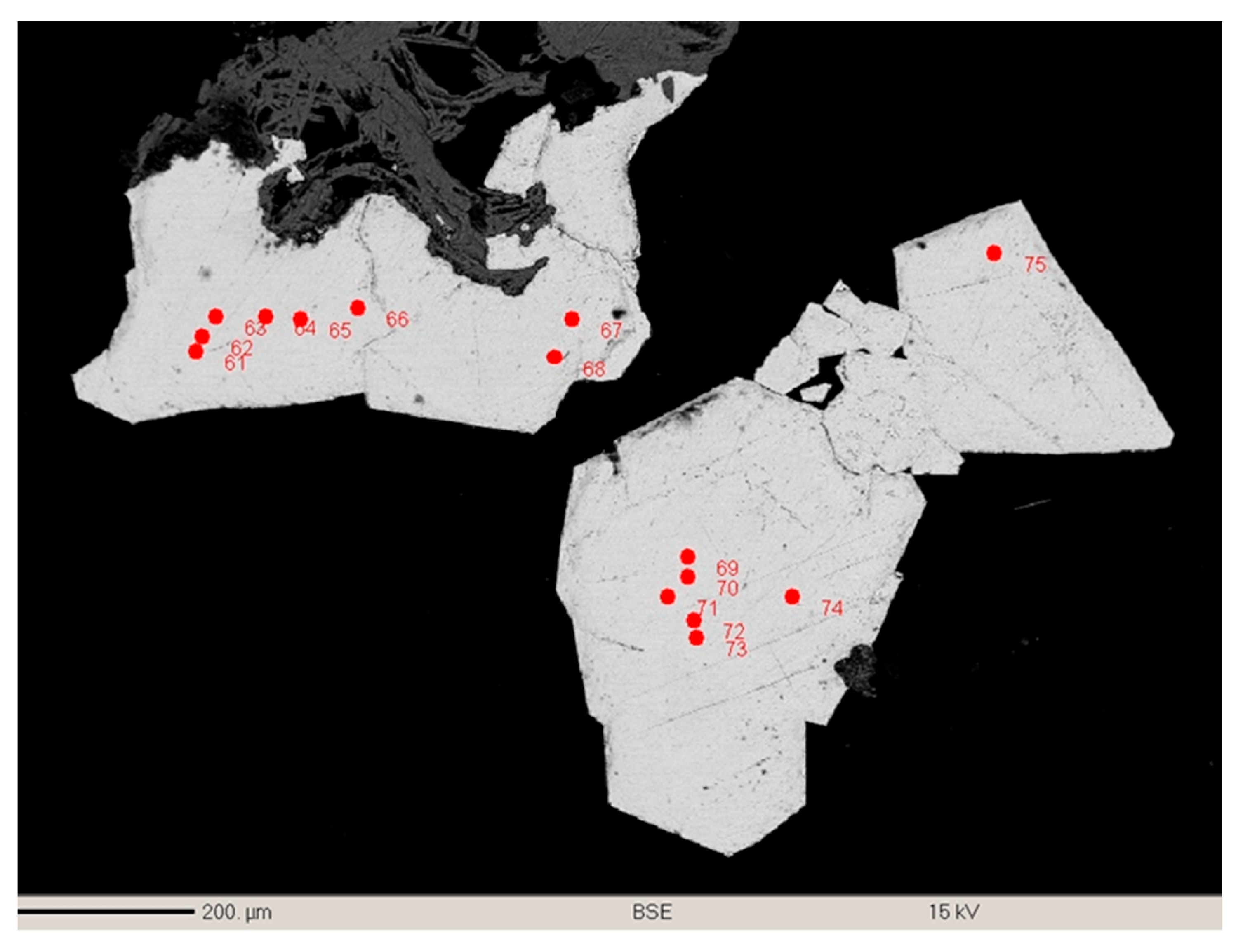
3.4. Zoning in Eddavidite–Murdochite
3.5. Eddavidite Crystal Structure
4. Discussion
4.1. Origin of Eddavidite
4.2. Bromine-Bearing Minerals
4.3. Identifying Eddavidite
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christ, C.L.; Clark, J.R. Crystal structure of murdochite (abstract). Am. Mineral. 1954, 39, 321. [Google Scholar]
- Fahey, J.J. Murdochite, a new copper lead oxide mineral. Am. Mineral. 1955, 40, 905–906. [Google Scholar]
- Christ, C.L.; Clark, J.R. The crystal structure of murdochite. Am. Mineral. 1955, 40, 907–916. [Google Scholar]
- Adib, D.; Otteman, J. Some new lead oxide minerals and murdochite from T. Khuni mine, Anarak, Iran. Miner. Depos. 1970, 5, 86–93. [Google Scholar] [CrossRef]
- Burke, E.A.J.; Maaskant, P. New data on murdochite. Neues Jb. Miner. Monat. 1970, 558–565. [Google Scholar]
- Dubler, E.; Verdani, A.; Oswald, H.R. New structure determination of murdochite, Cu6PbO8. Acta Crystallogr. 1983, C39, 1143–1146. [Google Scholar] [CrossRef]
- Taguchi, H. Relationship between crystal structure and electrical properties of murdochite-type Ni6+2xMn1−xO8. Solid State Commun. 1998, 108, 635–639. [Google Scholar] [CrossRef]
- Hayakawa, H.; Akiba, E.; Ono, S.; Ihara, H.; Izumi, F.; Asano, H. Refinement of the crystal structures of Cu6O8InCl and Cu6O8Cu2Cl by neutron powder diffraction. J. Ceram. Soc. Jpn. 1993, 101, 745–751. [Google Scholar] [CrossRef]
- Zouganelis, G.; Bushida, K.; Yazawa, I.; Terada, N.; Jo, M.; Hayakawa, H.; Ihara, H. Structure refinement of the Cu6O8·YCl compound. Solid State Commun. 1991, 80, 709–713. [Google Scholar] [CrossRef]
- Müller, M.; Thiele, G.; Zöllner, C. Strukturparameter von TlPd3O4 aus einem Neutronen-Pulverdiagramm. Z. Anorg. Allg. Chem. 1978, 443, 19–22. [Google Scholar] [CrossRef]
- Winkler, B.; Chall, M.; Pickard, C.J.; Milman, V.; White, J. Structure of Cu6PbO8. Acta Crystallogr. 2000, B56, 22–26. [Google Scholar] [CrossRef]
- Roberts, S.; David, E.E., Jr. Who elevated science under Nixon, dies at 92. New York Times, 28 February 2017; Section B. p. 13. [Google Scholar]
- Wilson, W.E.; Bartsch, J.A.; Mauthner, M. Masterpieces of the Mineral World; The Houston Museum of Natural Science and the Mineralogical Record, Inc.: Tucson, AZ, USA, 2004; 264p. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. Un nouveau modèle de calcul pour la micro-analyse quantitative par spectrométrie de rayons X. Partie I: Application a l’analyse d’énchantillons homogènes. Rech. Aérospatiale 1984, 3, 167–192. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Downs, R.T.; Bartelmehs, K.L.; Gibbs, G.V.; Boisen, M.B.J. Interactive software for calculating and displaying X-ray or neutron powder diffractometer patterns of crystalline materials. Am. Mineral. 1993, 78, 1104–1107. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Graeme, R.W. Famous mineral localities, Bisbee, Arizona. Mineral. Rec. 1981, 12, 258–319. [Google Scholar]
- Graeme, R. Bisbee revisited. Mineral. Rec. 1993, 24, 421–436. [Google Scholar]
- Lang, J.R.; Thompson, J.F.H.; Mortensen, J.K.; Baker, T.; Coulson, I.; Duncan, R.A.; Maloof, T.L.; James, J.; Friedman, R.M.; Lepitre, M.E. Regional and System-Scale Controls on the Formation of Copper and/or Gold Magmatic-Hydrothermal Mineralization; Mineral Deposit Research Unit Special, University of British Columbia: Vancouver, BC, Canada, 2001; Volume 2, 115p. [Google Scholar]
- Panczner, W.D. Minerals of Mexico; Van Nostrand Reinhold: New York, NY, USA, 1987; 459p. [Google Scholar]
- Hoffmann, V.J. The Mineralogy of the Mapimí Mining District, Durango, Mexico. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1967; 230p. [Google Scholar]
- Moore, T.P.; Megaw, P.K.M. Mexico II. The Ojuela mine. Mineral. Rec. 2003, 34, 1–120. [Google Scholar]
- Switzer, G. Paradamite, a new zinc arsenate from Mexico. Science 1956, 123, 1039. [Google Scholar] [CrossRef]
- Taggart, J.E.; Foord, E.E.; Rosenzweig, A.; Hanson, T. Scrutinyite, natural occurrences of αPbO2 from Bingham, New Mexico, U.S.A., and Mapimi, Mexico. Can. Mineral. 1988, 26, 905–910. [Google Scholar]
- Núñez-Regueiro, M.D.; Núñez-Regueiro, M. Folding CuO2 planes into fullerene-like clusters. J. Phys. I 1994, 4, 169–174. [Google Scholar]
- Paulus, E.F.; Miehe, G.; Fuess, H.; Yehia, I.; Löchner, U. The crystal structure of BaCuO2. J. Solid State Chem. 1991, 90, 17–26. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic study of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Welch, M.D.; Najorka, J.; Rumsey, M.S.; Spratt, J. The hexagonal ↔ orthorhombic structural phase transition in claringbullite, Cu4FCl(OH)6. Can. Mineral. 2021, 59, 265–285. [Google Scholar] [CrossRef]
- Elliott, P.; Cooper, M.A.; Pring, A. Barlowite, Cu4FBr(OH)6, a new mineral isostructural with claringbullite: Description and crystal structure. Mineral. Mag. 2014, 78, 1755–1762. [Google Scholar] [CrossRef]
- Slattery, J.S.; Cobban, W.A.; McKinney, K.C.; Harries, P.J.; Sandness, A.L. Early Cretaceous to Paleocene paleogeography of the Western Interior Seaway of North America and its relation to tectonics and eustasy. In Cretaceous Conference: Evolution and Revolution; Bingle-Davis, M., Ed.; Wyoming Geological Association: Casper, WY, USA, 2015; pp. 22–60. [Google Scholar]
- Blakely, R.C.; Ranney, W.D. Ancient Landscapes of Western North America; Springer: Berlin/Heidelberg, Germany, 2018; 228p. [Google Scholar]
- Dickinson, W.R.; Klute, M.A.; Swift, P.N. The Bisbee basin and its bearing on late Mesozoic paleogeographic and paleotectonic relations between the cordilleran and caribbean regions. In Cretaceous Stratigraphy Western North America; Abbott, P.L., Ed.; Pacific Section, Society of Economic Paleontologists and Mineralogists: Los Angeles, CA, USA, 1986; pp. 51–62. [Google Scholar]
- Braitsch, O. Salt Deposits: Their Origin and Composition; Springer: New York, NY, USA, 1971; 297p. [Google Scholar]
- McCaffrey, M.A.; Lazar, B.; Holland, H.D. The evaporation path of seawater and coprecipitation of Br– and K+ with halite. J. Sediment. Petrol. 1987, 57, 928–937. [Google Scholar]
- Babel, M.; Schreiber, B.C. Geochemistry of evaporites and evolution of seawater. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 9, pp. 483–560. [Google Scholar]
- Sanford, W.E.; Wood, W.W. Brine evolution and mineral deposition in hydrologically open evaporite basins. Am. J. Sci. 1991, 291, 687–710. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 1–51. [Google Scholar]
- Barnes, H.L. (Ed.) Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Wiley and Sons: New York, NY, USA, 1997; 992p. [Google Scholar]
- Palache, C.; Berman, H.; Frondel, C. Dana’s System of Mineralogy, 7th ed.; John Wiley & Sons: New York, NY, USA, 1951; Volume II, 1124p. [Google Scholar]
- Cooper, M.A.; Abdu, Y.A.; Hawthorne, F.C.; Kampf, A.R. The crystal structure of comancheite, Hg2+55N3–24(OH,NH2)4(Cl,Br)34 and crystal-chemical and spectroscopic discrimination of N3– and O2– anions in Hg2+ compounds. Mineral. Mag. 2013, 77, 3217–3237. [Google Scholar] [CrossRef]
- Demartin, F.; Gramaccioli, C.M.; Campostrini, I.; Orlandi, P. Demicheleite, BiSBr, a new mineral from La Fossa crater, Vulcano, Aeolian Islands, Italy. Am. Mineral. 2008, 93, 1603–1607. [Google Scholar] [CrossRef]
- Karpenko, V.Y.; Pautov, L.A.; Siidra, O.I.; Mirakov, M.A.; Zaitsev, A.N.; Plechov, P.Y.; Makhmadsharif, S. Ermakovite (NH4)(As2O3)2Br, a new exhalative arsenite bromide mineral from the Fan-Yagnob coal deposit, Tajikistan. Mineral. Mag. 2023, 87, 69–78. [Google Scholar] [CrossRef]
- Vasil’ev, V.I.; Usova, L.V.; Pal’chik, N.A. Grechishchevite—Hg3S2(Br,Cl,I)2—A new supergene mercury sulfohalide. Geol. Geofiz. 1989, 30, 61–69. (In Russian) [Google Scholar]
- Vasil’ev, V.I. Kadyrelite, Hg4(Br,Cl)2O—A new oxyhalide of mercury from the Kadyrelsky ore deposit (Tuvinskaya ASSR). Zap. Vsesoyuznogo Mineral. Obs. 1987, 116, 733–737. (In Russian) [Google Scholar]
- Pervukhina, N.V.; Borisov, S.V.; Magarill, S.A.; Naumov, D.Y.; Vasil’ev, V.I. The crystal structure of kelyanite, (Hg2)6(SbO6)BrCl2. Am. Mineral. 2008, 93, 1666–1669. [Google Scholar] [CrossRef]
- Vasil’ev, V.I.; Lavrent’ev, Y.G.; Pal’chik, N.A. Kuzminite—Hg2(Br,Cl)2—A new natural halide of mercury. Zap. Vsesoyuznogo Mineral. Obs. 1986, 115, 595–598. (In Russian) [Google Scholar]
- Garavelli, A.; Mitolo, D.; Pinto, D.; Vurro, F. Lucabindiite, (K,NH4)As4O6(Cl,Br), a new fumarole mineral from the “La Fossa” crater at Vulcano, Aeolian Islands, Italy. Am. Mineral. 2013, 98, 470–477. [Google Scholar] [CrossRef]
- Zelenski, M.; Balić-Žunić, T.; Bindi, L.; Garavelli, A.; Makovicky, E.; Pinto, D.; Vurro, F. First occurrence of iodine in natural sulfosalts: The case of mutnovskite, Pb2AsS3(I,Cl,Br), a new mineral from the Mutnovsky volcano, Kamchatka Peninsula, Russian Federation. Am. Mineral. 2006, 91, 21–28. [Google Scholar] [CrossRef]
- Mumme, W.G.; Nickel, E.H. Crystal structure and crystal chemistry of perroudite: A mineral from Coppin Pool, Western Australia. Am. Mineral. 1987, 72, 1257–1262. [Google Scholar]
- Cooper, M.A.; Hawthorne, F.C. The crystal structure of tedhadleyite, Hg2+Hg1+10O4I2(Cl,Br)2, from the Clear Creek Claim, San Benito County, California. Mineral. Mag. 2009, 73, 227–234. [Google Scholar] [CrossRef]
- Cooper, M.A.; Hawthorne, F.C. The crystal structure of vasilyevite, (Hg2)2+10O6I3(Br,Cl)3(CO3). Can. Mineral. 2003, 41, 1173–1181. [Google Scholar] [CrossRef]
- Graeme, R.W., III; Graeme, R.W., IV; Graeme, D.L. The Mineralogy of Bisbee, Arizona; Copper Czar Publishing: Bisbee, AZ, USA, 2021; Volume I. [Google Scholar]




| Spot Range (wt. %) | Mean of 9 (with s.d.) | |
|---|---|---|
| PbO2 | 25.35–31.31 | 29.74(189) |
| CuO | 58.07–65.61 | 61.71(194) |
| SiO2 | 0.17–0.37 | 0.23(8) |
| FeO | 0.09–0.59 | 0.30(16) |
| F | 0.00–0.06 | 0.02(2) |
| Cl | 1.52–3.91 | 2.04(74) |
| Br | 2.80–6.19 | 5.11(108) |
| total | 99.15 | |
| molar Br/(Br + Cl) | 0.64–0.24 | 0.53 |
| # spots eddavidite | - | 6 |
| # spots murdochite | - | 3 |
| dobs (Eddavidite) | Iobs | dcalc (Eddavidite) | dobs (Murdochite) | {hkl} |
|---|---|---|---|---|
| 5.296 | 40 | 5.336 | 5.30 | {111} |
| 4.739 | 15 | 4.621 | 4.59 | {200} |
| 3.260 | 9 | 3.268 | 3.25 | {220} |
| 2.788 | 5 | 2.787 | 2.776 | {311} |
| 2.668 | 100 | 2.668 | 2.659 | {222} |
| 2.305 | 31 | 2.311 | 2.303 | {400} |
| 2.120 | 13 | 2.120 | 2.109 | {331} |
| 2.063 | 6 | 2.067 | 2.059 | {420} |
| 1.882 | 3 | 1.887 | 1.880 | {422} |
| 1.773 | 2 | 1.779 | 1.772 | {333}, {511} |
| 1.632 | 35 | 1.634 | 1.629 | {440} |
| 1.561 | 6 | 1.562 | 1.556 | {531} |
| 1.536 | 3 | 1.540 | 1.537 | {442}, {600} |
| 1.456 | 2 | 1.461 | 1.457 | {620} |
| 1.394 | 28 | 1.393 | 1.404 | {622} |
| 1.334 | 7 | 1.334 | {444} | |
| 1.292 | 3 | 1.282 | {640} | |
| 1.154 | 5 | 1.155 | {800} | |
| 1.060 | 11 | 1.060 | {662} |
| Eddavidite | Murdochite | |
|---|---|---|
| locality | Southwest mine, Bisbee, Arizona | Hansonburg district, New Mexico |
| crystal size | 70 × 60 × 60 µm | 90 × 110 × 90 µm |
| idealized formula | Cu12Pb2O15Br2 | Cu6PbO8−x(Cl,Br)2x |
| refined unit cell content | Cu24Pb4O30.20Br1.98Cl1.78 | Cu24Pb4O30(Cl0.64Br0.36)4 |
| space group | m | m |
| a (Å) | 9.2407(9) | 9.224(2) |
| V (Å3) | 789.1(2) | 784.3(3) |
| R | 0.0112 | 0.027 |
| wR | 0.0348 * | 0.026 |
| maximum 2θ (°) | 66.6 | 100 |
| {hkl} span | −13 < h < 4 | -- |
| −5 < k < 13 | ||
| −14 < l < 1 | ||
| # reflections collected | 490 | 1723 |
| # independent reflections | 105 | 258 |
| # reflections I > 2s(I) | 105 | 255 |
| # parameters refined | 9 | 10 |
| Rint | 0.0151 | -- |
| GoF | 1.316 | -- |
| r(Pb-O) (Å) | 2.286(3) | 2.283(1) |
| r(Cu-O) (Å) | 1.9245(7) | 1.921(1) |
| r(Cu-X) (Å) | 3.2671(3) | 3.261(2) |
| X site chemistry | 0.495 Br + 0.445 Cl | 0.64(2) Cl + 0.36 Br |
| Site | Occupancy | Wyckoff | x | y | z |
|---|---|---|---|---|---|
| Cu | Cu1.00 | 24(d) | ¼ | ¼ | 0 |
| Pb | Pb1.00 | 4(a) | 0 | 0 | 0 |
| O | O0.944 | 32(f) | 0.1428(2) | x | x |
| X | Br0.495Cl0.445 | 4(b) | ½ | ½ | ½ |
| Atom | Ueq. | U11 = U22 | U33 | U12 | U13 = U23 |
|---|---|---|---|---|---|
| Cu | 0.00524(18) | 0.0056(2) | 0.0046(3) | −0.00222(18) | 0 |
| Pb | 0.00420(14) | 0.00420(14) | U11 | 0 | 0 |
| O | 0.0025(5) | 0.0025(5) | U11 | 0.0006(6) | U12 |
| X | 0.0237(5) | 0.0237(5) | U11 | 0 | 0 |
| Mineral | IMA Chemistry | Cl Analog | Reference |
|---|---|---|---|
| barlowite | Cu4BrF(OH)6 | claringbullite | [29] |
| bromargyrite | AgBr | chlorargyrite | [40] |
| comancheite | Hg2+55N3−24(NH2,OH)4(Cl,Br)34 | – | [41] |
| demicheleite-(Br) | BiSBr | demicheleite-(Cl) | [42] |
| eddavidite | Pb2Cu12O15Br2 | murdochite | this study |
| ermakovite | (NH4)(As2O3)2Br | – | [43] |
| grechishchevite † | Hg3S2BrCl0.5I0.5 | – | [44] |
| kadyrelite | ([Hg1+]2)3OBr3(OH) | eglestonite | [45] |
| kelyanite | Hg12SbO6BrCl2 | – | [46] |
| kuzminite | HgBr | calomel | [47] |
| lucabindiite * | (K,NH4)As4O6(Cl,Br) | – | [48] |
| mutnovskite * | Pb2AsS3(I,Cl,Br) | – | [49] |
| perroudite * | Ag4Hg5S5(I,Br)2Cl2 | – | [50] |
| tedhadleyite * | Hg2+Hg1+10O4I2(Cl,Br)2 | – | [51] |
| vasilyevite | (Hg2)2+10O6I3Br2Cl(CO3) | – | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenblatt, M.; Origlieri, M.J.; Graeme, R., III; Graeme, R., IV; Graeme, D.; Downs, R.T. Eddavidite, Cu12Pb2O15Br2, a New Mineral Species, and Its Solid Solution with Murdochite, Cu12Pb2O15Cl2. Minerals 2024, 14, 307. https://doi.org/10.3390/min14030307
Rosenblatt M, Origlieri MJ, Graeme R III, Graeme R IV, Graeme D, Downs RT. Eddavidite, Cu12Pb2O15Br2, a New Mineral Species, and Its Solid Solution with Murdochite, Cu12Pb2O15Cl2. Minerals. 2024; 14(3):307. https://doi.org/10.3390/min14030307
Chicago/Turabian StyleRosenblatt, Melli, Marcus J. Origlieri, Richard Graeme, III, Richard Graeme, IV, Douglas Graeme, and Robert T. Downs. 2024. "Eddavidite, Cu12Pb2O15Br2, a New Mineral Species, and Its Solid Solution with Murdochite, Cu12Pb2O15Cl2" Minerals 14, no. 3: 307. https://doi.org/10.3390/min14030307
APA StyleRosenblatt, M., Origlieri, M. J., Graeme, R., III, Graeme, R., IV, Graeme, D., & Downs, R. T. (2024). Eddavidite, Cu12Pb2O15Br2, a New Mineral Species, and Its Solid Solution with Murdochite, Cu12Pb2O15Cl2. Minerals, 14(3), 307. https://doi.org/10.3390/min14030307









