Oily Bubble Flotation of Coal Macerals of Shendong Jurassic Coal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
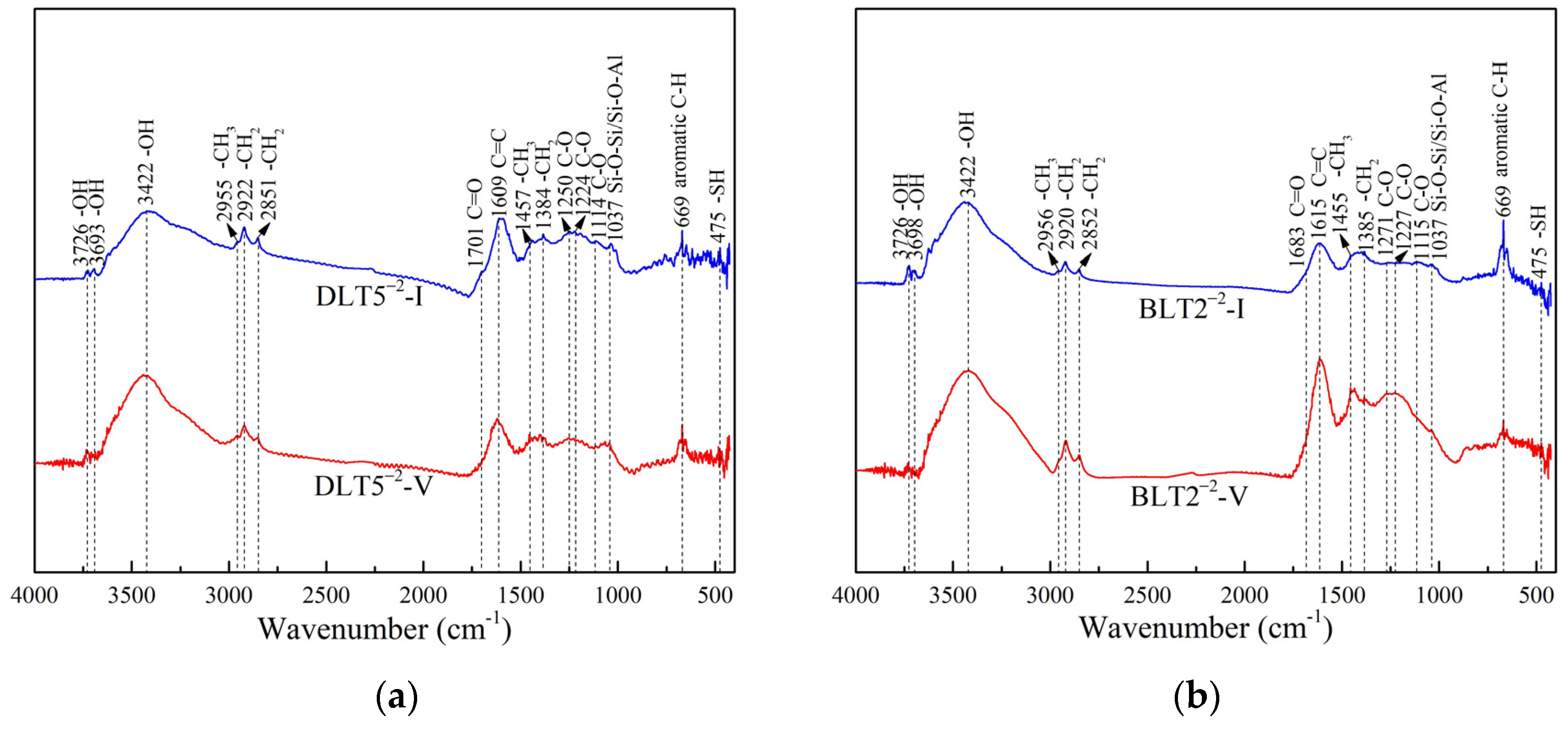
2.2. Fourier Transform Infrared (FT-IR) Analysis
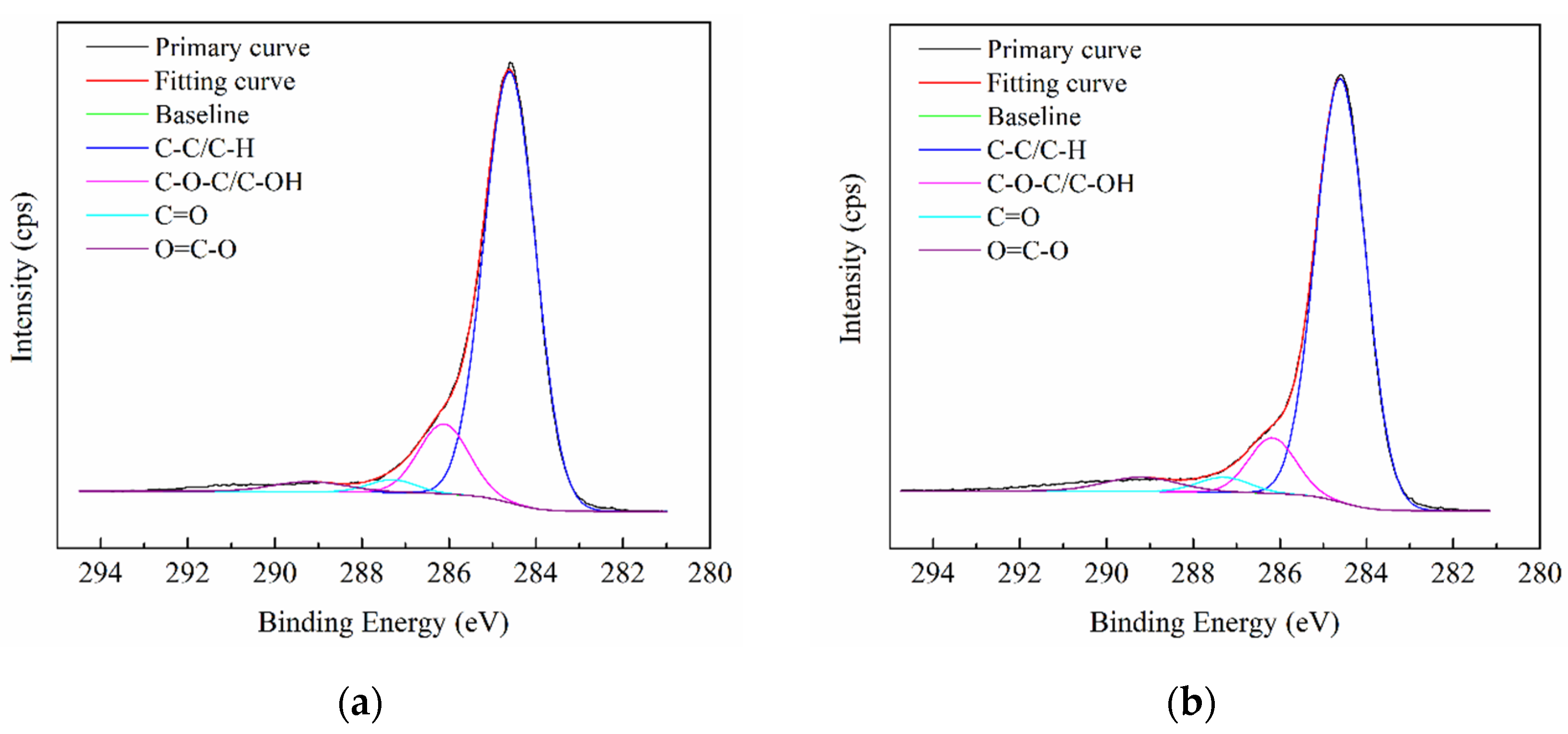
2.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.4. Contact Angle Measurements
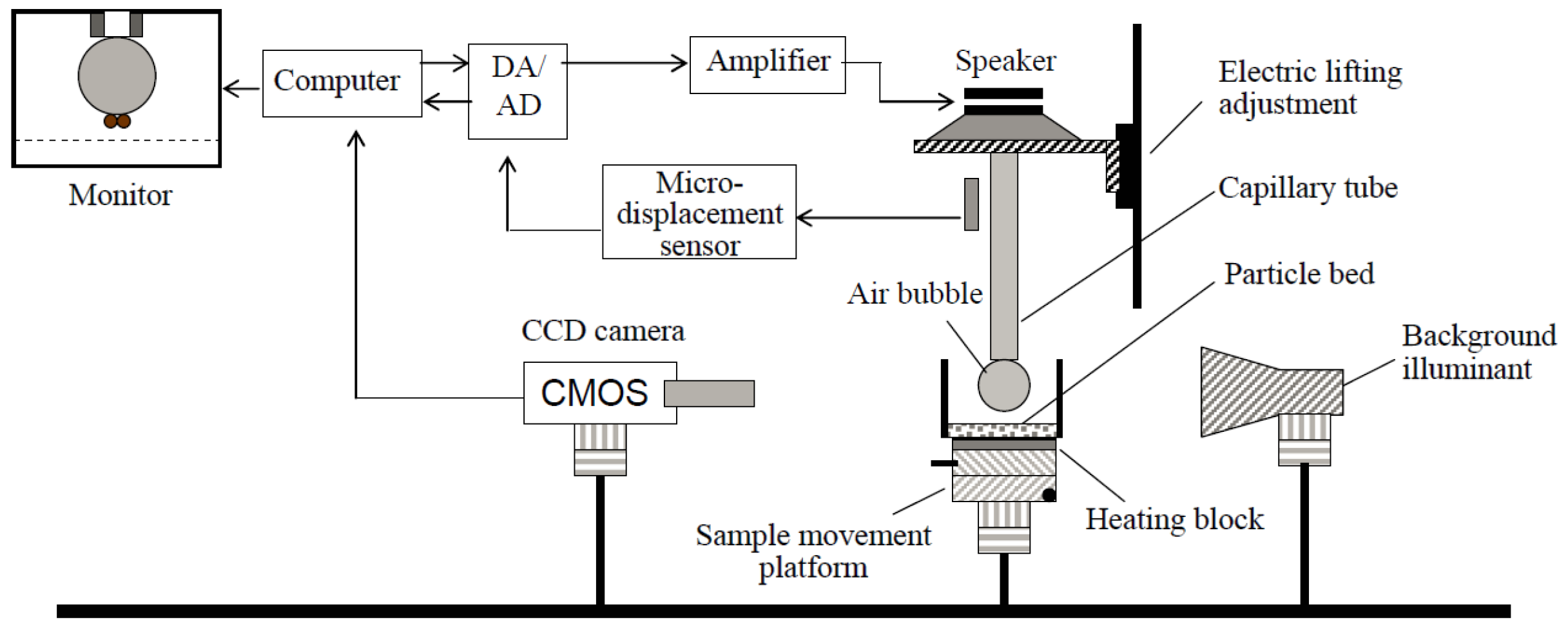
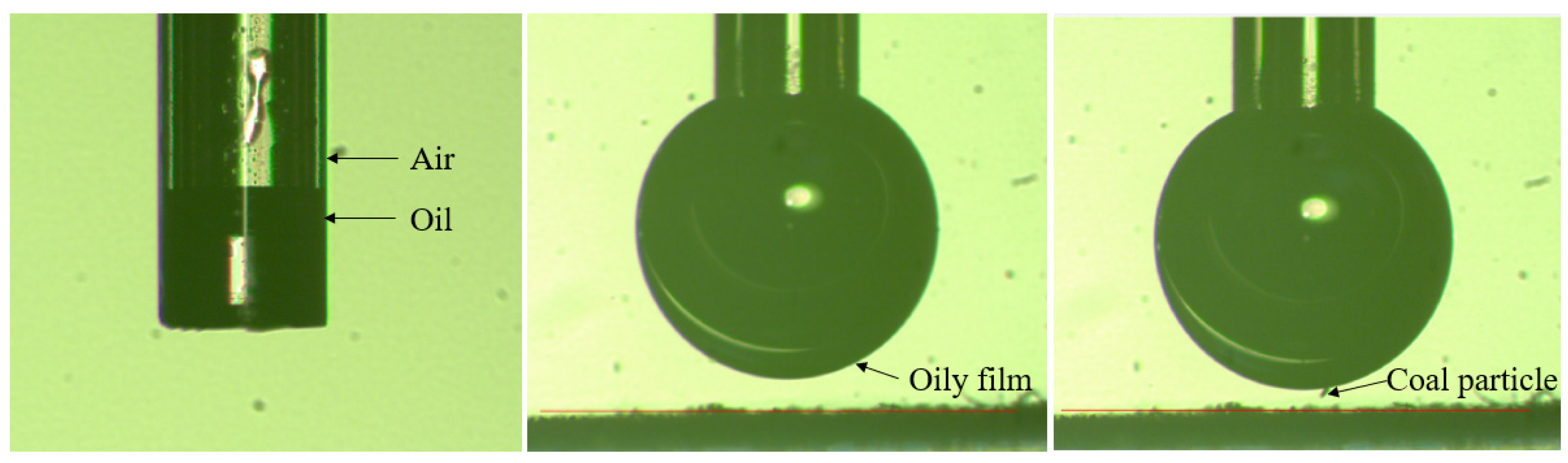
2.5. Induction Time Measurements
2.6. Conventional Flotation Tests
2.7. Oily Bubble Flotation and Modification
3. Results and Discussion
3.1. Analysis of the Surface Properties of the Coal Samples
3.1.1. Analysis of FT-IR Spectra
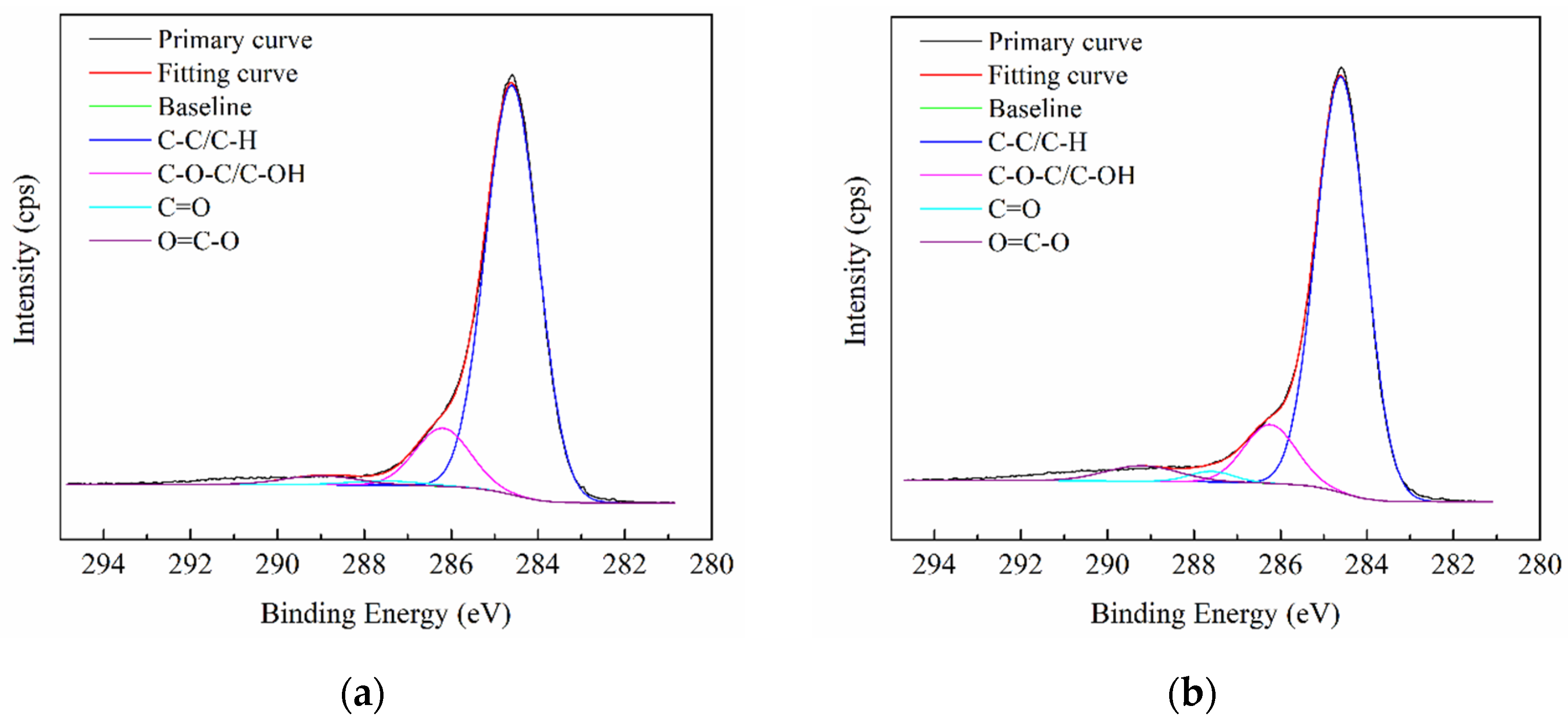
3.1.2. XPS Analysis
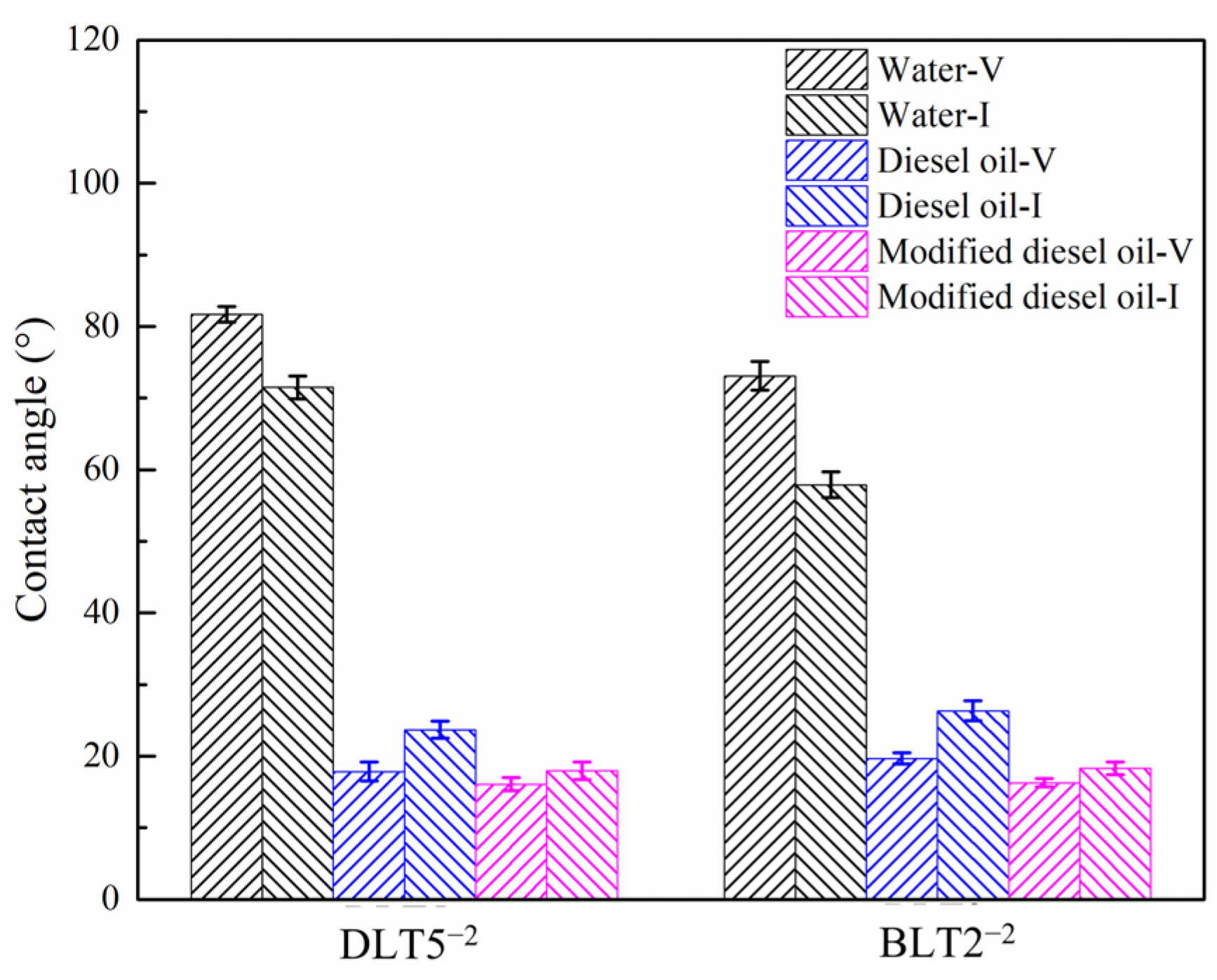
3.2. Wettability Analysis
3.3. Induction Times of Air and Oily Bubbles on Coal Macerals
3.4. Flotation Results of Coal Macerals
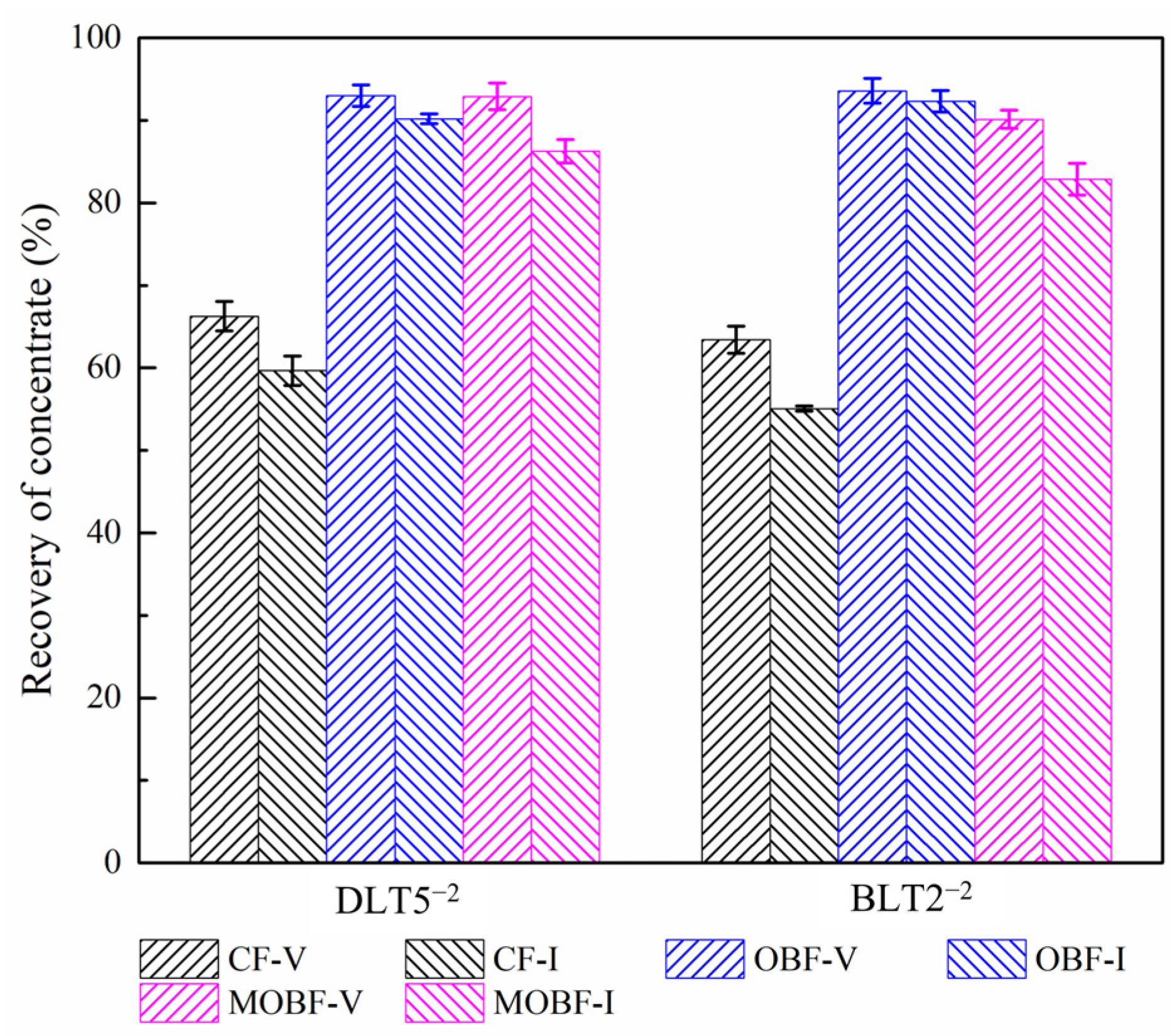
3.4.1. Conventional Flotation Tests
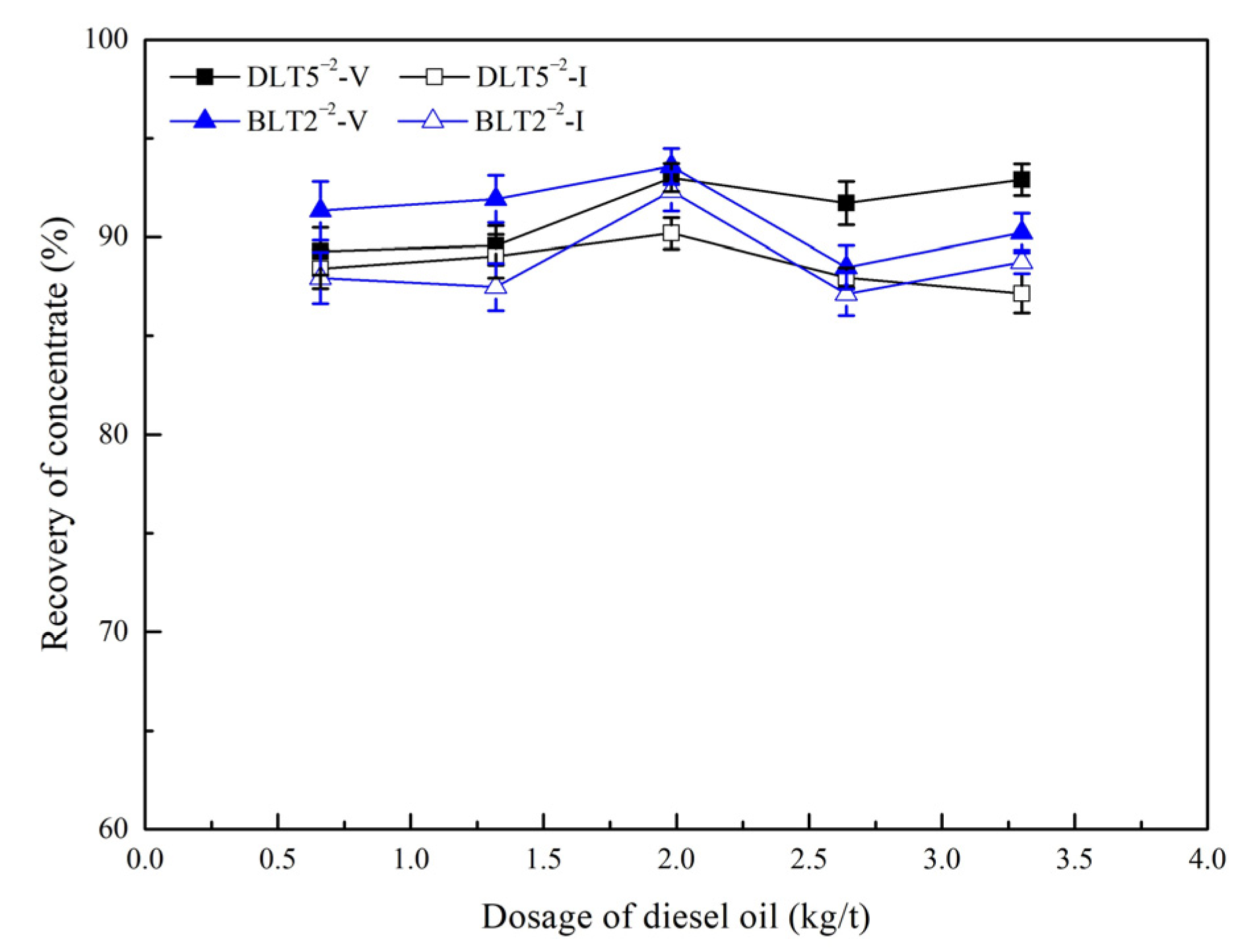
3.4.2. Oily Bubble Flotation Tests
4. Conclusions
- Enrichment rates exceeding 84% for both vitrinite-rich and inertinite-rich coal can be obtained by the density gradient centrifugation method with two rounds of enrichment.
- FT-IR and XPS analyses confirmed that there are clear differences in the contents of the C-C, C-H, and oxygen-containing functional groups between vitrinite-rich and inertinite-rich coal, and the O/C value of vitrinite-rich coal is lower than that of inertinite-rich coal.
- Vitrinite-rich coal has a higher hydrophobicity than inertinite-rich coal, and (modified) diesel oil droplets can further increase the hydrophobicity.
- The induction time on the coal surface is significantly reduced by diesel oily bubbles, and a further reduction in the induction time between coal macerals and oily bubbles can be obtained by the addition of diethyl phthalate (DP).
- Oily bubble flotation can significantly improve the recoveries of vitrinite-rich and inertinite-rich coal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| DLT5−2-V | the vitrinite-rich coal of the 5−2 coal seam of Daliuta mine |
| DLT5−2-I | the inertinite-rich coal of the 5−2 coal seam of Daliuta mine |
| BLT2−2-V | the vitrinite-rich coal of the 2−2 coal seam of Bulianta mine |
| BLT2−2-I | the inertinite-rich coal of the 2−2 coal seam of Bulianta mine |
| M | the moisture content (air-dry basis) |
| A | the ash content (air-dry basis) |
| V | the volatile matter content (air-dry basis) |
| FC | the fixed carbon content (air-dry basis) |
| C | the carbon content (dry basis) |
| H | the hydrogen content (dry basis) |
| O | the oxygen content (dry basis) |
| N | the nitrogen content (dry basis) |
| St | the total sulfur content (dry basis) |
| DP | diethyl phthalate |
| Mobf | the actual dosage of collector in oily bubble flotation |
| V0 | the maximum volume of air and collector after atomization and heating flash evaporation |
| V1 | the volume of air and collector discharged from the gas flowmeter |
| V2 | the volume of air and collector sucked into flotation cell |
| Ma | the atomizing rate of collector |
| t | the oily bubble flotation time, i.e., the skimming time |
| mc | the amount of coal sample used in a single flotation test |
| CF-V | the conventional (air) flotation of vitrinite-rich coal |
| CF-I | the conventional (air) flotation of inertinite-rich coal |
| OBF-V | the oily bubble flotation of vitrinite-rich coal |
| OBF-I | the oily bubble flotation of inertinite-rich coal |
| MOBF-V | the modified oily bubble flotation of vitrinite-rich coal |
| MOBF-I | the modified oily bubble flotation of inertinite-rich coal |
References
- Huang, W.; Tang, S.; Tang, X.; Chen, P.; Zhao, Z.; Wan, H.; Ao, W.; Xiao, X.; Liu, J.; Finkelman, B. The Jurassic coal petrology and the research significance of Northwest China. Coal Geol. Explor. 2010, 38, 1–6. [Google Scholar]
- Chen, P.; Ma, J. Petrographic characteristics of Chinese coals and their application in coal utilization processes. Fuel 2002, 81, 1389–1395. [Google Scholar] [CrossRef]
- Gan, J.; Ma, B.; Shang, J.; Ma, X.; Yang, Z. Formation and development of the coal grading conversion ideas. Coal Chem. Ind. 2013, 41, 3–6. [Google Scholar]
- Zhang, L. Study on Maceral Separation and Characteristics of Maceral Concentrates for Kailuan Coking Coals. Ph.D. Thesis, China University of Mining and Technology (Beijing), Beijing, China, 2015. [Google Scholar]
- Honaker, R.Q.; Mohanty, M.K.; Crelling, J.C. Coal maceral separation using column flotation. Miner. Eng. 1996, 9, 449–464. [Google Scholar] [CrossRef]
- Dormans, H.; Hunrjens, F.; van Krevelen, D.W. Chemical structure and properities of coal XX-composition of individual maceral (Vitrinites, Fusinites, Micrinites and Exinites). Fuel 1957, 36, 321–339. [Google Scholar]
- Dyrkacz, G.R.; Horwitz, E.P. Separation of coal macerals. Fuel 1982, 61, 3–12. [Google Scholar] [CrossRef]
- Men, D. Study on the Maceral Crushing and Liberation and on the Coal Blending Coking for Gas Coal. Ph.D. Thesis, China University of Mining and Technology (Beijing), Beijing, China, 2017. [Google Scholar]
- Gagarin, S.G. Coal enrichment with separation into fractions by density. Coke Chem. 2009, 52, 89–93. [Google Scholar] [CrossRef]
- Ma, F.; Tao, Y. Study on maceral characteristics and separation of low-rank coal. Int. J. Coal Prep. Util. 2023, 43, 847–862. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Z.; Xu, J. Separation and preparation of macerals in Shenfu coals by flotation. Fuel 2002, 81, 495–501. [Google Scholar] [CrossRef]
- Barraza, J.; Piñeres, J. A pilot-scale flotation column to produce beneficiated coal fractions having high concentration of vitrinite maceral. Fuel 2005, 84, 1879–1883. [Google Scholar] [CrossRef]
- Kopparthi, P.; Singh, R.; Nag, D.; Mukherjee, A.K. Vitrinite maceral separation using column flotation. Int. J. Coal Prep. Util. 2018, 38, 13–29. [Google Scholar] [CrossRef]
- Hower, J.C.; Kuehn, K.W.; Parekh, B.K.; Peters, W.J. Maceral and microlithotype beneficiation in column flotation at the Powell Mountain Coal Mayflower Preparation Plant, Lee County, Virginia. Fuel Process. Technol. 2000, 67, 23–33. [Google Scholar] [CrossRef]
- Liu, J.; Mak, T.; Zhou, Z.; Xu, Z. Fundamental study of reactive oily-bubble flotation. Miner. Eng. 2002, 15, 667–676. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, J.; Zhou, Z. Selective Reactive Oily Bubble Carriers in Flotation Processes and Methods of Generation and Uses Thereof. U.S. Patent No. 6,959,815, 1 November 2005. [Google Scholar]
- Wallwork, V.; Xu, Z.; Masliyah, J. Bitumen recovery with oily air bubbles. Can. J. Chem. Eng. 2003, 81, 993–997. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y. Study on oily bubbles flotation experiment with low rank Shenfu coal. Coal Sci. Technol. 2015, 43, 152–157. [Google Scholar]
- Qu, J. Research on Reactive Oily Bubble Flotation Behavior of Low Rank Coal and Its Flotation Technique. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2015. [Google Scholar]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Yang, Z.; Chen, L. Exploration on the mechanism of oily-bubble flotation of long-flame coal. Fuel 2018, 216, 427–435. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Liu, R.; Zhou, A.; Qu, J.; Liu, L.; Zhang, N.; Yu, Y.; Zhu, Z.; Chang, J.; et al. Comparison of attachment process of particles to air and oily bubbles in flotation. Adv. Powder Technol. 2023, 34, 104059. [Google Scholar] [CrossRef]
- Wang, S.; Tao, X. Comparison of flotation performances of low rank coal in air and oily bubble processes. Powder Technol. 2017, 320, 37–42. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Ma, X.; Tao, X. Comparison of flotation performances of low-rank coal with lower ash content using air and oily bubbles. Powder Technol. 2020, 374, 443–448. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, J.; Xing, Y.; Li, M.; Zhang, R.; Li, G.; Gui, X. Investigation of adhesion behavior between reactive oily bubble and low-rank coal. Colloid Surf. A 2022, 632, 127809. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Liu, Q.; Deng, M.; Chi, R. Application of reactive oily bubbles to bastnaesite flotation. Miner. Eng. 2014, 64, 139–145. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Liu, Q.; Chi, R. Reactive oily bubble technology for flotation of apatite, dolomite and quartz. Int. J. Miner. Process. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Ruan, Y.; Chi, R. A study on novel reactive oily bubble technology enhanced collophane flotation. Int. J. Miner. Process. 2017, 169, 85–90. [Google Scholar] [CrossRef]
- Ramirez, A.; Gutierrez, L.; Laskowski, J.S. Use of “oily bubbles” and dispersants in flotation of molybdenite in fresh and seawater. Miner. Eng. 2020, 148, 106197. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Bu, X.; Ni, C.; Xie, G. Study on inhibition mechanisms of detachment of coal particles from oily bubbles in flotation column. Powder Technol. 2024, 434, 119368. [Google Scholar] [CrossRef]
- Pietrzak, R.; Wachowska, H. The influence of oxidation with HNO3 on the surface composition of high-sulphur coals: XPS study. Fuel Process. Technol. 2006, 87, 1021–1029. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lee, C.Y.; Lee, H.C. Surface chemistry of polyacrylonitrile- and rayon-based activated carbon fibers after post-heat treatment. Mater. Chem. Phys. 2007, 101, 199–210. [Google Scholar] [CrossRef]
- Xiang, J.; Hu, S.; Sun, L.; Xu, M.; Li, P.; Su, S.; Sun, X. Evolution of carbon and oxygen functional groups during coal combustion. J. Chem. Ind. Eng. China 2006, 57, 2180–2184. [Google Scholar]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Cohen, A.D. Characterization of organically bound oxygen forms in lignites, peats, and pyrolyzed peats by X-ray photoelectron spectroscopy (XPS) and solid-state 13C NMR methods. Energy Fuel 2002, 16, 1450–1462. [Google Scholar] [CrossRef]
- Perry, D.L.; Grint, A. Application of XPS to coal characterization. Fuel 1983, 62, 1024–1033. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, B.; Tian, C.; Sheng, Q.; Li, Z.; Zhang, N.; Wang, J.; Yang, X. Polyaspartic acid, an eco-friendly regulator for the improvement of coking coal flotation: Applications and mechanism. Int. J. Coal Prep. Util. 2024, 1–20. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Z.; Chen, L.; Tao, X.; Tang, L.; He, H. Wetting thermodynamics of low rank coal and attachment in flotation. Fuel 2017, 207, 214–225. [Google Scholar] [CrossRef]
- Albijanic, B.; Bradshaw, D.J.; Nguyen, A.V. The relationships between the bubble–particle attachment time, collector dosage and the mineralogy of a copper sulfide ore. Miner. Eng. 2012, 36–38, 309–313. [Google Scholar] [CrossRef]
- Gu, G.; Xu, Z.; Nandakumar, K.; Masliyah, J. Effects of physical environment on induction time of air-bitumen attachment. Int. J. Miner. Process. 2003, 69, 235–250. [Google Scholar] [CrossRef]
- Zou, W.; Cao, Y.; Liu, J.; Li, W.; Liu, C. Wetting process and surface free energy components of two fine liberated middling bituminous coals and their flotation behaviors. Powder Technol. 2013, 246, 669–676. [Google Scholar] [CrossRef]
- Zhu, H.; Li, H.; Ou, Z.; Wang, D.; Lyu, X. Study on surface modification of different rank coals by using FTIR. J. Chin. Univ. Min. Technol. 2001, 30, 366–370. [Google Scholar]
- Wen, S. Spectral Analysis of Fourier Transform Infrared Spectroscopy, 2nd ed.; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]




| Samples | Vitrinite | Inertinite | Mineral | Total | |||
|---|---|---|---|---|---|---|---|
| Counts | Enrichment Rates (%) | Counts | Enrichment Rates (%) | Counts | Enrichment Rates (%) | ||
| DLT5−2-V | 834 | 85.45 | 127 | 13.01 | 15 | 1.54 | 976 |
| DLT5−2-I | 128 | 13.20 | 815 | 84.02 | 27 | 2.78 | 970 |
| BLT2−2-V | 811 | 85.55 | 119 | 12.55 | 18 | 1.90 | 948 |
| BLT2−2-I | 122 | 13.10 | 788 | 84.64 | 21 | 2.26 | 931 |
| Samples | Proximate Analysis, Air-Dry Basis | Ultimate Analysis, Dry Basis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M/% | A/% | V/% | FC/% | C/% | H/% | O 1/% | N/% | St/% | |
| DLT5−2-V | 7.04 | 2.40 | 45.92 | 44.64 | 77.10 | 4.61 | 16.69 | 1.15 | 0.44 |
| DLT5−2-I | 6.04 | 7.52 | 44.48 | 41.96 | 72.90 | 2.28 | 22.98 | 1.28 | 0.57 |
| BLT2−2-V | 6.72 | 2.48 | 41.76 | 49.04 | 75.44 | 4.81 | 17.99 | 1.29 | 0.47 |
| BLT2−2-I | 7.83 | 6.16 | 37.12 | 48.89 | 69.67 | 3.83 | 25.04 | 0.91 | 0.55 |
| Fitting Range (eV) | Background | L-G | FWHM Range (eV) | C1s Peak Range (eV) | Attribution |
|---|---|---|---|---|---|
| (281–295) ± 0.5 | Shirley | 0 | 0–2 | 284.6 | C-C/C-H |
| 285.8–286.3 | C-O-C/C-OH | ||||
| 287.3–287.6 | C=O | ||||
| 289.0–289.2 | O=C-O |
| Element | Element (Atomic) Contents/% | |||
|---|---|---|---|---|
| DLT5−2 Coal | BLT2−2 Coal | |||
| Vitrinite-Rich Coal | Inertinite-Rich Coal | Vitrinite-Rich Coal | Inertinite-Rich Coal | |
| C1s | 82.83 | 80.24 | 84.18 | 79.28 |
| O1s | 15.22 | 17.55 | 14.35 | 18.40 |
| N1s | 1.95 | 2.22 | 1.47 | 2.33 |
| O/C | 0.18 | 0.22 | 0.17 | 0.23 |
| Functional Groups | Contents in Total Sample/% | |||
|---|---|---|---|---|
| DLT5−2 Coal | BLT2−2 Coal | |||
| Vitrinite-Rich Coal | Inertinite-Rich Coal | Vitrinite-Rich Coal | Inertinite-Rich Coal | |
| C-C/C-H | 67.24 | 60.24 | 65.82 | 58.90 |
| C-O-C/C-OH | 9.91 | 8.83 | 10.37 | 7.25 |
| C=O | 0.22 | 0.39 | 0.40 | 0.66 |
| O=C-O | 0.04 | 0.11 | 0.05 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Luo, C.; Zhu, Z.; An, Q.; Li, X.; Zhu, H.; Chang, J.; Li, Z.; Zhou, A.; Chen, S.; et al. Oily Bubble Flotation of Coal Macerals of Shendong Jurassic Coal. Minerals 2024, 14, 328. https://doi.org/10.3390/min14040328
Qu J, Luo C, Zhu Z, An Q, Li X, Zhu H, Chang J, Li Z, Zhou A, Chen S, et al. Oily Bubble Flotation of Coal Macerals of Shendong Jurassic Coal. Minerals. 2024; 14(4):328. https://doi.org/10.3390/min14040328
Chicago/Turabian StyleQu, Jinzhou, Chang Luo, Zhanglei Zhu, Quan An, Xinyi Li, Honglin Zhu, Jing Chang, Zhen Li, Anning Zhou, Songjiang Chen, and et al. 2024. "Oily Bubble Flotation of Coal Macerals of Shendong Jurassic Coal" Minerals 14, no. 4: 328. https://doi.org/10.3390/min14040328





