Swelling Stress of Bentonite: Thermodynamics of Interlayer Water in K-Montmorillonite in Consideration of Alteration
Abstract
:1. Introduction
1.1. Research Background
1.1.1. Overview of Radioactive Waste Disposal
1.1.2. Geological Disposal System
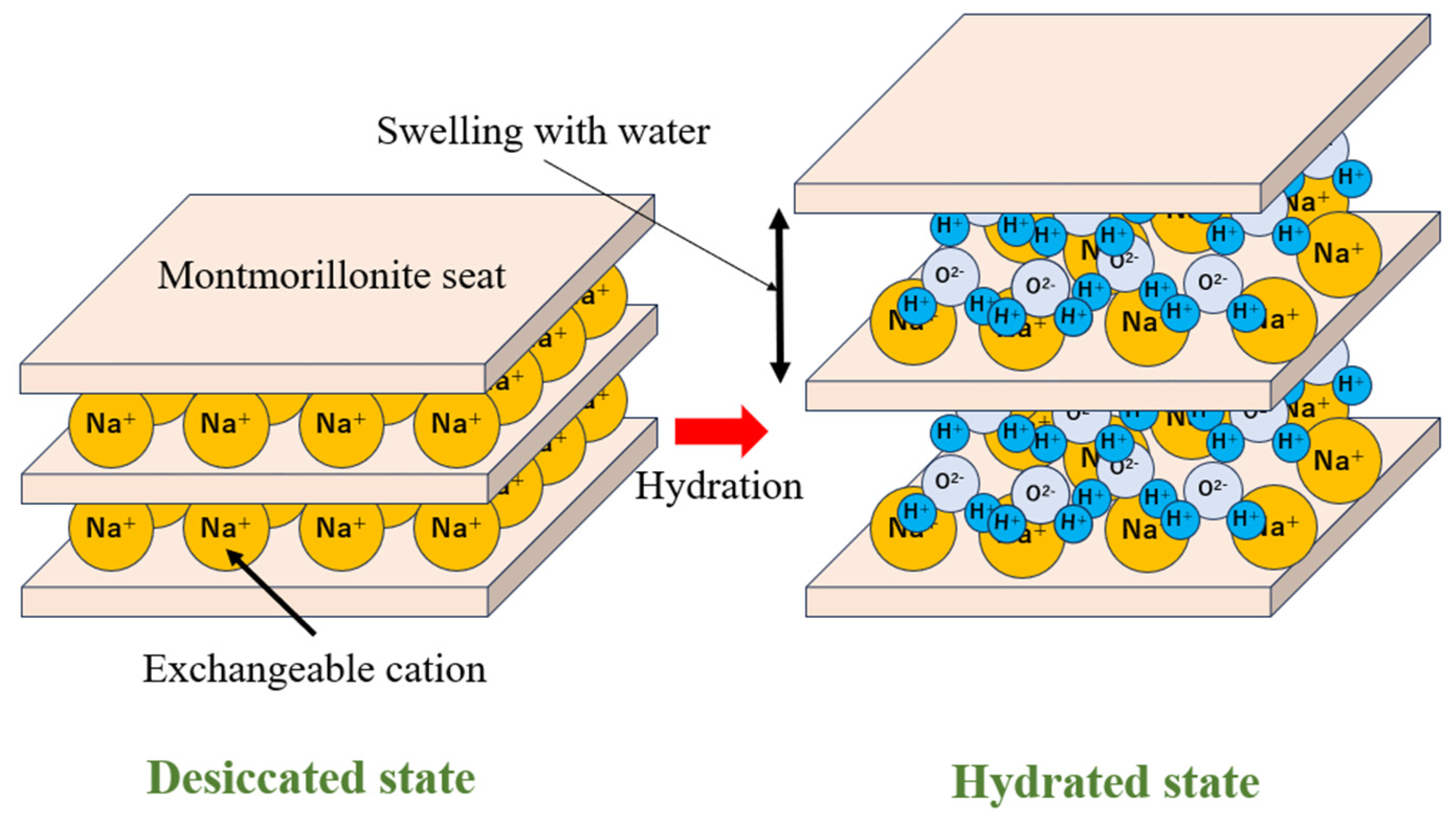
1.1.3. Functions and Properties of Bentonite
1.2. Previous Research
1.3. Purpose of Research
2. Thermomechanical Analysis of Swelling Stress
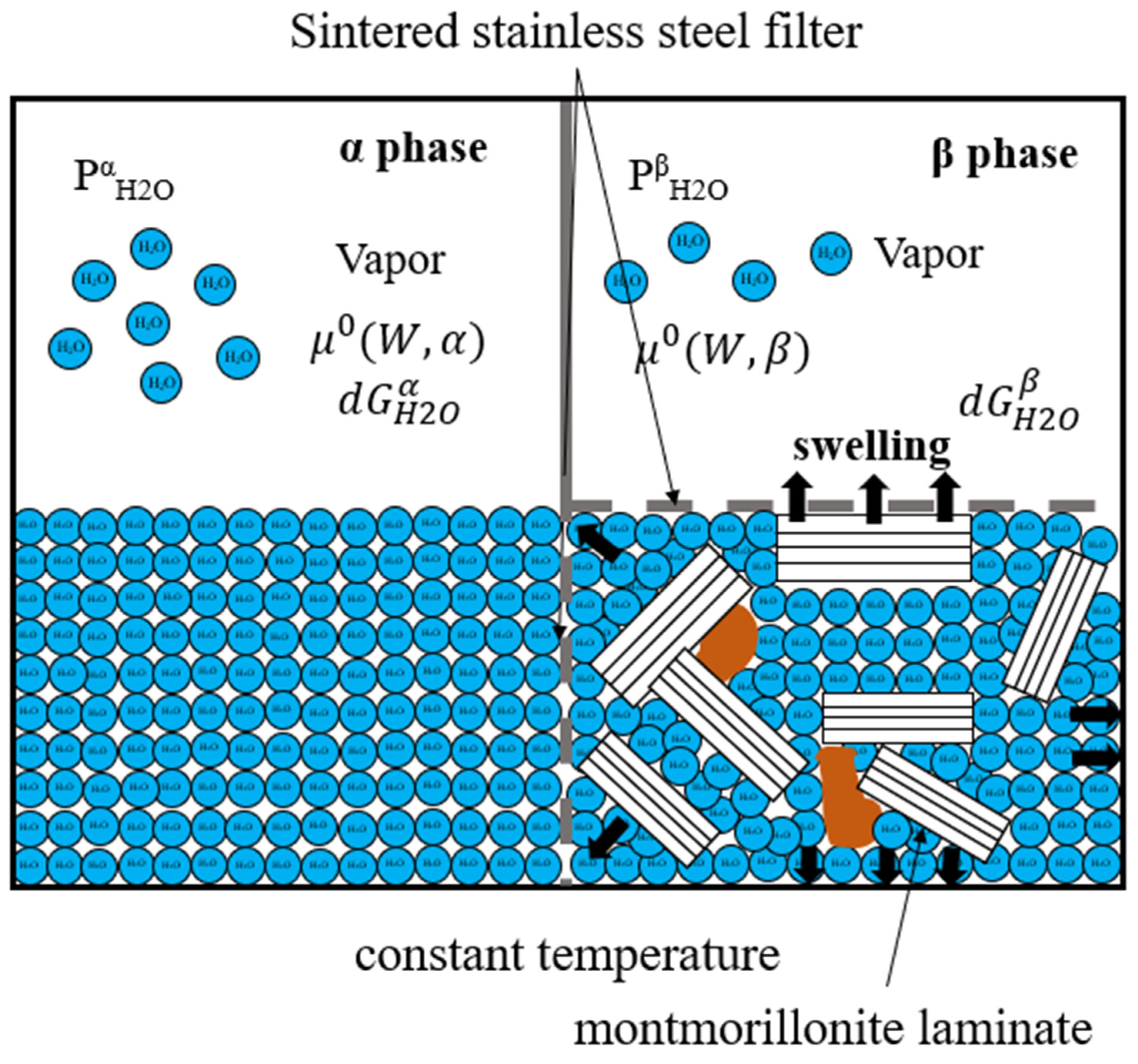
2.1. Thermodynamic Model for Swelling Stress at a Constant Temperature
2.2. Analysis Conditions
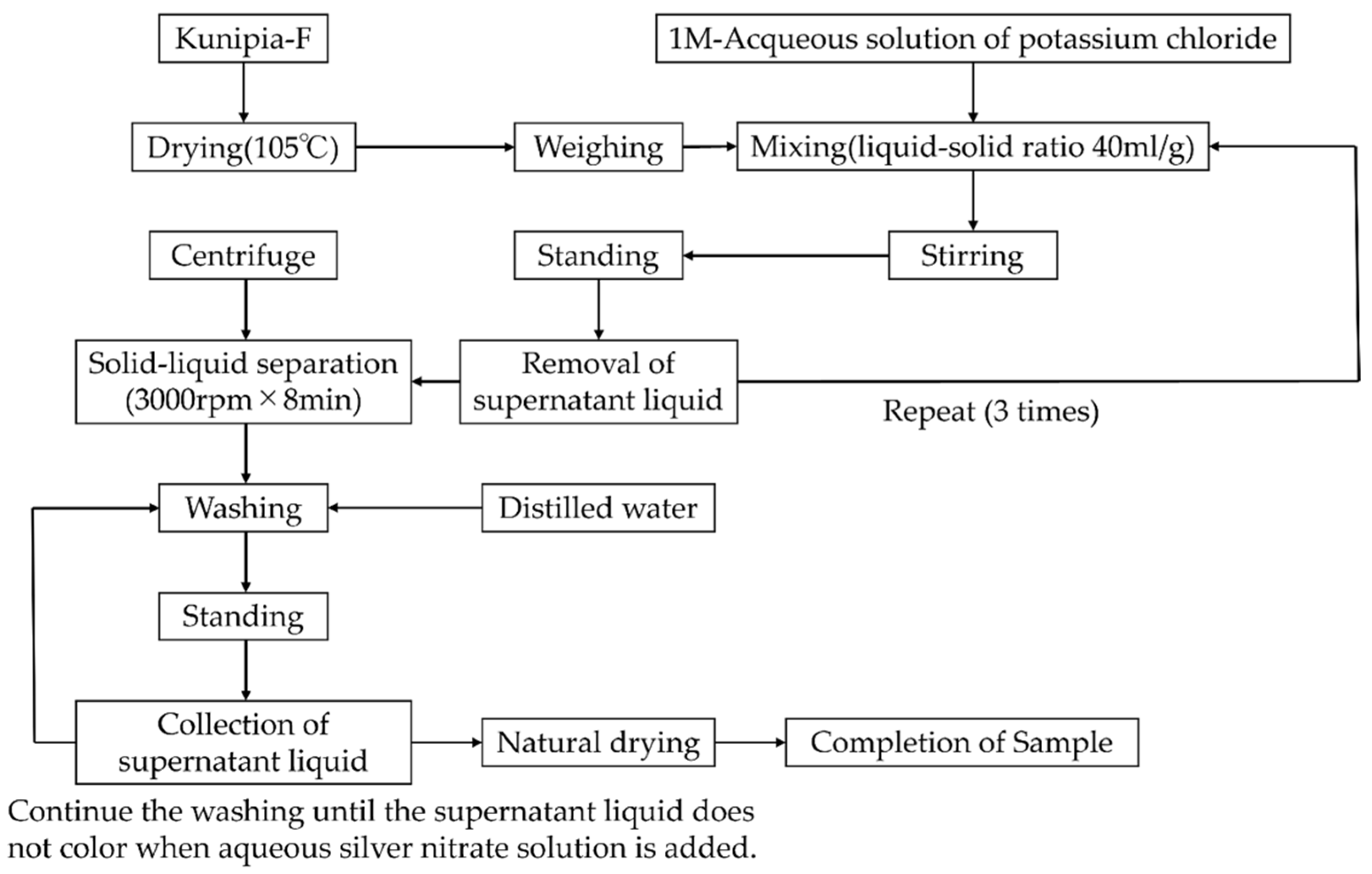
2.3. Substitutional Synthesis of K-Montmorillonite
2.4. Vapor Pressure Measurement by Relative Humidity Method
3. Measurement Results and Discussion
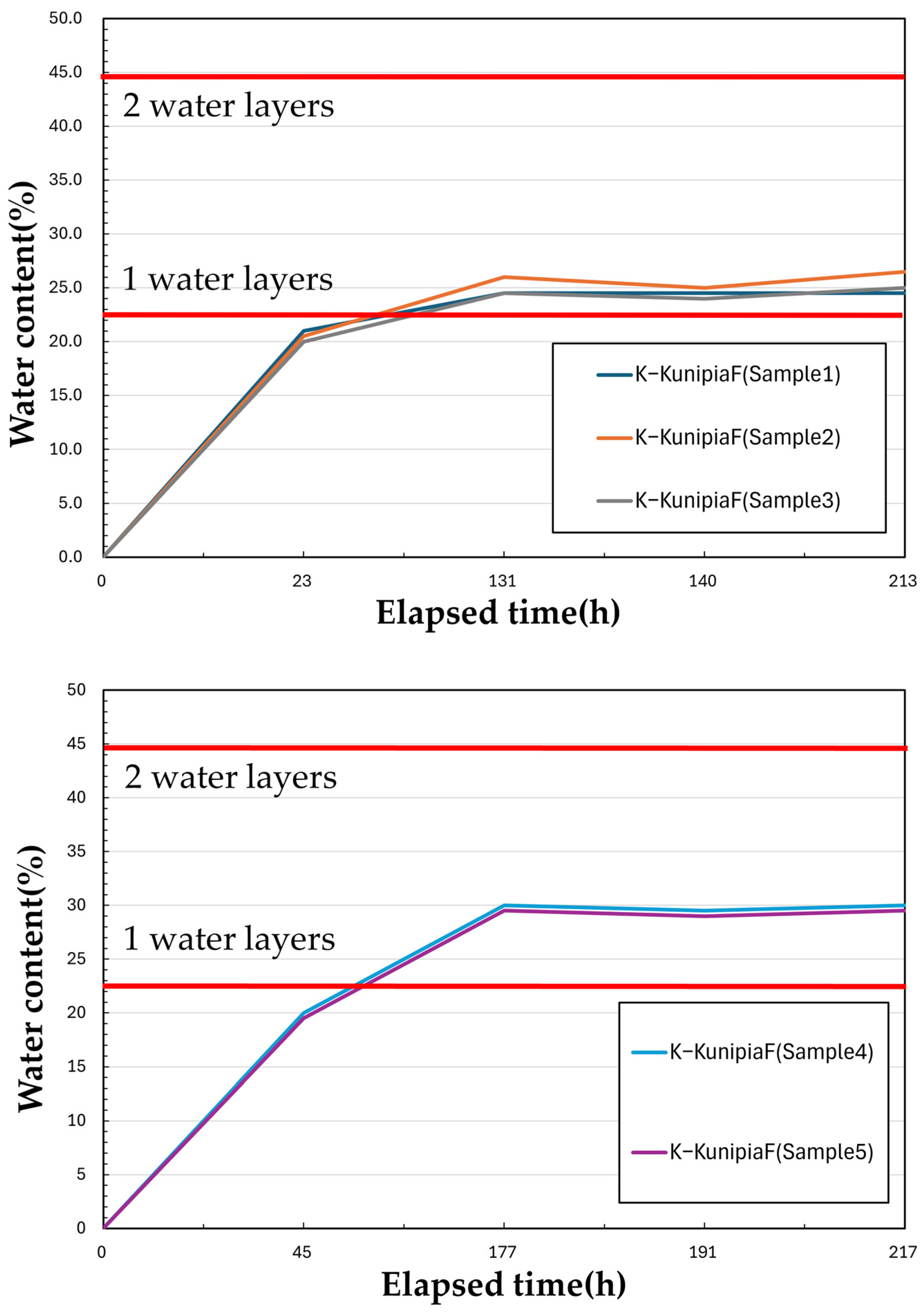
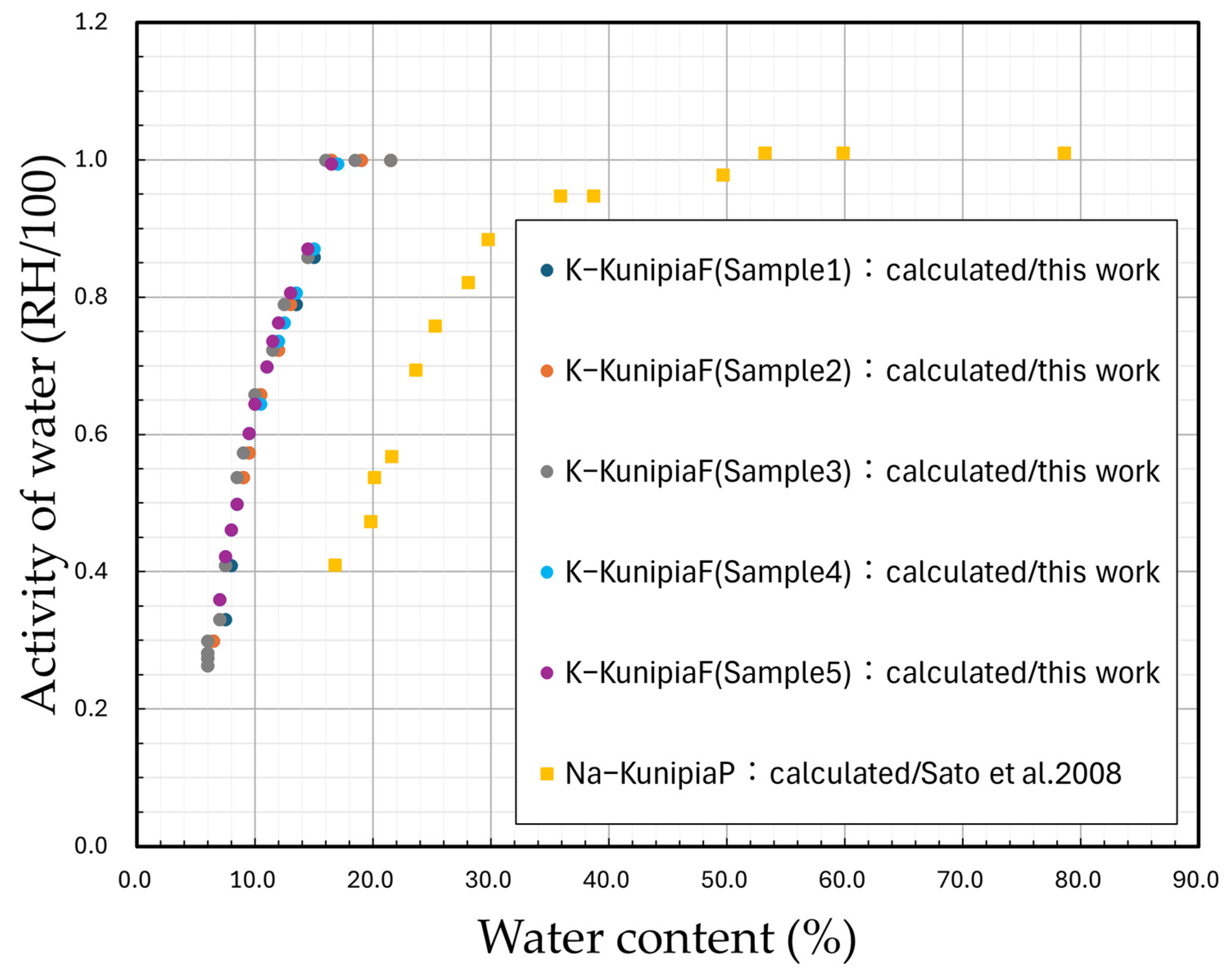
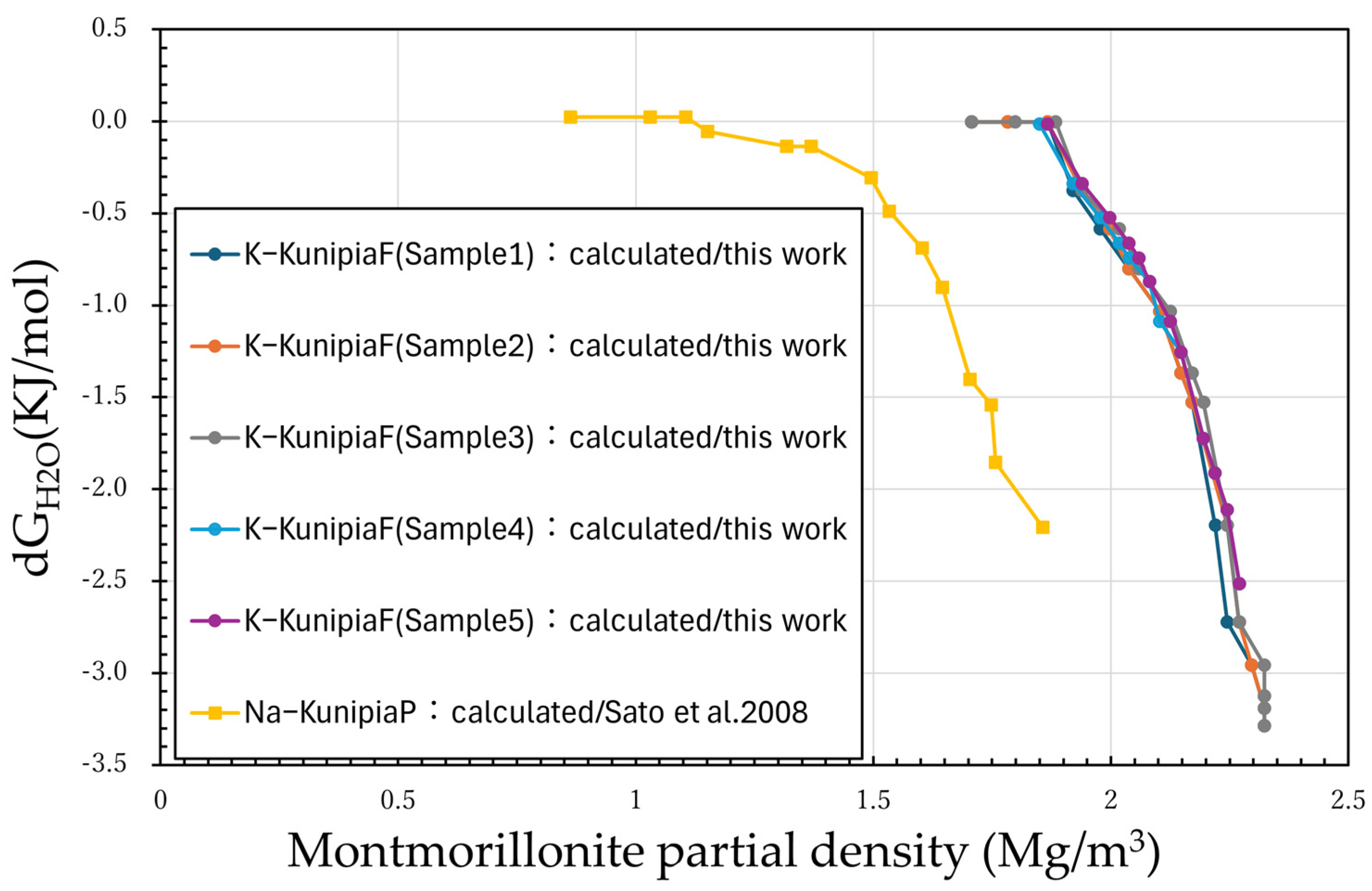
3.1. Measurement Results
3.2. Discussion
4. Conclusions
4.1. Summary
4.2. Future Issues
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Japan Nuclear Cycle Development Institute. Technical Reliability on Geological Disposal of HLW in Japan—Second Progress Report on Research and Development for the Geological Disposal of HLW in Japan—General Report, JNC TN1400 99-020. 1999. Available online: https://jopss.jaea.go.jp/pdfdata/JNC-TN1400-99-020.pdf (accessed on 15 February 2024). (In Japanese).
- Sato, H. Function for Controlling Nuclide Migration of Buffer Material in the Geological Disposal for High-Level Radioactive Waste. J. MMIJ 2009, 125, 1–12. Available online: https://www.jstage.jst.go.jp/article/journalofmmij/125/1/125_1_1/_pdf/-char/ja (accessed on 15 February 2024). (In Japanese). [CrossRef]
- Sato, H. Quantification of Exchangeable Cations in Interlayer of Tsukinuno Sodium-Montmorillonite. Mater. Res. Soc. Symp. Proc. 2009, 1193, 529. [Google Scholar] [CrossRef]
- Sato, H. Measurements of Thermodynamic Data of Water in Na-Bentonite in the Pressure Release System and Standard Condition by Relative Humidity Method, Atomic Energy Society of Japan [2022 Fall Meeting], 3C12. 2022. Available online: https://confit.atlas.jp/guide/event-img/aesj2022f/3C12/public/pdf?type=in (accessed on 15 February 2024). (In Japanese).
- Ito, M.; Okamoto, M.; Shibata, M.; Sasaki, Y.; Danbara, T.; Suzuki, K.; Watanabe, T. Mineral Composition of Bentonite, PNC TN8430 93-003; Japan Nuclear Cycle Development Institute (JNC): Ibaraki, Japan, 1993. (In Japanese) [Google Scholar]
- Kikuchi, H.; Tanai, K. Basic Characteristic Test of Buffer/Backfill Material under Horonobe Groundwater Condition (Testing Document), JNC TN8430 2004-005; Japan Nuclear Cycle Development Institute (JNC): Ibaraki, Japan, 2005. (In Japanese) [Google Scholar]
- Suzuki, H.; Fujita, T. Swelling Characteristics of Buffer Material, JNC TN8400 99-038; Japan Nuclear Cycle Development Institute (JNC): Ibaraki, Japan, 1999. (In Japanese) [Google Scholar]
- Japan Atomic Energy Agency (JAEA). Database on Basic Characteristics of Buffer Material. Available online: https://bufferdb.jaea.go.jp/bmdb/ (accessed on 20 January 2023).
- Agency for Natural Resources and Energy, Ministry of Economy, Trade and Industry. The Project for Validating Assessment Methodology in Geological Disposal System. 2013. Available online: https://www.enecho.meti.go.jp/category/electricity_and_gas/nuclear/rw/library/2013/25-7-1.pdf (accessed on 15 February 2024). (In Japanese).
- Morodome, S.; Kawamura, K. In Situ X-ray Diffraction Study of the Swelling of Montmorillonite as Affected by Exchangeable Cations and Temperature. Clays Clay Miner. 2011, 59, 165–175. Available online: https://link.springer.com/article/10.1346/CCMN.2011.0590205 (accessed on 15 February 2024). [CrossRef]
- Saito, Y.; Okawara, M. Study on State of Water in Homo-Ionic Montmorillonite by X-Ray Diffraction and Near-Infrared Spectroscopy. Clay Sci. 2019, 58, 43–59. Available online: https://www.jstage.jst.go.jp/article/jcssjnendokagaku/58/2/58_580203/_pdf/-char/ja (accessed on 15 February 2024). (In Japanese).
- The Japan Society of Mechanical Engineers. 1980 JSME Steam Tables in SI; JSME: Tokyo, Japan, 1981. (In Japanese) [Google Scholar]
- Nihon, K.; Kagaku, B.; Kisohen, K. Edition Handbook of Chemistry, Basic Version, Revised 6th Edition; Maruzen Publishing Co., Ltd.: Tokyo, Japan, 2021. (In Japanese) [Google Scholar]
- Sato, H. A Thermodynamic Approach on Effect of Salinity on Swelling Pressure of Bentonite. In Proceedings of the 4th Japan-Korea Joint Workshop on Radioactive Waste Disposal 2008: Perspective of Science and Engineering, Hakone, Japan, 27–28 May 2008; pp. 1–17. [Google Scholar]
- Sato, H. Thermodynamic Understanding on Swelling Pressure of Bentonite Buffer. In Proceedings of the 15th International Conference on Nuclear Engineering (ICONE15), Nagoya, Japan, 22–26 April 2007. ICONE15-10207; Available online: https://www.jstage.jst.go.jp/article/jsmeicone/2007.15/0/2007.15__ICONE1510_99/_pdf (accessed on 15 February 2024).
- Sato, H. Thermodynamic Model on Swelling of Bentonite Buffer and Backfill Materials. Phys. Chem. Earth 2008, 33, S538–S543. [Google Scholar] [CrossRef]
- Sato, H. Purification of Na-smectite and preparation of oriented samples for diffusion experiments, JAEA-Research 2005-004. 2006. Available online: https://jopss.jaea.go.jp/pdfdata/JAEA-Research-2005-004.pdf (accessed on 15 February 2024). (In Japanese).
- Sato, H. Swelling and Thermodynamic of Buffer Material as the Engineered Barrier in the Geological Disposal. Nucl. Backend Res. 2020, 27, 105–114. Available online: https://nuce.aesj.or.jp/jnuce/vol27/Jnuce-Vol27-2-p105-114.pdf (accessed on 15 February 2024). (In Japanese).
- National Institutes of Natural Sciences, National Astronomical Observatory of Japan. Chronological Scientific Tables, Desktop version; Maruzen Publishing Company Ltd.: Tokyo, Japan, 2012; p. 383. (In Japanese) [Google Scholar]
- Suzuki, H.; Shibata, M.; Yamagata, J.; Hirose, I.; Terakado, K. Characteristic Test of Buffer Material (1), PNC TN8410 92-057. 1992, pp. 1–103. Available online: https://jopss.jaea.go.jp/pdfdata/PNC-TN8410-92-057.pdf (accessed on 15 February 2024). (In Japanese).
- Maeda, M.; Tanai, K.; Ito, M.; Mihara, M.; Tanaka, M. Mechanical properties of the Ca exchanged and Ca bentonite—Swelling pressure, hydraulic conductivity, compressive strength and elastic modulus-, Power Reactor and Nuclear Fuel Development Corporation, PNC TN8410 98-021. 1998, pp. 1–136. Available online: https://jopss.jaea.go.jp/pdfdata/PNC-TN8410-98-021.pdf (accessed on 15 February 2024). (In Japanese).
- Shirozu, H. Clay Mineralogy—The Basis of Clay Science; Asakura Publishing: Tokyo, Japan, 2010. (In Japanese) [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, M.; Sato, H. Swelling Stress of Bentonite: Thermodynamics of Interlayer Water in K-Montmorillonite in Consideration of Alteration. Minerals 2024, 14, 430. https://doi.org/10.3390/min14040430
Endo M, Sato H. Swelling Stress of Bentonite: Thermodynamics of Interlayer Water in K-Montmorillonite in Consideration of Alteration. Minerals. 2024; 14(4):430. https://doi.org/10.3390/min14040430
Chicago/Turabian StyleEndo, Misato, and Haruo Sato. 2024. "Swelling Stress of Bentonite: Thermodynamics of Interlayer Water in K-Montmorillonite in Consideration of Alteration" Minerals 14, no. 4: 430. https://doi.org/10.3390/min14040430





