1. Introduction
Metals in nature are generally found in oxidized form, except gold, which is often found in its native metallic form. When referring to ore, it generally means a complex mixture containing various metal oxides, sometimes combined with other components. This chemical diversity makes the process of extracting metals, known as extractive metallurgy, a complex and meticulous undertaking [
1]. The main aim of extractive metallurgy is to reduce these metal oxides to recover pure metals. This process generally involves several stages, each designed to separate the metals from the other elements present in the ore and return them to their metallic form. Oxides can be reduced by various means, such as chemical reactions with reducing agents or by heating at high temperatures in a controlled environment [
1].
Worldwide crude steel production currently stands at 1500 million tons per year, followed by other base metals and alloys. Most of these metals, including iron, aluminum, silicon, manganese, chromium, nickel, titanium, vanadium, and many others, are extracted from oxidized minerals. This extraction process typically utilizes carbon as a reducing agent, posing challenges in terms of future supply and environmental pollution. For instance, steel production alone contributes to 5% of total CO
2 emissions [
2].
Thermal plasma represents a versatile decarbonization solution for the large-scale manufacture of various advanced materials required in highly specialized industrial sectors. The growing demand for materials with improved physical and mechanical properties to meet the stringent requirements of cutting-edge industries such as aerospace, chemicals, electronics, semiconductors, transport, and nuclear energy is steadily increasing [
3].
Moreover, plasmas are recognized for their superior efficiency compared to conventional heating methods and could eventually supplant them: the torch used in these experiments boasts an efficiency of 75% [
4]. In addition, the plasma used in this study offers further advantages by consuming polluting gases such as CO
2 and CH
4.Industrial plasmas can operate at pressures ranging from near vacuum to several atmospheres. Several significant industrial applications occur at or near atmospheric pressure, sometimes even in the air. These applications utilize non-equilibrium plasmas (non-LTE) with electron temperatures many orders of magnitude higher than the temperatures of the heavy components of the plasma, as well as thermal plasmas approaching local thermal equilibrium (LTE) [
5]. For instance, one study examined the effects of low-temperature, non-equilibrium plasma generated by a dielectric barrier discharge in ambient air at atmospheric pressure on the acid–base, adsorption, and flotation properties of natural iron sulfides such as pyrite and arsenopyrite [
6]. In another study, the application of the plasma–chemical ore treatment method using nanosecond pulsed dielectric barrier discharge (DBD) plasma at low temperatures in the air under atmospheric pressure showed promising prospects for selective separation processes of semiconducting ores, particularly sulfides and oxides [
7].
When the temperature of a plasma is high enough, a local thermodynamic equilibrium (LTE) is reached. For plasma species in motion, LTE is the thermodynamic state achieved when collisions prevail in regions with modest spatial variations (where the plasma does not absorb its own radiation). High-density arc discharges are the only means of creating the plasmas required for industrial-scale pyrometallurgical processes [
8]. These heated plasma arcs are created between a cathode and an anode. There is a wide variety of electrodes, each with its own shape and composition. The most common types of electrodes are rods, buttons, tubes, and rings. Rod and button electrodes are often made from thoriated tungsten (2%–3% ThO
2) or graphite. Currently, plasma tools are either transferred arc systems or non-transferred arc devices. The process calls for electricity, which may be provided by the device’s operating current and voltage [
9].
There are now two primary areas where thermal plasma technology is being applied: materials processing and waste treatment. Many high-tech companies rely on the large-scale, high-purity manufacturing of novel materials, and thermal plasma synthesis provides a flexible and cost-effective approach for this purpose. High-tech sectors, including aircraft, chemicals, electronics, semiconductors, transportation, and nuclear power, are increasingly aware of the need for materials with enhanced physical and mechanical qualities for demanding applications [
10]. Over the past decade, plasma waste treatment has emerged as a crucial technology in the face of growing waste disposal challenges. This method has gained importance, not least due to a growing awareness of the potential for waste recovery and the production of valuable co-products [
11]. For example, thermal plasma represents an essential tool in the transformation of waste-derived fuels, providing the optimum conditions for efficient pyrolysis and gasification processes, thus contributing to more sustainable waste management and the production of renewable energy [
12]. A recent study brilliantly illustrates the successful integration of hybrid thermal plasma technology with carbon capture, utilization, and storage (CCUS) for sewage sludge treatment. This innovative approach offers the ability to transform sewage sludge into syngas while capturing a proportion of the carbon in solid form and converting CO
2 to CO within the syngas [
13]. A study has been carried out into the gasification of various organic materials in a steam plasma generated in a special plasma torch equipped with a water-stabilized arc. This unique configuration produces a thermal plasma characterized by very high enthalpy and low mass flow thanks to an arc discharge in direct contact with water [
14]. In conjunction with this, a new process has been proposed and investigated using an equilibrium thermochemical modeling study to assess the effectiveness of an innovative technology that merges thermal plasma and thermochemical looping. This innovative approach aims to use hydrogen in the iron reduction process, opening up new prospects for the steel industry [
15]. Recently, thermal plasma has gained worldwide popularity for the treatment of complex waste streams due to its many intrinsic advantages. These include the ability to produce hydrogen-enriched synthesis gas (H
2) [
16].
In comparison to existing DC thermal plasma torches, the newly developed DC torch represents a significant breakthrough in technology. By utilizing molecular gases, it provides both high plasma enthalpy (50 MJ/kg at 7000 K) and high thermal conductivity (≈4 W/m·K at 7000 K) [
17]. One disadvantage of using argon as a plasma gas is its low thermal conductivity. This reduces the heat transfer rate to the treated materials. To overcome this, a small percentage of hydrogen or helium is typically added. While this addition improves heat transfer, it also accelerates electrodes’ erosion. During arc burning, hydrocarbons completely dissociate into free carbon and hydrogen. Under appropriate conditions, carbon ions from the gas phase diffuse to the cathode surface, establishing a dynamic equilibrium between carbon sublimation and precipitation [
18]. The electrode is preserved and has a longer service life due to the carbon coating. For combustion, this torch utilizes a gas similar to carbon dioxide, which can be obtained in near-pure form from certain chemical firms as waste gas [
19].
Potential uses for CO
2/CH
4 plasma torches are mainly unexplored. However, it is critical to emphasize their prospective capacity to reduce greenhouse gas (GHG) emissions, such as CO
2, as well as other harmful gases, such as NOx [
20]. Furthermore, these plasma torches might increase the consumption of the most harmful gases found across several industrial sectors. In this study, the CO
2/CH
4 torch utilized up to 3.6 m
3·h
−1 of gas, which is much less than gas burners, which may burn over 2000 m
3·h
−1 of fossil fuels [
21]. However, using oxygen-enriched air may significantly decrease consumption by up to 25% [
22].
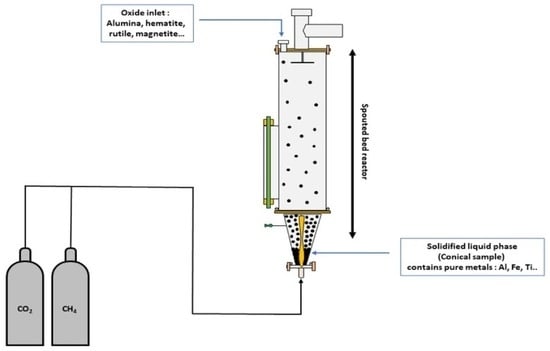
The main purpose of this study is to demonstrate the viability of the concept of reducing oxides using a plasma torch powered by greenhouse gases such as CO2 and CH4. What particularly sets this research apart is its innovative exploration of the use of this type of plasma torch to reduce specific oxides, an approach that has not been attempted yet. Alumina, hematite, magnetite, and titanium oxide are only a few of the oxides that were studied for their reducing potential in this report. Metallic phases have been produced by adopting this approach. The reducing power of the plasma torch was increased, and high temperatures were attained by using a CO2/CH4 molar ratio of 1:1. After plasma treatment, metal phases including titanium, iron, and aluminum were detected using XRD analysis. SEM–EDS observations were performed to analyze the microstructure and identify the elemental composition of materials before and after plasma treatment, proving the technology’s efficacy in the field of material processing.
4. Conclusions
In various sectors, the quest for tools that combine high efficiency with low cost has become crucial. Plasma technology stands out as one such tool, developed for a multitude of applications, particularly in material processing. This study took an innovative approach, using a new DC plasma torch for reducing oxides such as alumina, iron oxide, and titanium oxide. The fundamental aim of this research is to demonstrate the feasibility of this oxide reduction method using a plasma torch powered by greenhouse gases such as CO2 and CH4. This study stands out for its innovative exploration of the use of this specific type of plasma torch for the reduction of specific oxides, an approach that had never been attempted before. The conical portion of the plasma reactor proved critical in preventing overheating, thus reducing processing time. This innovative approach successfully reduced a wide range of oxides, including alumina, hematite, magnetite, and rutile, and their mixtures. The thermodynamic investigation confirmed the production of many chemical species, indicating that pure metals might be produced utilizing the CO2/CH4 mixture as a plasmagenic gas. XRD and SEM–EDS studies were used to investigate the reduction of alumina, hematite, magnetite, and rutile. The results show that in the reactor’s conical section, titanium oxide is partially reduced, and a small amount of titanium is produced, while aluminum oxide and iron oxide (magnetite and hematite) are reduced to produce aluminum and iron metal, respectively. The significant variation in oxygen levels before and after treatment confirms the XRD studies, achieved by using the plasma torch’s power as a successful tool for oxide reduction. The oxide mixtures also showed encouraging results, allowing the separation of pure metals such as iron and titanium. Regarding plasma composition, a detailed analysis utilizing mass spectrometry and thermodynamic calculations showed that CH4 and CO2 decompose into H2 and CO. This process paves the way for an innovative procedure that promises to significantly reduce the environmental footprint associated with the thermal treatment of oxides. Using these greenhouse gases for reduction avoids generating new emissions, thereby mitigating adverse environmental impacts. Despite these hopeful findings, further research and development in this area are needed before this technology can realize its full potential.