Minerals of the Au-Cu-Ag System in Grains from the Placers of the Olkhovaya-1 River (Eastern Kamchatka, Russia)
Abstract
:1. Introduction
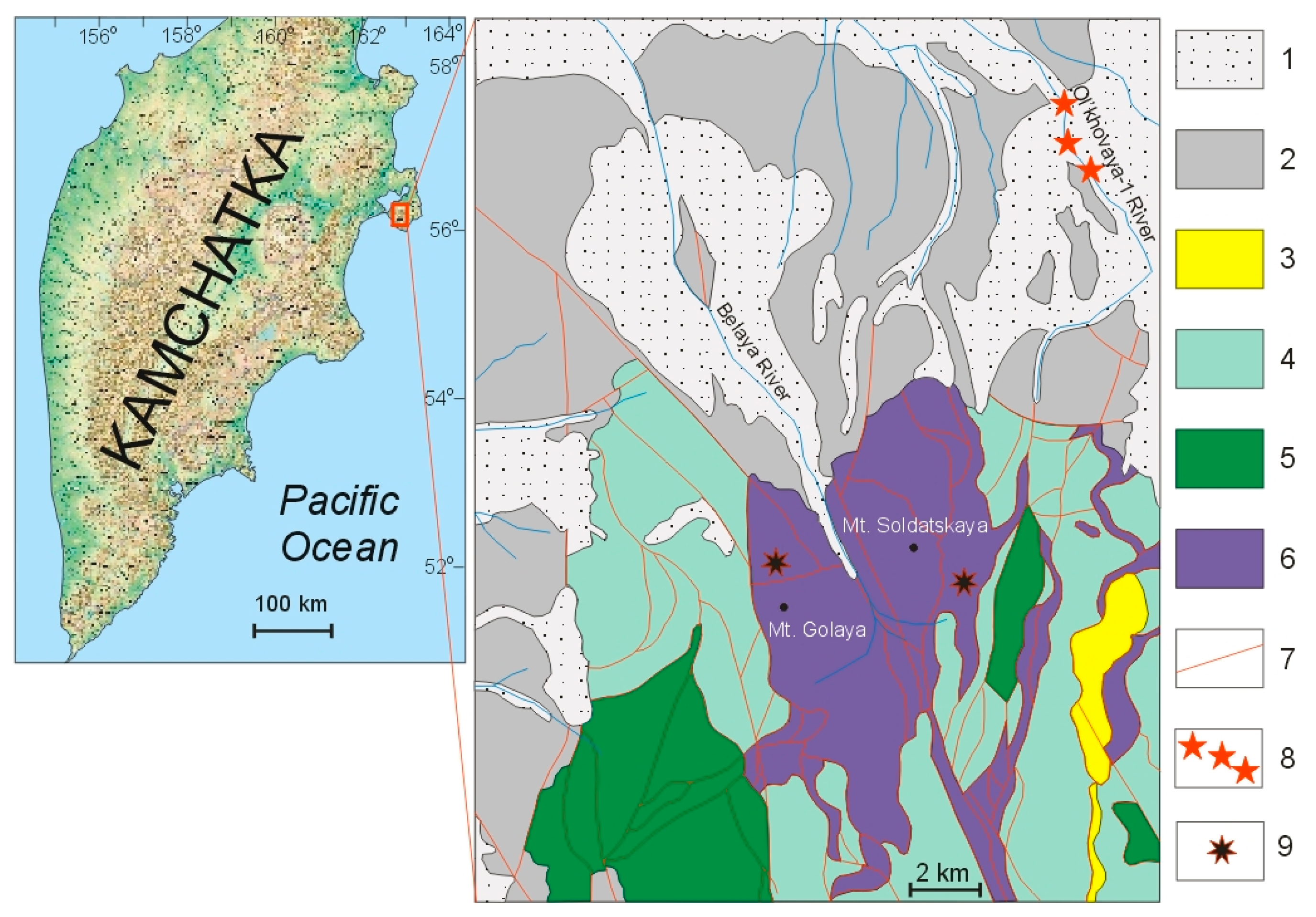
2. Geological Background
3. Materials for Research and Analytical Techniques
3.1. Materials for Research
3.2. Analytical Techniques
4. Results
4.1. Colors and Internal Textures of Grains
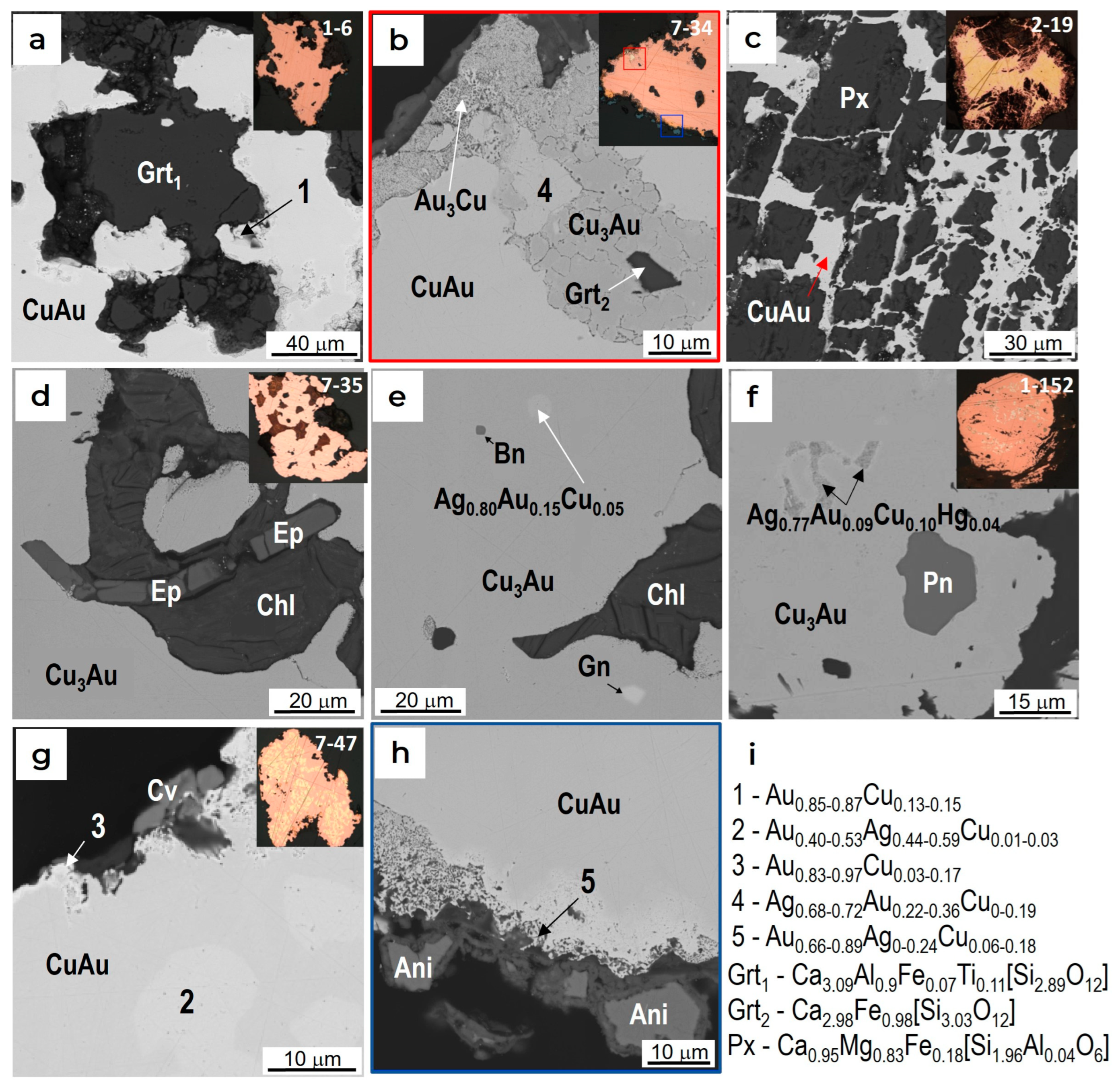
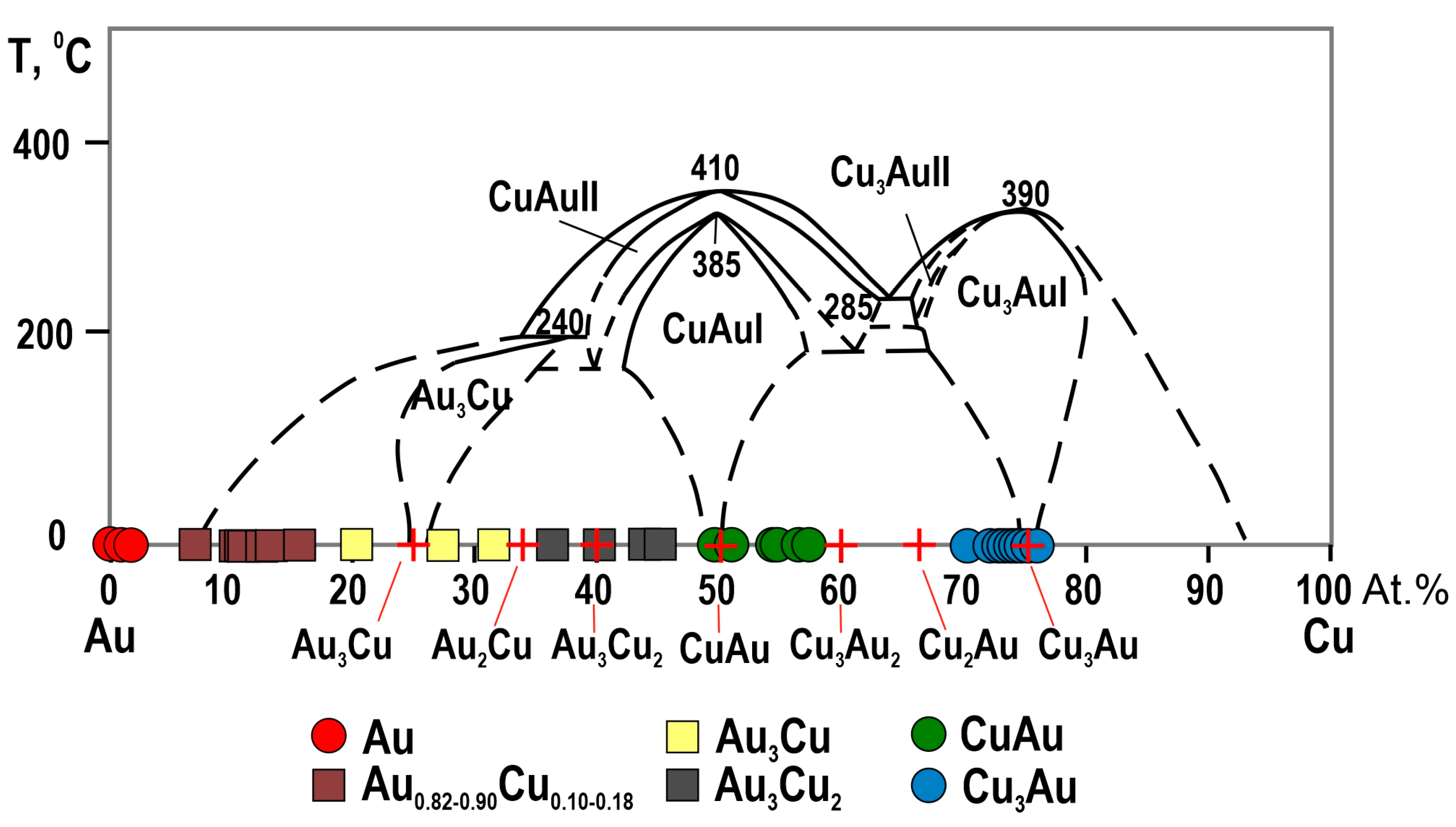
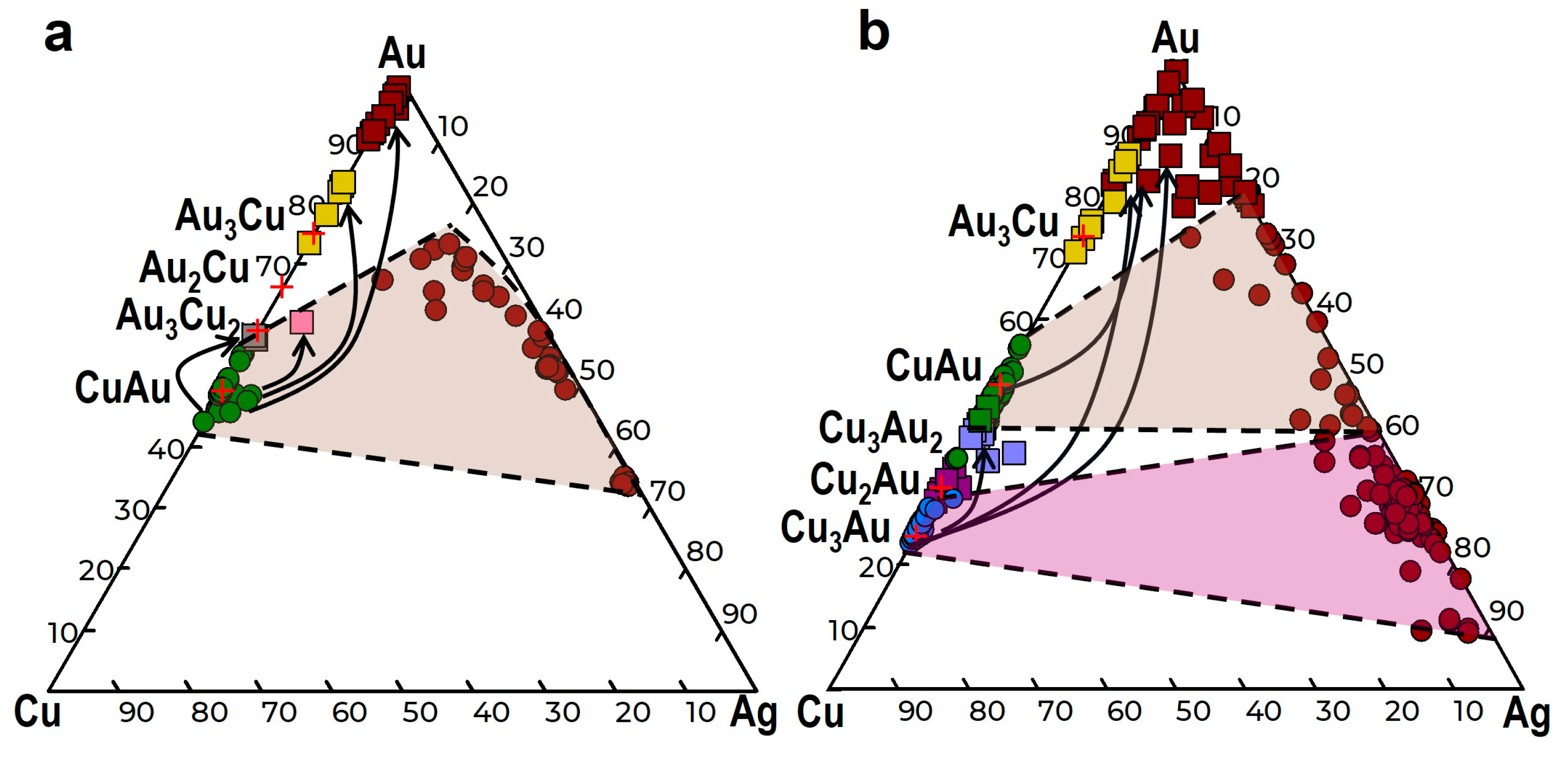
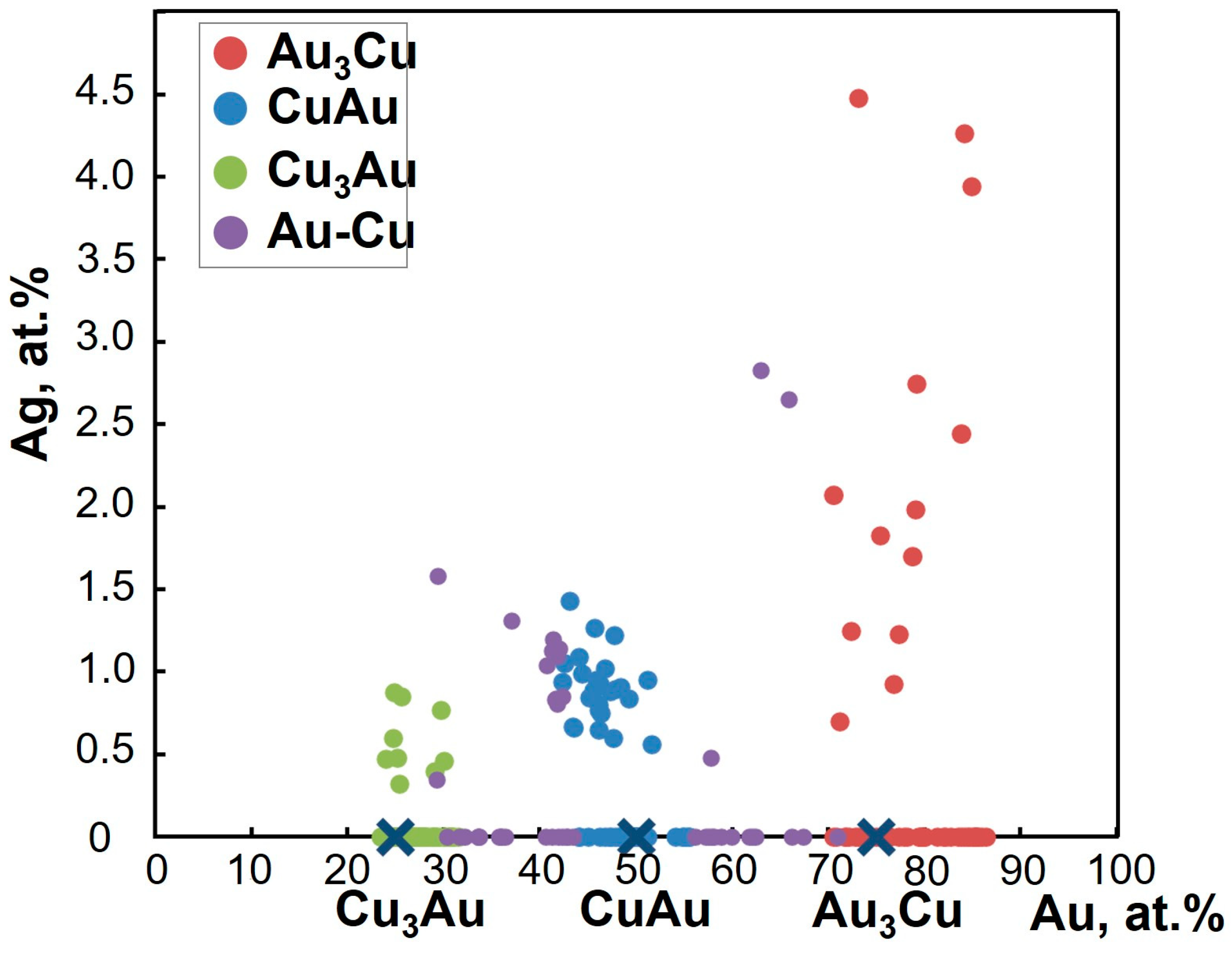
4.2. The Compositions of Minerals of the Au-Cu-Ag System

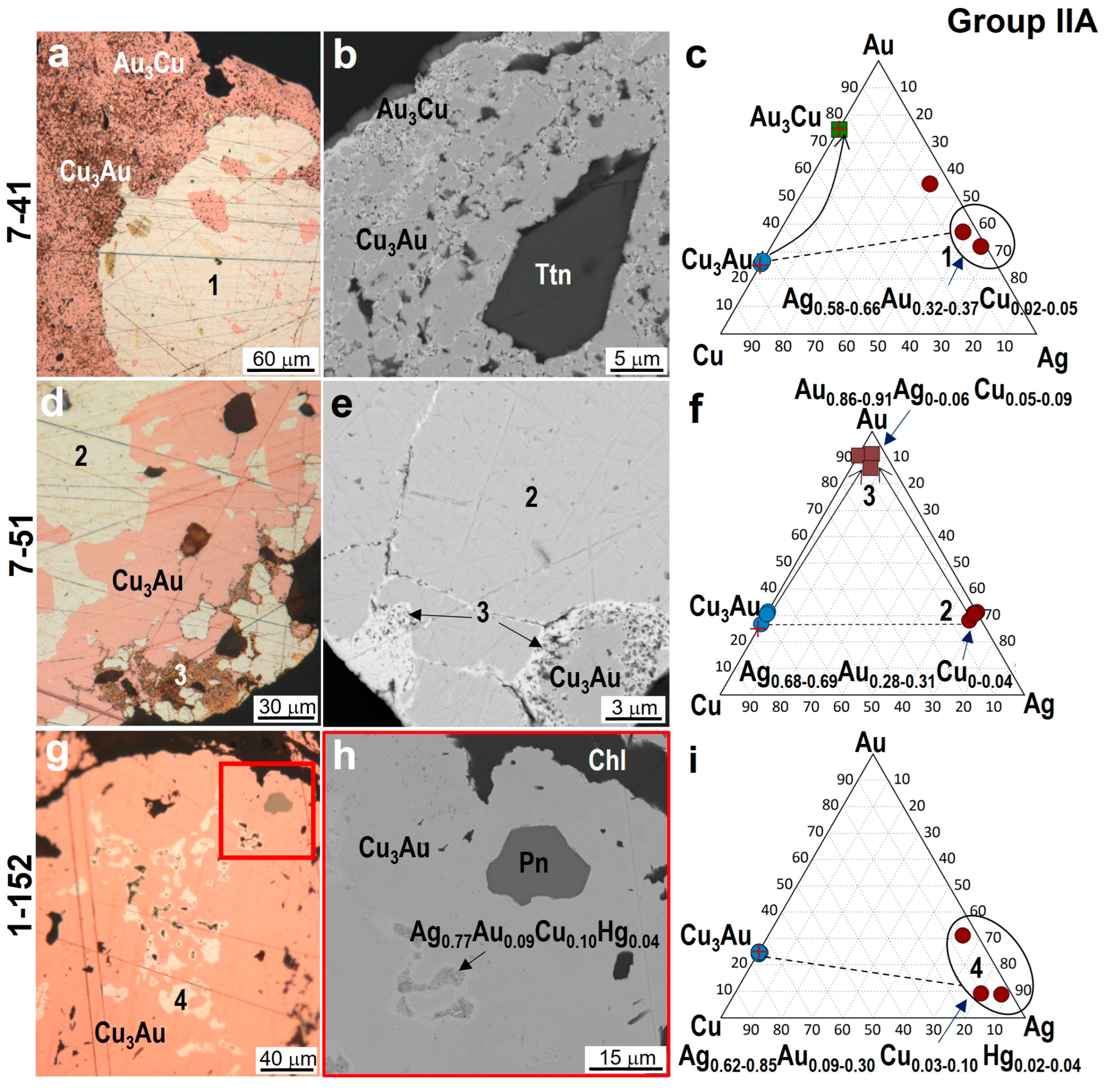
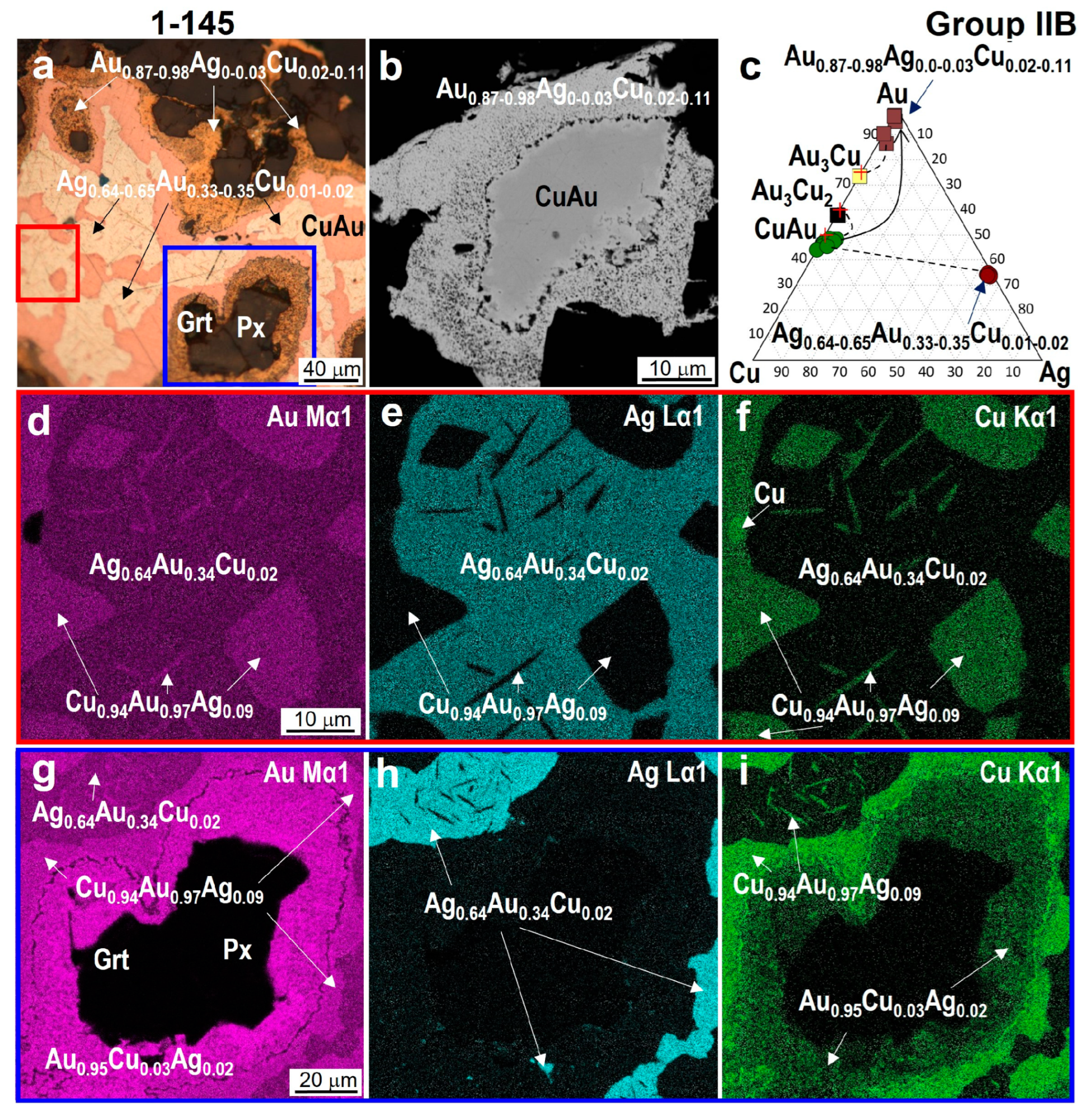
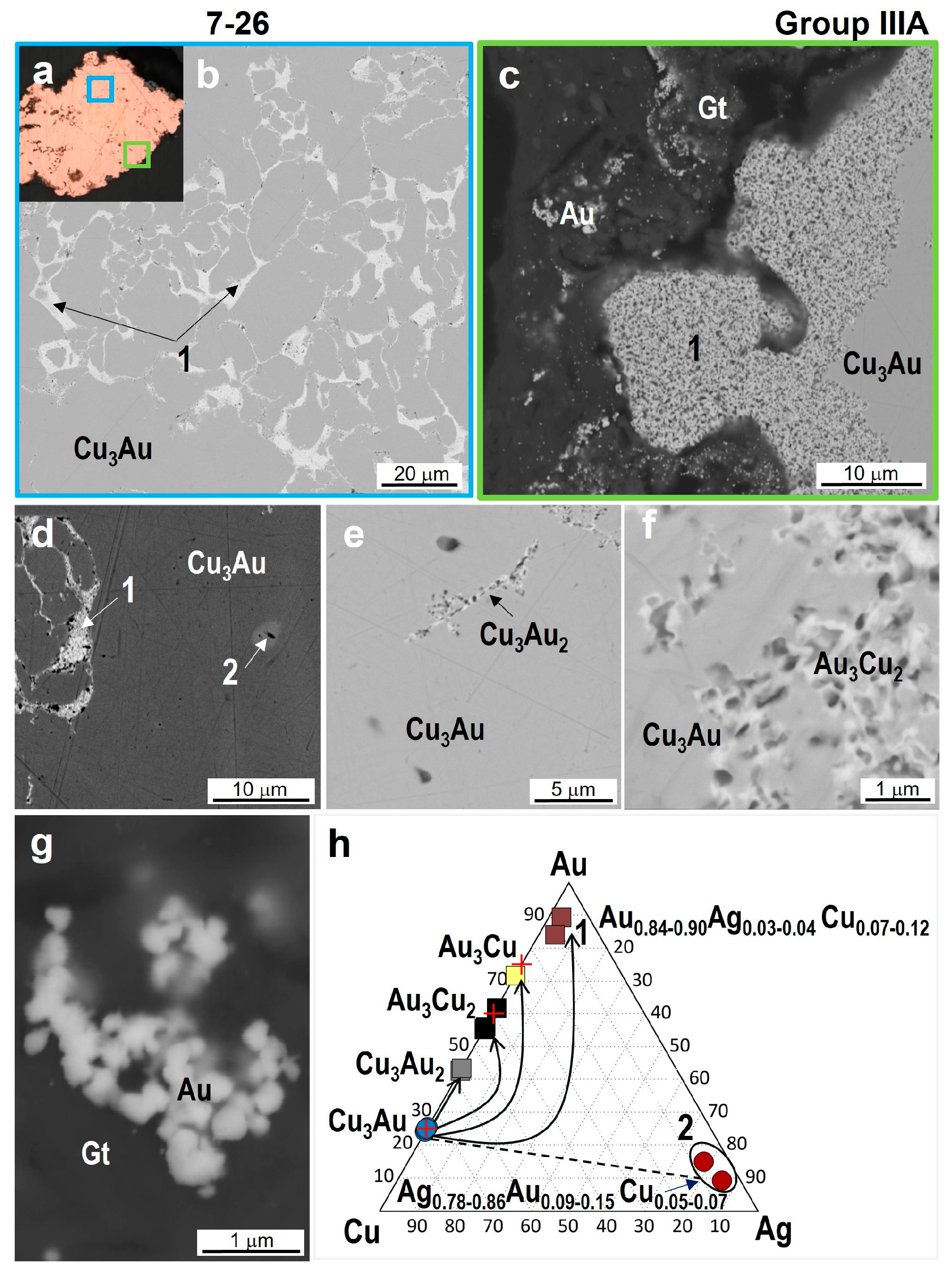
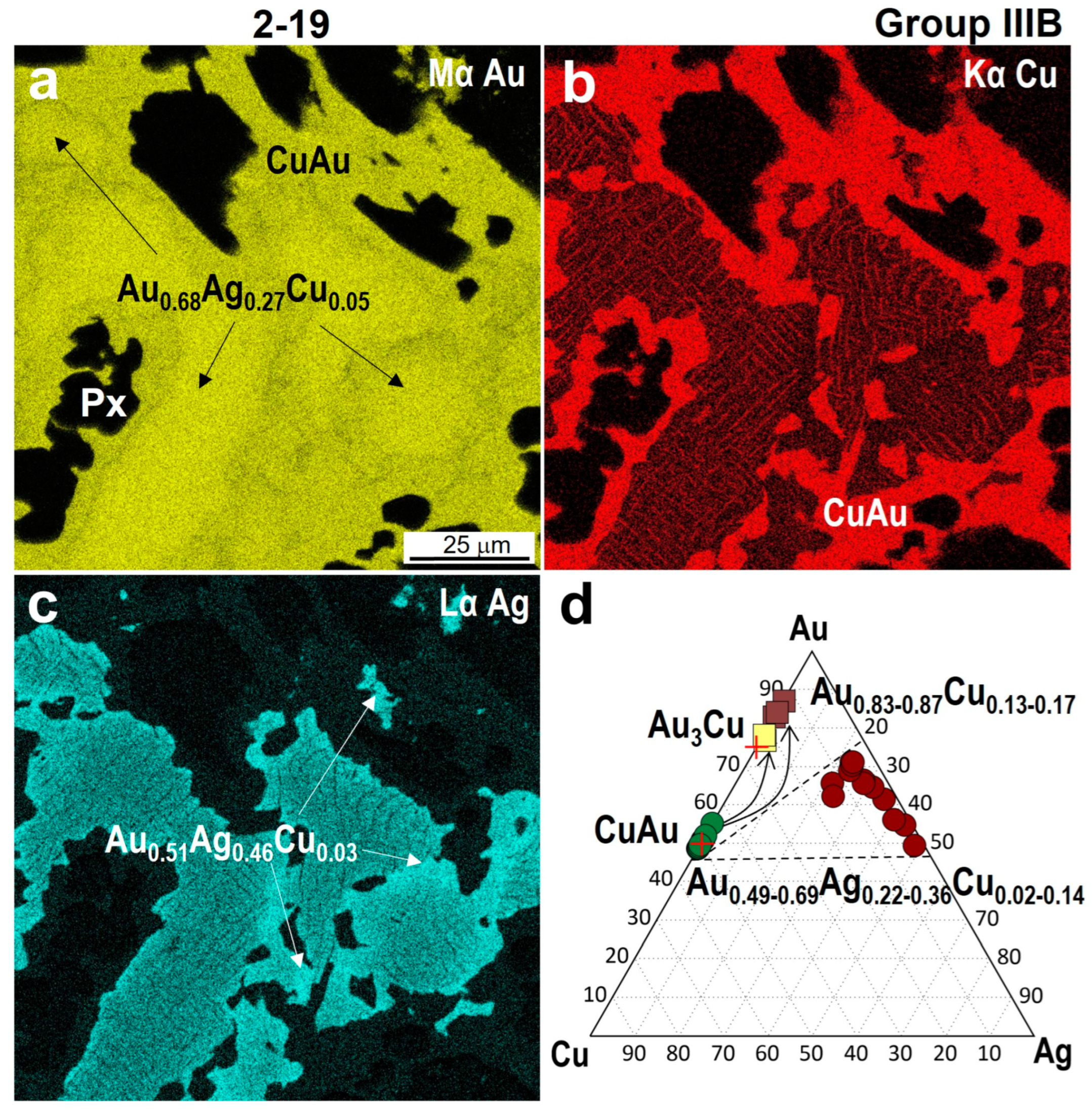
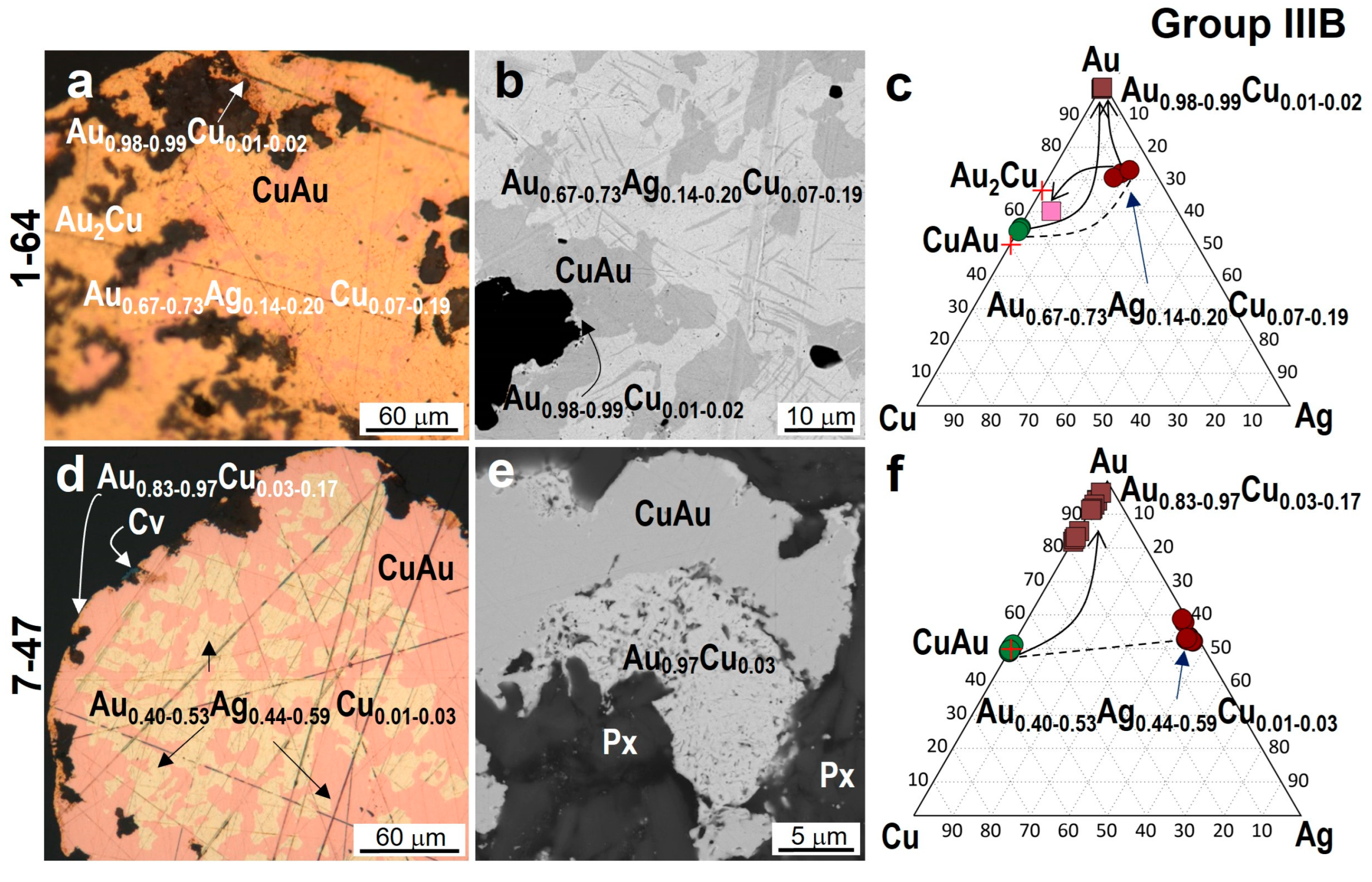
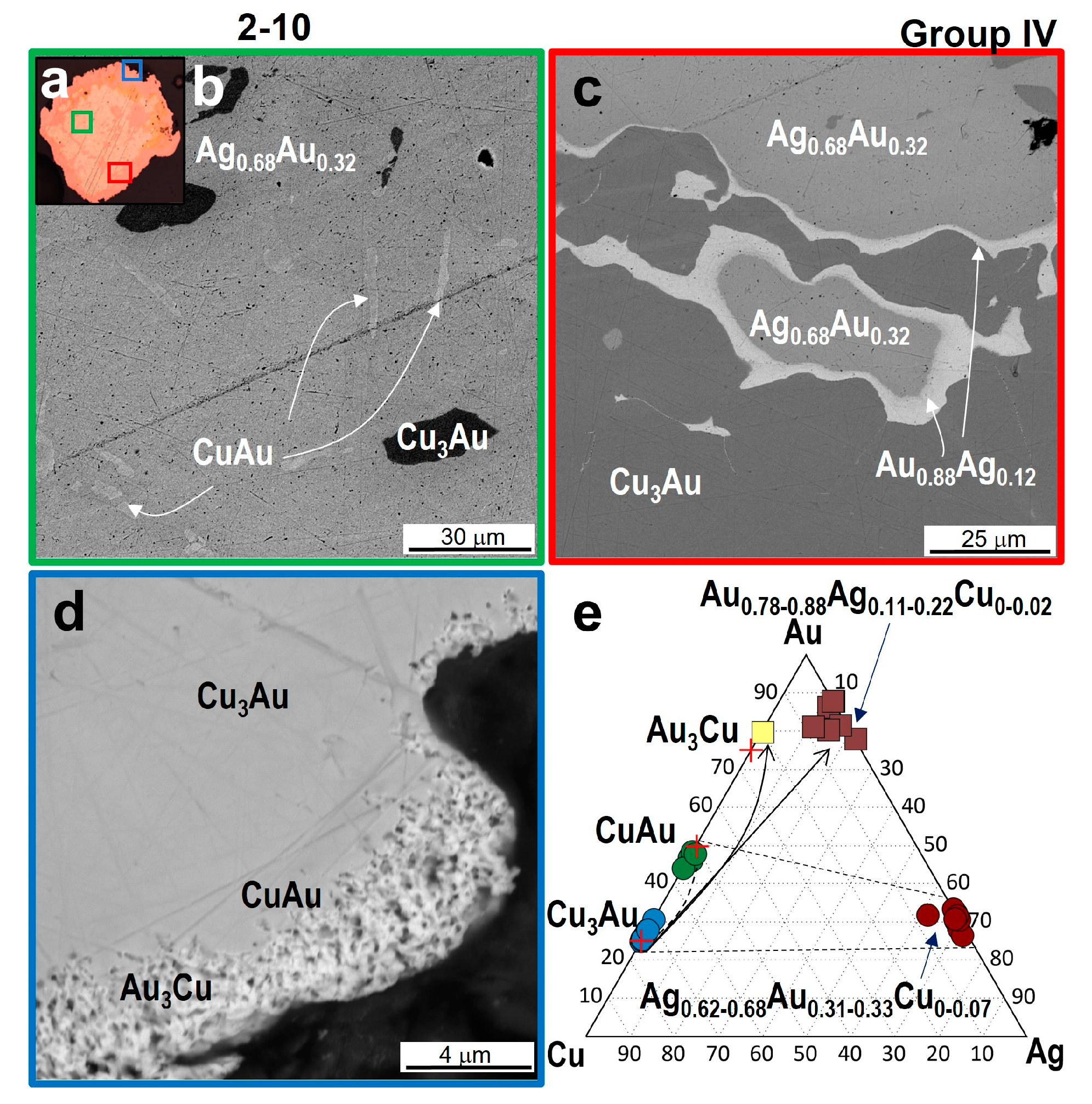
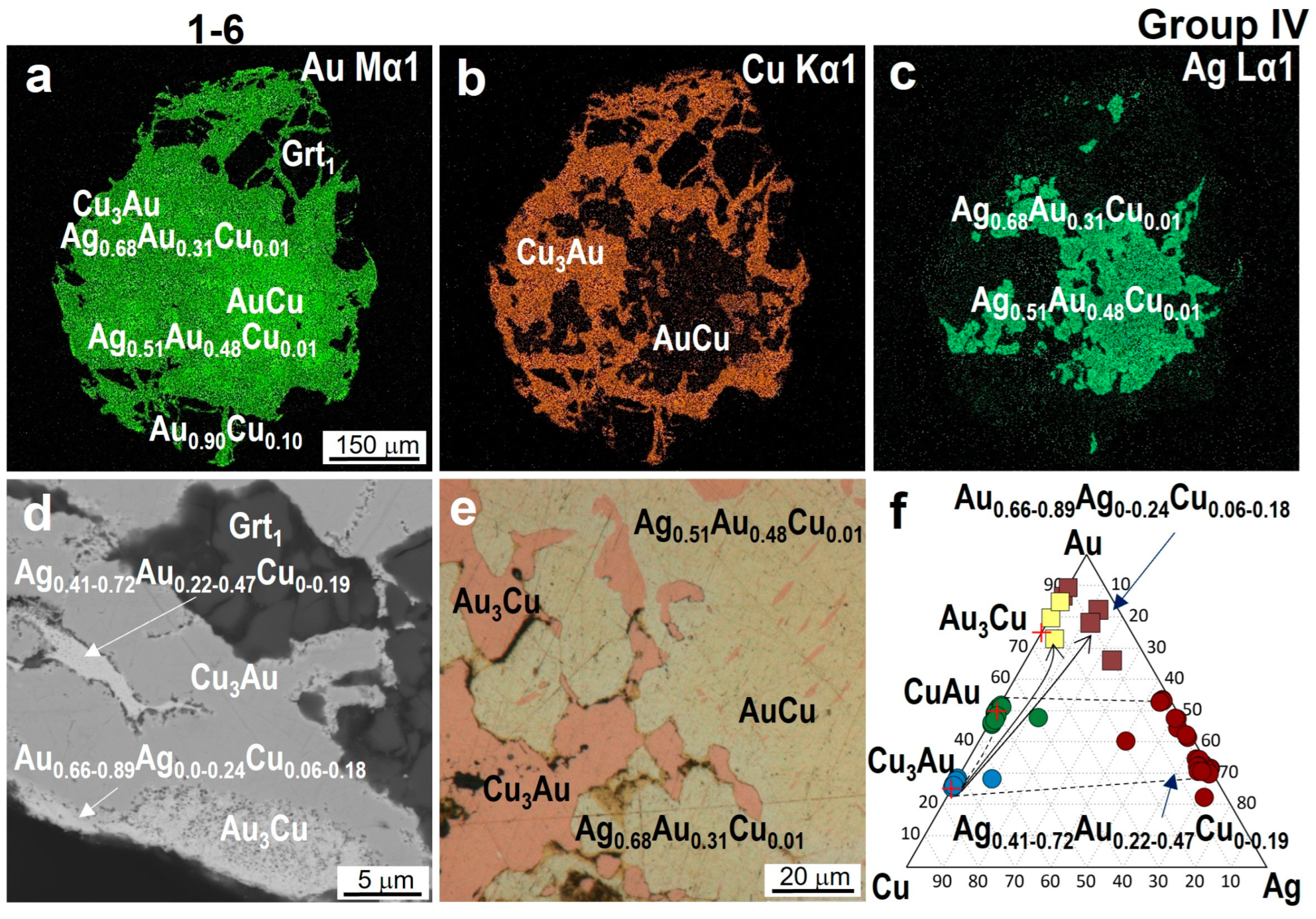
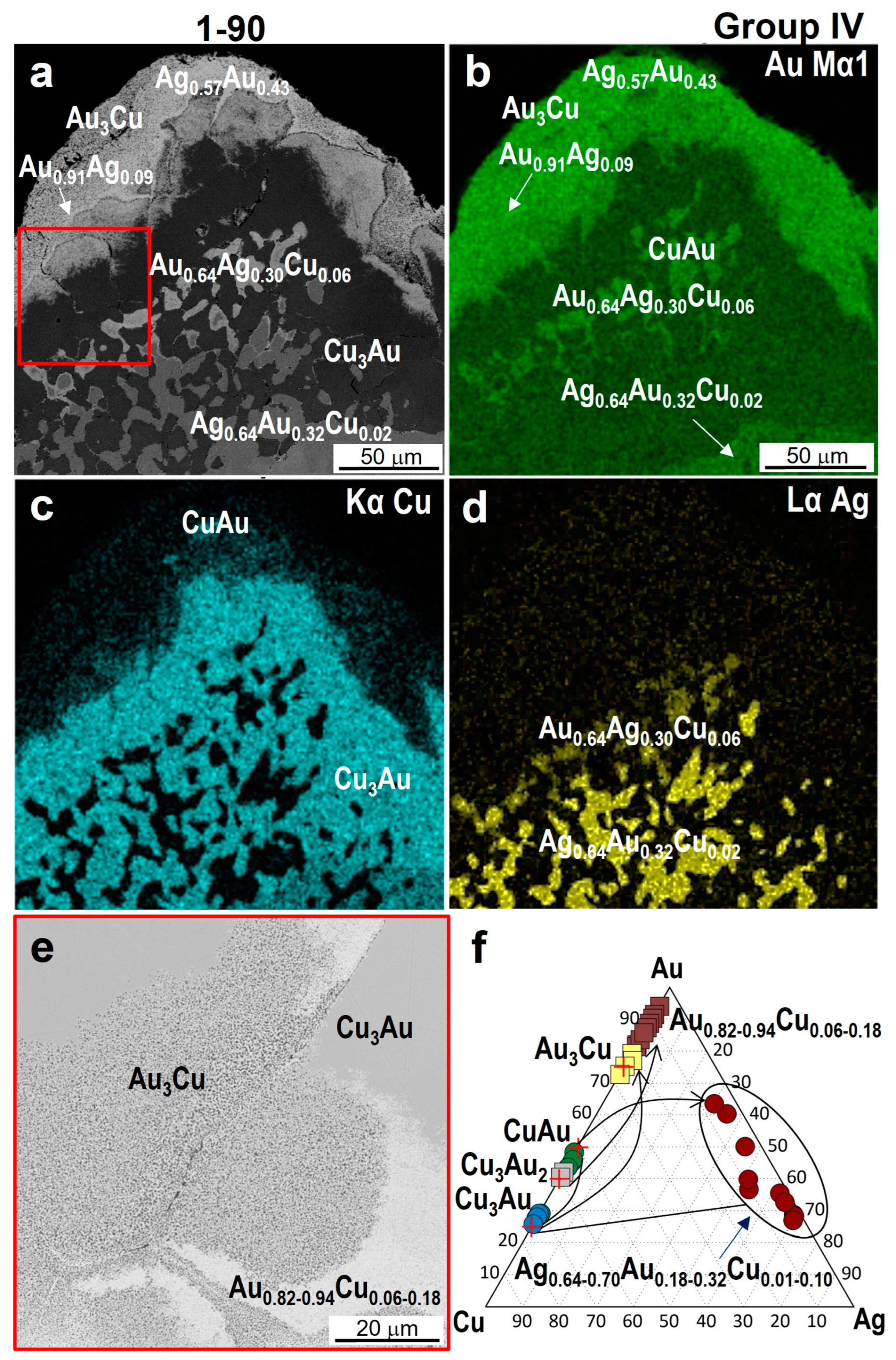
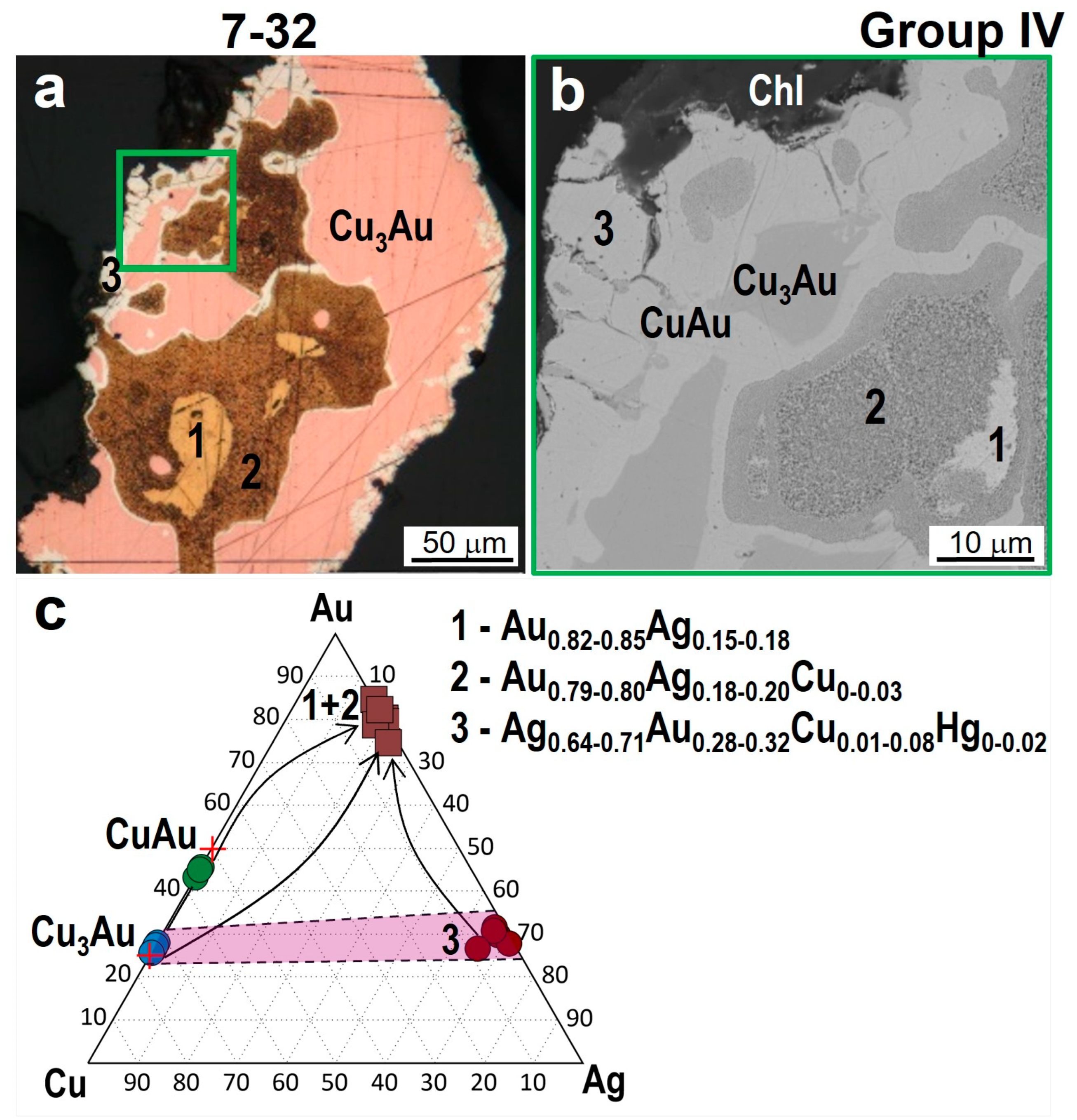

4.3. Minerals in Association with Au-Ag-Cu Phases
4.4. XRD Results
5. Discussion
5.1. The Relationships of Au-Ag-Cu Phases
5.2. Genesis of Cuprian Gold
5.3. Source of Au-Cu-Ag Mineralization
6. Conclusions
- The study of the decomposition structures of solid solutions in grains from the Olkhovaya-1 river revealed that Au-Cu-Ag solid solutions decompose to form two two-phase mineral parageneses: (1) auricupride (Cu3Au) + Ag0.86–0.67Au0.09–0.33Cu<0.05 (160‰–480‰) and (2) tetra-auricupride (CuAu) + Au0.73–0.62Ag0.2–0.38Cu<0.07 (520‰–850‰).
- One possible mechanism for the formation of various Cu-Au-Ag alloys is controlled by the fluid composition, while the other is the result of the decomposition products of the Au-Ag-Cu solid solution.
- Au-Cu and Au-Cu-Ag phases, formed after auricupride and tetra-auricupride and containing high concentrations of gold—Au3Cu2, Au2Cu, and Au3Cu up to high-fineness Cu,Ag-containing gold (930‰–980‰)—occur in supergene environments in processes related to the selective leaching of Ag and Cu from the rim zone with dissolution–precipitation. The Au3Cu phase and other gold-rich phases appear to be isostructural with gold.
- The presence of thin rims with high-fineness gold in the grains suggests that the distance of their transfer cannot be significant. Mineral inclusions—silicates (pyroxene, garnet, chlorite, epidote, and titanite)—in the Au, Cu, and Ag particles indicate a genetic connection with ultramafic rocks. The probable source of Au-Ag-Cu minerals of the Olkhovaya-1 river placers is located in the upper reaches of water courses that erode the ultrabasic massif of Mounts Soldatskaya and Golaya (Kamchatka Cape Peninsula).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phase, n | wt.%, Min–Max/Mean | NAu, ‰ | Formula | Phases in Intergrowth | ||
|---|---|---|---|---|---|---|
| Cu | Au | Ag | ||||
| CuAu, 12 | 19.00–26.00 24.15 | 63.71–76.98 73.93 | 0.0 | 745–770 754 | Cu0.96–1.03Au1.04–0.97 | Au0.49–0.69Ag0.36–0.22Cu0.14–0.02, Au0.83–0.87Cu0.17–0.13, Au3Cu |
| Au-Cu, 3 | 4.07–6.15 5.24 | 85.68–92.42 89.48 | 0.0 | 938–955 945 | Au0.83–0.87Cu0.17–0.13 | CuAu |
| Au-Ag-Cu, 16 | 0.73–9.87 2.72 | 64.75–82.82 77.90 | 12.47–34.75 19.39 | 644–830 779 | Au0.49–0.71Ag0.48–0.22Cu0.14–0.02 | CuAu |
| Au3Cu, 2 | 8.07 | 86.41–89.44 87.93 | 0.0–0.57 0.29 | 909–917 913 | Au3.07–3.13Cu0.89–0.87Ag0.04–0.0 | CuAu |
| Phase, n | wt.%, (Min–Max)/Mean | NAu, ‰ | Formula | Phase in Intergrowth | ||
|---|---|---|---|---|---|---|
| Cu | Au | Ag | ||||
| CuAu, 4 | 23.78–25.85 24.55 | 73.83–74.85 74.50 | 0.0–0.51 0.13 | 737–759 751 | Cu0.99–1.03Au1.01–0.95Ag<0.02 | Au0.81–0.90Ag<0.04Cu0.19–0.08, Au0.51–0.55Ag0.43–0.38Cu0.11–0.02 |
| Cu3Au, 14 | 41.82–47.23 44.96 | 50.49–55.04 52.74 | 0.0 | 523–568 540 | Cu2.81–2.95Au1.19–1.05 | Au2Cu, Cu3Au2, Au3Cu, Au0.81–0.90Ag<0.04Cu0.19–0.08 |
| Au-Ag-Cu, 5 | 1.34–6.83 4.28 | 88.85–96.7 91.22 | 0.87–7.11 2.58 | 913–964 935 | Au0.81–0.90Ag0.06–0.12Cu0.19–0.04 | Cu3Au, CuAu |
| Au-Ag-Cu, 4 | 0.64–4.78 1.79 | 64.90–70.19 67.19 | 27.02–32.57 29.83 | 662–694 680 | Au0.51–0.55Ag0.43–0.38Cu0.11–0.02 | CuAu |
| Au3Cu, 2 | 9.00–9.67 9.34 | 82.11–90.68 86.22 | 0.0–0.78 0.39 | 887–906 900 | Au2.89–3.03Cu1.06–0.97Ag<0.05 | Cu3Au |
| Cu3Au2, 3 | 32.10–36.03 33.71 | 61.50–64.48 62.92 | 0.0 | 635–668 651 | Cu3.01–3.20Au1.97–1.80 | Cu3Au |
| Au2Cu, 1 | 14.54 | 83.00 | 2.04 | 834 | Cu1.03Au1.89Ag0.08 | Cu3Au |
| N Block-Grain | Cu3Au | Au3Cu | Cu3Au2, Au3Cu2, Au4Cu, Au9Cu | Au-Ag | Au-Ag-Cu |
|---|---|---|---|---|---|
| 1-73 | Cu2.95–2.98Au1.05–1.02 | Au2.82Cu1.18 | Au0.82–0.90Cu0.18–0.10 | - | - |
| 7-50 | Cu2.98–3.00Au1.02–1.00 | - | Cu2.86Au2.14 Au2.99Cu2.01 | - | - |
| 7-35 | Cu2.99–3.01Au1.01–0.99 | Au3.14–3.17Cu1.86–1.83 | Au0.87Cu0.13 | Au | Ag0.72–0.85Au0.23–0.15Cu<0.11 |
| 7-31 | Cu3.01–3.02Au0.99–0.98 | Au2.88–2.96Cu1.12–1.04 | - | Au0.96Ag0.04 | Au0.95–0.96Ag0.03Cu0.01–0.02 |
| 7-33 | Cu3.00Au1.00 | - | - | - | Au0.89Ag0.07Cu0.03 |
| N Block-Grain | Cu3Au/ CuAu | Au3Cu | Cu2Au, Au3Cu2, Au2Cu, Au4Cu | Au-Ag(-Hg) | Au-Ag-Cu(-Hg) |
|---|---|---|---|---|---|
| Group IIA (main phases Cu3Au + Ag-Au alloys) | |||||
| 7-41 | Cu2.93–2.98Au1.07–1.02 | Au3.00Cu1.00 | - | - | Ag0.58–0.66Au0.37–0.32Cu0.02–0.05 |
| 7-51 | Cu2.74–2.93Au1.26–1.07 | - | - | Ag0.69Au0.31 | Ag0.68–0.69Au0.28–0.31Cu0.01–0.04 Au0.86–0.91Ag0.06–0.0Cu0.05–0.09 |
| 1-15 | Cu2.90–2.95Au1.10–1.05 | - | Cu1.89–1.99Au1.11–1.01 | Ag0.73Au0.24Hg0.03 | - |
| 1-146 | Cu2.96Au1.04 | - | - | - | Au0.70Ag0.27Cu0.03 |
| 1-27 | Cu2.88–2.96Au1.12–1.04 | - | Au0.80Cu0.20 | Ag0.74Au0.24Hg0.02 | |
| 7-5 | Cu2.98Au1.02 | - | - | - | Ag0.67–0.66Au0.32Cu0.01–0.02 Au0.82–0.89Ag0.17–0.09Cu0.02 |
| 7-3 | Cu3.00–3.04Au1.00–0.96 | Au2.95Cu1.05 | Au1.97Cu1.03 | - | Ag0.65–0.70Au0.33–0.27Cu0.03–0.04 Au0.79–0.81Ag0.13–0.10Cu0.08–0.09 |
| 1-152 | Cu2.99–3.02Au1.01–0.98 | - | - | - | Ag0.62–0.85Au0.3–0.09Cu0.03–0.10Hg0.02–0.04 |
| Group IIB (main phases CuAu + Ag-Au alloys) | |||||
| 1-145 | /Cu0.94–1.12Au1.06–0.88 | Au2.94Cu1.06 | Au2.91Cu2.09 | - | Ag0.64–0.65Au0.35–0.33Cu0.01–0.02 Au0.87–0.98Cu0.11–0.02Ag<0.03 |
| N Block-Grain | Cu3Au/CuAu | Au3Cu | Cu2Au, Au3Cu2, Au4Cu, … | Au-Ag | Au-Ag-Cu(-Hg) |
|---|---|---|---|---|---|
| Group IIIA (main phases Cu3Au + Au-Ag-Cu alloys) | |||||
| 7-7 | Cu2.83–2.85Au1.17–1.15 | - | - | - | Ag0.68–0.70Au0.29–0.27Cu<0.01Hg<0.01 Au0.63Ag0.32Cu0.05 |
| 1-183 | Cu2.99–3.00Au1.01–1.00 | - | - | Au0.82Ag0.18 | Ag0.54–0.62Au0.44–0.38Cu0.01–0.04 Au0.62–0.69Ag0.35–0.29Cu0.01–0.09 |
| 1-23 | Cu3.00–3.06Au1.00–0.94 | - | Cu1.93Au1.07 | - | Au0.73–0.76Ag0.18–0.05Cu0.08–0.22 |
| 1-184 | Cu2.79–3.01Au1.21–0.99 | - | - | - | Au0.73–0.82Ag0.16–0.05Cu0.12–0.13 |
| 7-26 | Cu2.98–3.04Au1.02–0.96 | Au2.86–3.18Cu1.14–0.82 | Au2.88–3.11Cu2.18–1.89 | Au | Au0.84–0.90Ag0.04–0.03Cu0.07–0.12 |
| Group IIIB (main phases CuAu + Au-Ag alloys) | |||||
| 7-47 | /Cu0.99–1.02Au1.01–0.98 | Au3.28–3.31Cu0.72–0.69 | Au0.83–0.97Cu0.17–0.03 | - | Au0.40–0.53Ag0.59–0.44Cu0.01–0.03 |
| 1-64 | /Cu0.90–0.92Au1.10–1.08 | Au1.81Cu1.02Ag0.16 Au0.98–0.99Cu0.02–0.01 | - | Au0.67–0.73Ag0.20–0.14Cu0.07–0.19 | |
| 2-19 | /Cu0.90–1.03Au1.10–0.97 | Au3.07–3.13Cu0.93–0.87 | Au0.83–0.87Cu0.17–0.13 | - | Au0.49–0.69Ag0.36–0.22Cu0.02–0.14 |
| N Block-Grain | Cu3Au | CuAu | Au3Cu | Cu3Au2, Au3Cu2, Au2Cu, Au4Cu, … | x in AuxAg1-x | Au-Ag-Cu(-Hg) |
|---|---|---|---|---|---|---|
| 1-7 | Cu2.81–2.95Au1.19–1.05 | Cu0.99–1.05Au1.01–0.95 | Au3.02Cu0.98 | Au0.81Cu0.19 Cu3.12–3.48Au1.88–1.52 | - | Au0.82–0.90Ag0.12–0.01Cu0.04–0.16 |
| 1-6 | Cu2.86–3.01Au1.14–0.99 | Cu0.94–1.04Au1.06–0.96 | Au3.19Cu0.81 | - | 0.82–0.90 | Au0.66–0.89Ag<0.24Cu0.06–0.18 Ag0.51Au0.48Cu0.01 Ag0.72–0.41Au0.22–0.47Cu0<0.19 |
| 1-10 | Cu2.71–2.95Au1.29–1.05 | Cu1.08–1.03Au0.92–0.97 | Au2.89–3.09Cu1.11–0.91 | Au0.96–0.98Cu0.04–0.02 Au2.94Cu2.06 | 0.92–0.93 | Ag0.60–0.68Au0.32–0.30Cu0.01–0.10 Au0.90–0.94Ag0.04–0.02Cu0.02–0.08 |
| 1-90 | Cu2.82–2.97Au1.18–1.03 | Cu0.90–0.97Au1.03–1.10 | Au2.96–3.18Cu1.04–0.82 | Au0.82–0.94Cu0.18–0.06 | 0.91 | Au0.64Ag0.30Cu0.06 Ag0.64–0.70Au0.32–0.18Cu0.01–0.10 |
| 7-8 | Cu2.89–2.94Au1.11–1.06 | Cu0.97–1.10Au1.03–0.90 | - | Au0.85Cu0.15 | - | Ag0.45–0.67Au0.50–0.28Cu0.01–0.04Hg<0.03 |
| 7-36 | Cu2.89–2.94Au1.11–1.06 | Cu1.00Au0.99Ag0.01 | - | - | - | Au0.71Ag0.26Cu0.03 |
| 7-32 | Cu2.88–2.98Au1.12–1.02 | Cu1.09–1.14Au0.91–0.86 | - | - | 0.82–0.85 | Au0.79–0.80Ag0.18–0.20Cu0–0.03 Ag0.64–0.71Au0.28–0.32Cu0.01–0.08Hg<0.02 |
| 2-10 | Cu2.78–3.01Au1.11–0.99 | Cu1.06Au0.94–0.72Ag<0.22 | Au3.04–3.20Cu0.96–0.80 | - | 0.31–0.33 0.78–0.88 | Ag0.62–0.72Au0.32–0.26Cu0.01–0.07 Au0.78–0.86Ag0.17–0.11Cu0.02–0.10 |
| 7-34 | Cu2.81–2.91Au1.19–1.09 | Cu1.01–1.09Au0.99–0.91 | Au3.01Cu0.91Ag0.07 | Au0.87Cu0.13 | - | Ag0.57–0.59Au0.39–0.38Cu0.03–0.04 |
| 1-182 | Cu2.99–3.01Au1.01–0.99 | Cu0.89–0.97Au1.11–1.03 | - | Cu2.35–3.18Au1.95–1.62Ag<0.18 | - | Ag0.70–0.81Au0.26–0.14Cu0.03–0.07 |
References
- Petrovskaya, N.V. Native Gold; Nauka: Moscow, Russia, 1973; 348p. (In Russian) [Google Scholar]
- Boyle, R.W. The Geochemistry of Gold and Its Deposits; Bulletin/Geological Survey 280; Geological Survey of Canada: Ottawa, ON, Canada, 1979.
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Spiridonov, E.; Yanakieva, D. Modern mineralogy of gold: Overview and new data. Archéosciences 2009, 33, 67–73. [Google Scholar] [CrossRef]
- Razin, L.V.; Begizov, V.D. Gold-silver mineralization of the Talnakh and Oktyabrsky deposits of the Norilsk type. Geol. Ore Dep. 1973, 6, 32–42. (In Russian) [Google Scholar]
- Murzin, V.V.; Malyugin, A.A. New data on the unstability of natural solid solutions of the system Ag–Au–Cu at temperatures below 350 °C. Dokl. Akad. Nauk SSSR 1983, 269, 723–724. [Google Scholar]
- Murzin, V.V.; Malyugin, A.A. Typomorphism of Gold in the Hypergenesis Zone (on the Example of the Urals); UNC AN USSR: Sverdlovsk, Russia, 1987; 96p. (In Russian) [Google Scholar]
- Knipe, S.W.; Fleet, M.E. Gold-copper alloy minerals from the Kerr Mine, Ontario. Can. Mineral. 1997, 35, 573–586. [Google Scholar]
- Knight, J.; Leitch, C.H.B. Phase relations in the system Au–Cu–Ag at low temperatures, based on natural assemblages. Can. Mineral. 2001, 39, 889–905. [Google Scholar] [CrossRef]
- Kazachenko, V.T.; Miroshnichenko, N.V.; Perevoznikova, E.V.; Karabtsov, A.A. Mineral forms of noble metals in metal-bearing deposits of the Triassic-Jurassic carbonaceous sequence of Sikhote-Alin. Dokl. RAS 2008, 421, 383–386. [Google Scholar]
- Onishchenko, S.A.; Kuznetsov, S.K.; Tropnikov, E.M. Epigenetic alteration of cupreous gold in the Au-Ag-Cu-Pd exsolution texture. Dokl. Earth Sci. 2020, 492, 418–421. [Google Scholar] [CrossRef]
- Onishchenko, S.A.; Kuznetsov, S.K. Exsolution in the Au–Ag–Cu system in a gold-rich area. Geochem. Int. 2022, 60, 657–671. [Google Scholar] [CrossRef]
- Pandey, S.; Koch, R.J.; Li, G.; Misture, S.T.; Wang, H.; Phillpot, S.R. Thermodynamics and kinetics of ordered and disordered Cu/Au alloys from first principles calculations. J. Alloys Compd. 2019, 809, 151615. [Google Scholar] [CrossRef]
- Bonev, I.K.; Kerestedjian, T.; Atanassova, R.; Andrew, C.J. Morphogenesis and composition of native gold in the Chelopech volcanic-hosted Au-Cu epithermal deposit, Srednogorie zone, Bulgaria. Miner. Depos. 2002, 37, 614–629. [Google Scholar] [CrossRef]
- Arif, J.; Baker, T. Gold paragenesis and chemistry at Batu Hijau, Indonesia: Implications for gold-rich porphyry copper deposits. Miner. Depos. 2004, 39, 523–535. [Google Scholar] [CrossRef]
- Moles, N.R.; Chapman, R.J.; Warner, R. The significance of copper concentrations in natural gold alloy for reconnaissance exploration and understanding gold-depositing hydrothermal systems. Geochem. Explor. Environ. Anal. 2013, 13, 115–130. [Google Scholar] [CrossRef]
- Chudnenko, K.V.; Pal’yanova, G.A. Thermodynamic properties of solid solutions in the Ag–Au–Cu system. Russ. Geol. Geophys. 2014, 55, 349–360. [Google Scholar] [CrossRef]
- Chapman, R.J.; Moles, N.R.; Bluemel, B.; Walshaw, R.D. Detrital Gold as an Indicator Mineral. Geol. Soc. Spec. Publ. 2022, 516, 313–336. [Google Scholar] [CrossRef]
- Sluzhenikin, S.F.; Mokhov, A.V. Gold and silver in PGE-Cu-Ni and PGE ores of the Noril’sk deposits, Russia. Miner. Depos. 2015, 50, 465–492. [Google Scholar] [CrossRef]
- Palyanova, G.A. Gold and silver minerals in sulfide ore. Geol. Ore Dep. 2020, 62, 383–406. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Pletnev, P.A. The Zolotaya Gora Deposit of Copper-Containing Gold (Gold–Rodingite Mineralization); Nauchnyi Mir: Moscow, Russia, 2002; 220p. (In Russian) [Google Scholar]
- Murzin, V.V.; Chudnenko, K.V.; Palyanova, G.A.; Varlamov, D.A.; Naumov, E.A.; Pirajno, F. Physicochemical model for the genesis of Cu-Ag-Au-Hg solid solutions and intermetallics in the rodingites of the Zolotaya Gora gold deposit (Urals, Russia). Ore Geol. Rev. 2018, 93, 81–97. [Google Scholar] [CrossRef]
- Palyanova, G.; Murzin, V.; Kuznetsov, S.; Karmanov, N. Native gold of the Au-Pd-REE Chudnoye deposit (Subpolar Ural, Russia): Composition, mineral associations, genesis. Minerals 2021, 11, 451. [Google Scholar] [CrossRef]
- Chudnenko, K.V.; Palyanova, G.A. Thermodynamic modeling of native formation Cu-Ag-Au-Hg solid solutions. Appl. Geochem. 2016, 66, 88–100. [Google Scholar] [CrossRef]
- Pal’yanova, G.A.; Murzin, V.V.; Zhuravkova, T.V.; Varlamov, D.A. Au–Cu–Ag mineralization in rodingites and nephritoids of the Agardag ultramafic massif (southern Tuva, Russia). Russ. Geol. Geophys. 2018, 59, 237–255. [Google Scholar] [CrossRef]
- Murzin, V.V.; Palyanova, G.A.; Varlamov, D.A.; Shanina, S.N. Gold-Bearing Rodingites of the Agardag Ultramafic Massif (South Tuva, Russia) and Problems of Their Genesis. Geol. Ore Dep. 2020, 62, 204–224. [Google Scholar] [CrossRef]
- Koneev, R.I. Gold Nanomineralogy of Epithermal Deposits of the Chatkala–Kurama Region, Uzbekistan; Del’ta: Sankt-Peterburg, Russia, 2006; 220p. [Google Scholar]
- Nesterenko, G.V.; Kuznetsova, A.I.; Lavrentyev, Y.G. The nature of native gold of the Sinyukha deposit. Sov. Geol. Geophys. 1980, 10, 129–133. (In Russian) [Google Scholar]
- Chekryzhov, I.Y.; Safronov, P.P.; Panichev, A.M.; Golokhvast, K.S.; Vedyagin, A.A.; Bukhtiyarov, V.I. Geological and biological aspects of a find of natural alloy (Au-Cu-Ag) nanoparticles in Cenozoic zeolitized tuff of the Vanchinskaya basin (Primor’e area). Dokl. Earth Sci. 2011, 436, 55–57. [Google Scholar] [CrossRef]
- Zhmodik, S.M.; Mironov, A.G.; Zhmodik, A.S. Gold-Concentrating Systems of Ophiolite Belts (Using the Example of the Sayan-Baikal-Muya Belt); Publishing House “Geo”: Novosibirsk, Russia, 2008; 304p. [Google Scholar]
- Oen, I.S.; Kieft, C. Nickeline with pyrrhotite and cubanite exsolutions, Ni-Co rich loellingite and an Au-Cu alloy in Cr-Ni ores from Beni-Bousera, Morocco. Neues Jahrb. Miner. Monatsh. 1974, 115, 1–8. [Google Scholar]
- Nekrasov, I.Y.; Ivanov, V.V.; Lennikov, A.M.; Sapin, V.I.; Safronov, P.P.; Oktyabr’skii, R.A. Rare natural polycomponent alloys based on gold and copper from a platinum placer in the Konder alkaline-ultrabasic massif, southeastern Aldan shield, Russia. Geol. Ore Dep. 2001, 43, 406–417. [Google Scholar]
- Liu, H.; Beaudoin, G. Geochemical signatures in native gold derived from Au-bearing ore deposits. Ore Geol. Rev. 2021, 132, 104066. [Google Scholar] [CrossRef]
- Townley, B.K.; Hérail, G.; Maksaev, V.; Palacios, C.; De Parseval, P.; Sepulveda, F.; Orellana, R.; Rivas, P.; Ulloa, C. Gold grain morphology and composition as an exploration tool: Application to gold exploration in covered areas. Geochem. Explor. Environ. Anal. 2003, 3, 29–38. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Sandimirova, E.I.; Chubarov, V.M.; Anikin, L.P.; Ibragimova, E.K.; Antonov, A.V. Typomorphic features of gold in placer occurrences of the Olkhovaya River 1- I (Kamchatsky Cape, Eastern Kamchatka). Vestnik KRAUNTS. Geosci. 2013, 1, 34–43. [Google Scholar]
- Tolstykh, N.; Sidorov, E.; Kozlov, A. Platinum group minerals from the Olkhovaya-1 placer related to the Karaginsky ophiolite complex, the Kamchatskiy Mys peninsula, Russia. Can. Mineral. 2009, 47, 793–811. [Google Scholar] [CrossRef]
- Craig, J.R. Ore-mineral textures and the tales they tell. Can. Mineral. 2001, 39, 937–956. [Google Scholar] [CrossRef]
- Chapman, R.J.; Leake, R.C.; Bond, D.P.G.; Stedra, V.; Fairgrieve, B. Chemical and mineralogical signatures of gold formed in oxidizing chloride hydrothermal systems and their significance within populations of placer gold grains collected during reconnaissance. Econ. Geol. 2009, 104, 563–585. [Google Scholar] [CrossRef]
- Boyarinova, M.E.; Veshnyakov, N.A.; Korkin, A.G.; Savelyev, D.P. State Geological Map of the Russian Federation. Scale 1:200,000; East Kamchatka Series. Sheets O-58-XXVI, XXXI, XXXII. Explanatory Note; Cartographic Factory VSEGEI: Sankt-Peterburg, Russia, 2007; 226p. (In Russian) [Google Scholar]
- Novakov, R.M.; Ivanov, V.V.; Trukhin, Y.P.; Panova, E.G. Copper-nickel and noble metals mineralization in olivine websterite from the Kamchatsky Mys peninsular (Kamchatka). Bull. St. Petersburg State Univ. 2015, 7, 83–97, (In Russian, abstract in English). [Google Scholar]
- Savelyev, D.P.; Philisofova, T.M.V.M. Microinclusions of platinum group elements minerals and gold in rocks from ophiolite complex in the Kamchatsky Mys Peninsula. Vestnik KRAUNTS Geosci. 2017, 34, 5–13. [Google Scholar]
- Rapson, W.S. The metallurgy of the coloured carat gold alloys. Gold Bull. 1990, 23, 125–133. [Google Scholar] [CrossRef]
- Shuster, J.; Reith, F. Reflecting on gold geomicrobiology research: Thoughts and considerations for future endeavors. Minerals 2018, 8, 401. [Google Scholar] [CrossRef]
- Melchiorre, E.B.; Orwin, P.M.; Reith, F.; Rea, M.A.D.; Yahn, J.; Allison, R. Biological and geochemical development of placer gold deposits at Rich Hill, Arizona, USA. Minerals 2018, 8, 56. [Google Scholar] [CrossRef]
- Kubiak, R.; Janczak, J. X-ray study of ordered phase formation in Au31.6 Cu68.4, Au50Cu50 and Au75Cu25. J. Alloys Compd. 1991, 176, 133–140. [Google Scholar] [CrossRef]
- Bayliss, P. Revised unit-cell dimensions, space group, and chemical formula of some metallic minerals. Can. Mineral. 1990, 28, 751–755. [Google Scholar]
- Spiridonov, E.M.; Chvileva, T.N. Bogdanovite, Au5(Cu,Fe)3(Te,Pb)2, a new mineral of the group of inter-metallic compounds of gold. Vestn. Mosk. Univ. Geol. 1979, 1, 44–52. [Google Scholar]
- Lu, H.S.; Liang, C.K. The superlattice formation and lattice spacing changes in copper-gold alloys. Chin. J. Phys. 1966, 22, 505–526. (In English) [Google Scholar]
- Chapman, R.J.; Banks, D.A.; Styles, M.T.; Walshaw, R.D.; Piazolo, S.; Morgan, D.J.; Grimshaw, M.R.; Spence-Jones, C.P.; Matthews, T.J.; Borovinskaya, O. Chemical and physical heterogeneity within native gold: Implications for the design of gold particle studies. Miner. Depos. 2021, 56, 1563–1588. [Google Scholar] [CrossRef]
- Chapman, R.; Torvela, T.; Savastano, L. Insights into Regional Metallogeny from Detailed Compositional Studies of Alluvial Gold: An Example from the Loch Tay Area, Central Scotland. Minerals 2023, 13, 140. [Google Scholar] [CrossRef]
- Nikolaeva, L.A.; Nekrasova, A.N.; Milyaev, S.A.; Yablokova, S.V.; Gavrilov, A.M. Geochemical features of native gold from deposits of various types. Geol. Ore Depos. 2013, 55, 176–184. [Google Scholar] [CrossRef]
- Nikiforova, Z.S.; Kalinin, Y.A.; Makarov, V.A. Evolution of native gold in exogenous conditions. Russ. Geol. Geophys. 2020, 61, 1244–1259. [Google Scholar] [CrossRef]
- Nikiforova, Z. Internal Structures of Placer Gold as an Indicator of Endogenous and Exogenous Processes. Minerals 2023, 13, 68. [Google Scholar] [CrossRef]
- Savva, N.E.; Kravtsova, R.G.; Anisimova, G.S.; Palyanova, G.A. Typomorphism of Native Gold (Geological-Industrial Types of Gold Deposits in the North-East of Russia). Minerals 2022, 12, 561. [Google Scholar] [CrossRef]
- Onishchenko, S.A.; Kuznetsov, S.K. Native gold of the Chudnoe gold–palladium deposit (Subpolar Urals, Russia). Russ. Geol. Geophys. 2023, 64, 233–254. [Google Scholar] [CrossRef]
- Lalomov, A.; Grigorieva, A.; Kotov, A.; Ivanova, L. Typomorphic Features and Source of Native Gold from the Sykhoi Log Area Placer Deposits, Bodaibo Gold-Bearing District, Siberia, Russia. Minerals 2023, 13, 707. [Google Scholar] [CrossRef]
- Groen, J.C.; Craig, J.R.; Rimstidt, J.D. Gold-rich rim formation on electrum grains in placers. Can. Mineral. 1990, 28, 207–228. [Google Scholar]
- Stewart, J.; Kerr, G.; Prior, D.; Halfpenny, A.; Pearce, M.; Hough, R.; Craw, D. Low temperature recrystallisation of alluvial gold in paleoplacer deposits. Ore Geol. Rev. 2017, 88, 43–56. [Google Scholar] [CrossRef]
- Dunn, S.C.; von der Heyden, B.P.; Rozendaal, A.; Taljaard, R. Secondary gold mineralization in the Amani placer gold deposit, Tanzania. Ore Geol. Rev. 2019, 107, 87–107. [Google Scholar] [CrossRef]
- Okamoto, H.; Chakrabarti, D.J.; Laughlin, D.E.; Massalski, T.B. The Au−Cu (gold-copper) system. J. Phase Equilibria 1987, 8, 454–474. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Volkov, S.N. Au-Cu Phase Diagram. Russian J. Inorg. Chem. 2016, 61, 772–775. [Google Scholar] [CrossRef]
- Prince, A.; Velikanova, T.; Turchanin, M.A. Silver-Gold-Copper; Silver–Gold–Copper. Ternary Alloy Systems. Phase Diagrams, Crystallographic and Thermodynamic Data. Landolt Boernstein, Numerical Data and Functional Relationships in Science and Technology (New Series); Group IV: Physical Chemistry; Martiensen, W., Effenberg, G., Ilyenko, S., Eds.; Library of Congress Cataloging: Germany, Berlin, 2006; pp. 10–41. [Google Scholar]
- Marquez-Zavalia, M.F.; Southam, G.; Craig, J.R.; Galliski, M.A. Morphological and chemical study of placer gold from the San Luis Range, Argentina. Can. Mineral. 2004, 42, 169–182. [Google Scholar] [CrossRef]
- Alves, K.d.S.; Sánchez, S.B.; Barreiro, J.G.; Palomares, R.M.; Prieto, J.M.C. Morphological and compositional analysis of alluvial gold: The Fresnedoso gold placer (Spain). Ore Geol. Rev. 2020, 121, 103489. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Metals in Avachinsky peridotite xenoliths with implications for redox heterogeneity and metal enrichment in the Kamchatka mantle wedge. Lithos 2022, 412–413, 1066. [Google Scholar] [CrossRef]
- Yudovskaya, M.A.; Distler, V.V.; Chaplygin, I.V.; Mokhov, A.V.; Trubkin, N.V.; Gorbacheva, S.A. Gaseous transport and deposition of gold in magmatic fluid: Evidence from the active Kudryavy volcano, Kurile Islands. Mineral. Depos. 2006, 40, 828–848. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Kepezhinskas, N.P.; Berdnikov, N.V.; Krutikova, V.O. Native metals and intermetallic compounds in subduction-related ultramafic rocks from the Stanovoy mobile belt (Russian Far East): Implications for redox heterogeneity in subduction zones. Ore Geol. Rev. 2020, 127, 103800. [Google Scholar] [CrossRef]
- Berdnikov, N.; Kepezhinskas, P.; Krutikova, V.; Kozhemyako, N.; Konovalova, N. Cu-Ag-Au Microspherules in Igneous Rocks: Morphology, Composition, Diagnostic Criteria and Possible Origin. Minerals 2023, 13, 819. [Google Scholar] [CrossRef]
- Marshakov, I.K.; Vvedensky, A.V.; Kondrashin, V.Y.; Bokov, G.A. Anodic Dissolution and Selective Corrosion of Alloys; Voronezh State Unuversitet: Voronezh, Russia, 1988; 205p. [Google Scholar]




| Types of Deposits | Deposits | Alloy Compositions (wt.%) | References | |
|---|---|---|---|---|
| Ag | Cu (Other Metals) | |||
| Au-Ag epithermal (low-sulfidation) | Kochbulak (Uzbekistan) Kauldy (Uzbekistan) | <7.2 <15.1 | <0.29 (Fe < 0.1) <0.83 (Fe < 0.3) | [27] - “ - |
| Au-Ag epithermal (high-sulfidation) | Chelopech (Bulgaria) | <5.3 <33.9 | <2.96 (Fe < 0.6) <0.6 (Fe < 0.3) | [14] |
| Au-Cu-porphyry | Batu Hijau (Indonesia) | <10.9 | <3.1 | [15] |
| Au-Cu-skarn | Sinyukhinskoye (Russia) | 3–11 | 0.5 | [28] |
| Zeolitized tuffites | Beresovoe (Russia) | 7.2–11.8 | <14.2–29.5 | [29] |
| Au-Cu, ultrabasic lithologies | Kerr (Canada) | <4.2 | 22.3 (Hg < 0.6) | [8] |
| Au-bearing rodingites, talc-filled shear zones | the Wheaton Creek (British Columbia, Canada) | <24.9 | <23.2 (Hg < 0.2) | [9] |
| Au-bearing rodingites | Zolotaya Gora (Urals, Russia) | <13.7 | 23.7 (Hg < 2.6) | [21,22] |
| Cu-Ni-PGE | Norilsk-1 (Russia) Talnakh (Russia) | <1.2 <16.1 | <19.2 (Pt < 5.4, Pd < 8.4) <12.7 (Pd < 2.1) | [19] |
| Au-Pd-REE | Chudnoe (Urals, Russia) | <13.4 | <11.3 (Pd < 2.8) | [12,26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palyanova, G.A.; Beliaeva, T.V.; Savelyev, D.P.; Seryotkin, Y.V. Minerals of the Au-Cu-Ag System in Grains from the Placers of the Olkhovaya-1 River (Eastern Kamchatka, Russia). Minerals 2024, 14, 448. https://doi.org/10.3390/min14050448
Palyanova GA, Beliaeva TV, Savelyev DP, Seryotkin YV. Minerals of the Au-Cu-Ag System in Grains from the Placers of the Olkhovaya-1 River (Eastern Kamchatka, Russia). Minerals. 2024; 14(5):448. https://doi.org/10.3390/min14050448
Chicago/Turabian StylePalyanova, Galina A., Tatiana V. Beliaeva, Dmitry P. Savelyev, and Yurii V. Seryotkin. 2024. "Minerals of the Au-Cu-Ag System in Grains from the Placers of the Olkhovaya-1 River (Eastern Kamchatka, Russia)" Minerals 14, no. 5: 448. https://doi.org/10.3390/min14050448






