Biomineralization Patterns of Intracellular Carbonatogenesis in Cyanobacteria: Molecular Hypotheses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Sample Preparation
2.2. Transmission Electron Microscopy Analyses
2.3. Sample Preparation for Conventional Ultramicrotomy
2.4. Scanning Transmission X-Ray Microscopy Analyses
3. Results
3.1. Chemical Composition of Carbonate and Polyphosphate Inclusions in Ca. Gloeomargarita lithophora and Ca. Synechococcus calcipolaris




3.2. Spatial Arrangement and Total Volume of Ca-Carbonate Inclusions

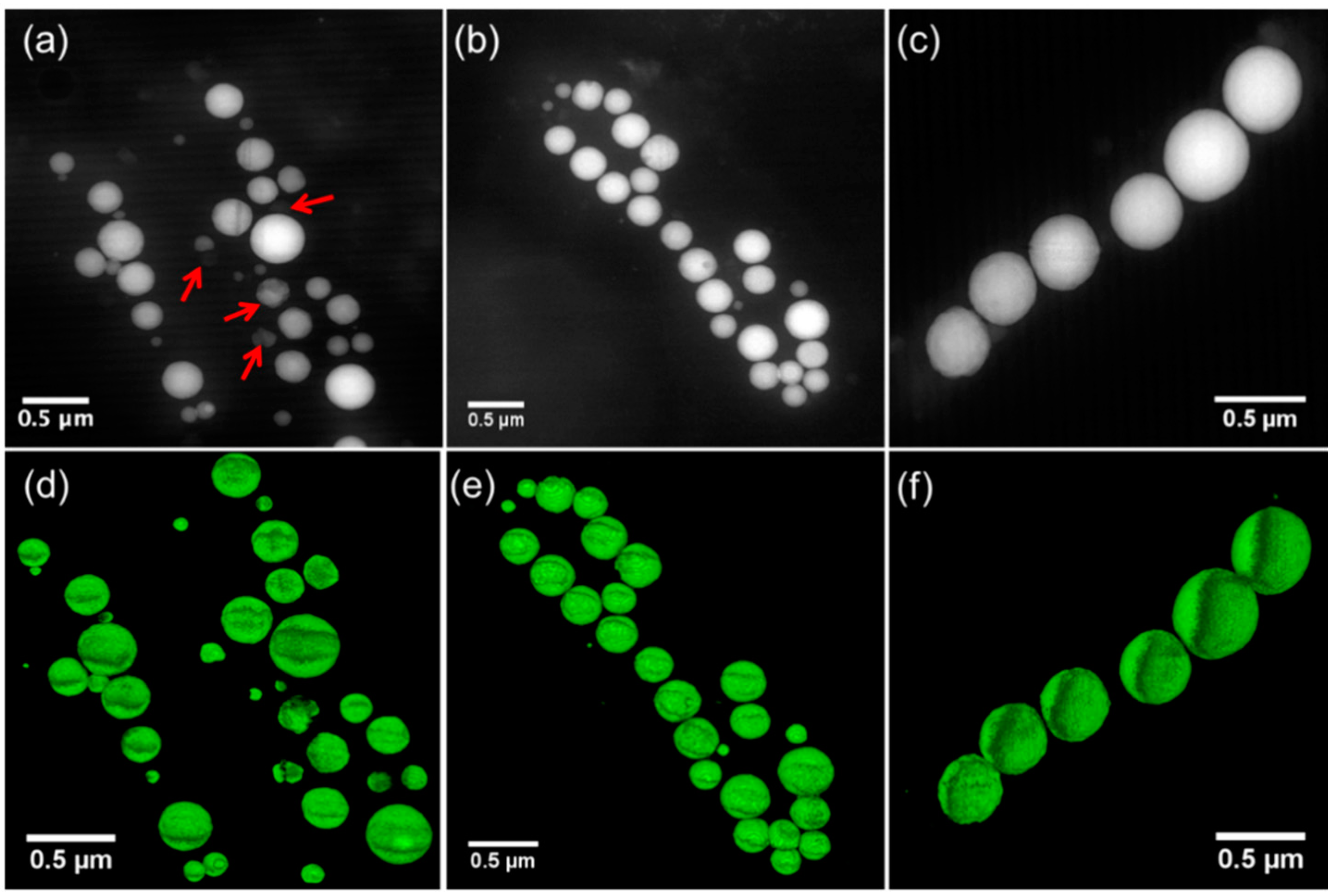

3.3. Morphology and Size Distribution of Ca-Carbonate Inclusions

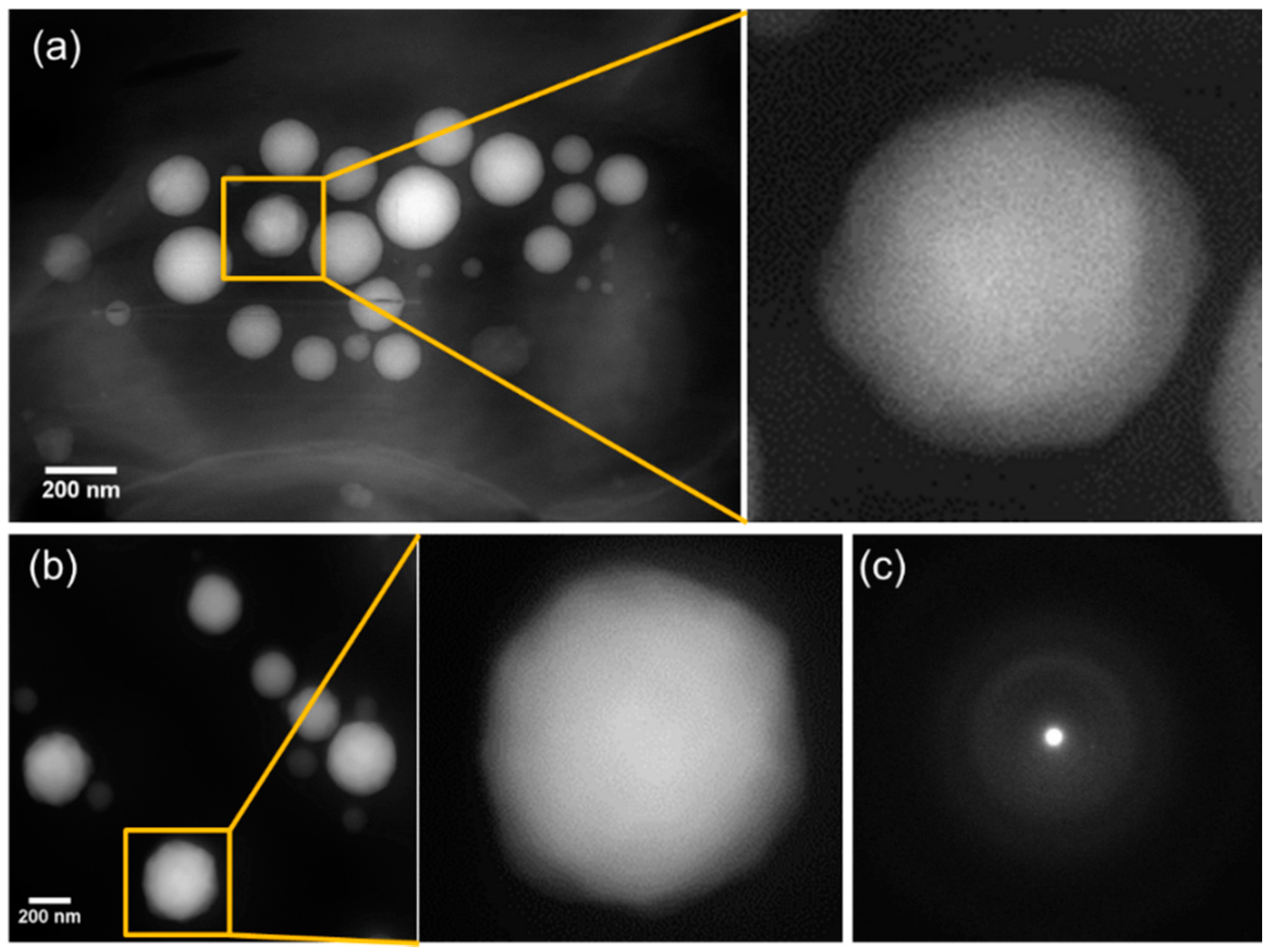

3.4. Conventional Ultramicrotomy of Intracellularly Calcifying Cyanobacteria


4. Discussion
4.1. Inaccuracy of Standard Ultramicrotomy to Study Amorphous Calcium Carbonates in Cyanobacteria
4.2. Possible Biomineralization Processes Operating in Intracellularly Calcifying Cyanobacteria
4.2.1. Possible Involvement of a Membrane-Limited Compartment in the Formation of Intracellular Carbonates
4.2.2. Carboxysomes as Possible Nucleation Sites for Ca-Carbonate Inclusions in Ca. Gloeomargarita lithophora
4.2.3. Division Proteins as Nucleation Sites for Ca-Carbonate Inclusions in Ca. Synechococcus calcipolaris
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Inclusions | Mg K | P K | K K | Ca K |
|---|---|---|---|---|
| Polyphosphate-1 | 13.29 | 62.84 | 8.71 | 15.13 |
| Polyphosphate-2 | 12.79 | 60.85 | 10.42 | 15.94 |
| Polyphosphate-3 | 17.84 | 59.40 | 7.63 | 15.14 |
| Polyphosphate-4 | 17.61 | 60.51 | 6.89 | 14.99 |
| Polyphosphate-5 | 16.48 | 60.14 | 7.77 | 15.62 |
| Polyphosphate-6 | 15.05 | 61.51 | 7.91 | 15.54 |
| Polyphosphate-7 | 16.04 | 61.87 | 8.29 | 13.80 |
| Polyphosphate-8 | 15.17 | 62.23 | 7.01 | 15.6 |
| Ca-carbonate-1 | 16.23 | 0.57 | 2.78 | 80.42 |
| Ca-carbonate-2 | 19.52 | 1.47 | 2.78 | 76.23 |
| Ca-carbonate-3 | 19.37 | 0.79 | 1.67 | 78.17 |
| Ca-carbonate-4 | 15.39 | 0.55 | 3.34 | 80.71 |
| Ca-carbonate-5 | 14.29 | 0.59 | 1.53 | 83.59 |
| Ca-carbonate-6 | 18.45 | - | 1.68 | 79.82 |
| Ca-carbonate-7 | 16.86 | - | 1.27 | 81.87 |
| Ca-carbonate-8 | 13.94 | 1.77 | 1.32 | 82.96 |
| Inclusions | Mg K | Ca K |
|---|---|---|
| Ca-carbonate-1 | 16.81 | 83.19 |
| Ca-carbonate-2 | 15.75 | 84.25 |
| Ca-carbonate-3 | 13.51 | 86.49 |
| Ca-carbonate-4 | 18.59 | 81.41 |
| Ca-carbonate-5 | 18.09 | 81.91 |
| Ca-carbonate-6 | 15.39 | 84.61 |
| Ca-carbonate-7 | 16.04 | 83.96 |
| Ca-carbonate-8 | 14.84 | 85.16 |
| Ca-carbonate-9 | 17.78 | 82.22 |
| Ca-carbonate-10 | 16.95 | 83.05 |
| Ca-carbonate-11 | 19.37 | 80.63 |
| Ca-carbonate-12 | 16.64 | 83.36 |
| Ca-carbonate-13 | 12.92 | 87.08 |
| Ca-carbonate-14 | 18.66 | 81.34 |
| Ca-carbonate-15 | 13.27 | 86.73 |
| Ca-carbonate-16 | 16.87 | 83.13 |
| Ca-carbonate-17 | 11.01 | 88.99 |
| Ca-carbonate-18 | 13.48 | 86.52 |
| Ca-carbonate-19 | 14.99 | 85.01 |
| Ca-carbonate-20 | 11.86 | 88.14 |
| Ca-carbonate-21 | 13.36 | 86.64 |
| Inclusions | Mg K | P K | K K | Ca K |
|---|---|---|---|---|
| Polyphosphate-1 | 5.66 | 56.16 | 12.72 | 25.45 |
| Polyphosphate-2 | 4.92 | 57.62 | 13.86 | 23.58 |
| Polyphosphate-3 | 6.75 | 57.99 | 13.88 | 21.37 |
| Polyphosphate-4 | 5.82 | 53.74 | 11.51 | 28.93 |
| Polyphosphate-5 | 2.99 | 57.71 | 13.95 | 25.35 |
| Polyphosphate-6 | 2.15 | 60.61 | 12.70 | 24.54 |
| Polyphosphate-7 | 4.32 | 55.84 | 14.06 | 25.79 |
| Polyphosphate-8 | 6.09 | 56.68 | 13.45 | 23.78 |
| Polyphosphate-9 | 4.89 | 56.89 | 12.58 | 25.64 |
| Ca-carbonate-1 | 3.88 | 4.34 | 1.71 | 90.07 |
| Ca-carbonate-2 | 6.43 | - | 3.19 | 90.38 |
| Ca-carbonate-3 | - | - | 2.76 | 97.22 |
| Ca-carbonate-4 | 3.69 | 2.71 | 1.38 | 92.23 |
| Ca-carbonate-5 | 2.46 | 1.62 | 1.34 | 94.85 |
| Ca-carbonate-6 | - | - | 2.21 | 97.79 |
| Ca-carbonate-7 | - | - | 0.58 | 99.42 |
| Ca-carbonate-8 | 3.49 | 1.51 | 0.98 | 94.02 |
| Ca-carbonate-9 | 3.47 | - | - | 96.43 |
| Ca-carbonate-10 | 0.96 | - | 0.27 | 98.77 |
| Inclusions | Mg K | Ca K |
|---|---|---|
| Ca-carbonate-1 | 6.18 | 93.82 |
| Ca-carbonate-2 | 4.6 | 95.4 |
| Ca-carbonate-3 | 1.46 | 98.54 |
| Ca-carbonate-4 | 4.53 | 95.47 |
| Ca-carbonate-5 | 7.46 | 92.54 |
| Ca-carbonate-6 | 2.98 | 97.02 |
| Ca-carbonate-7 | 5.99 | 94.01 |
| Ca-carbonate-8 | 3.74 | 96.26 |
| Ca-carbonate-9 | 7.15 | 92.85 |
| Ca-carbonate-10 | 5.59 | 94.41 |
| Ca-carbonate-11 | 3.26 | 96.74 |
| Ca-carbonate-12 | 5.9 | 94.1 |
| Ca-carbonate-13 | 1.19 | 98.81 |
| Ca-carbonate-14 | 2.37 | 97.63 |
| Ca-carbonate-15 | 6.59 | 93.41 |
| Ca-carbonate-16 | 4.64 | 95.36 |
| Ca-carbonate-17 | 0.85 | 99.15 |
| Ca-carbonate-18 | 1.62 | 98.38 |
| Ca-carbonate-19 | 6.23 | 93.77 |
References
- Golubic, S.; Seong-Joo, L. Early cyanobacterial fossil record: Preservation, palaeoenvironments and identification. Eur. J. Phycol. 1999, 34, 339–348. [Google Scholar] [CrossRef]
- Blank, C.E.; Sánchez-Baracaldo, P. Timing of morphological and ecological innovations in the cyanobacteria—A key to understanding the rise in atmospheric oxygen. Geobiology 2010, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Merz, M. The biology of carbonate precipitation by cyanobacteria. Facies 1992, 26, 81–101. [Google Scholar] [CrossRef]
- Arp, G.; Thiel, V.; Reimer, A.; Michaelis, W.; Reitner, J. Biofilm exopolymers control microbialite formation at thermal springs discharging into the alkaline Pyramid Lake, Nevada, USA. Sediment Geol. 1999, 126, 159–176. [Google Scholar] [CrossRef]
- Altermann, W.; Kazmierczak, J.; Oren, A.; Wright, D.T. Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology 2006, 4, 147–166. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria-the potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bundeleva, I.A.; Shirokova, L.S.; Pokrovsky, O.S.; Benezeth, P.; Menez, B.; Gerard, E.; Balor, S. Experimental modeling of calcium carbonate precipitation by cyanobacterium Gloeocapsa sp. Chem. Geol. 2014, 374, 44–60. [Google Scholar] [CrossRef]
- Freytet, P.; Verrecchia, E.P. Freshwater organisms that build stromatolites: A synopsis of biocrystallization by prokaryotic and eukaryotic algae. Sedimentology 1998, 45, 535–563. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A. Carbonic anhydrase: Enzyme that has transformed the biosphere. Russ. J. Plant Physiol. 2011, 58, 197–209. [Google Scholar] [CrossRef]
- Bosak, T.; Liang, B.; Wu, T.D.; Templer, S.P.; Evans, A.; Vali, H.; Guerquin-Kern, J.L.; Klepac-Ceraj, V.; Friedman, J.; Sim, M.S.; et al. Cyanobacterial diversity and activity in modern conical microbialites. Geobiology 2013, 11, 100–100. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.B.; Schultze-Lam, S.; Beveridge, T.J.; des Marais, D.J. Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton. Limnol. Oceanogr. 1997, 42, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kamennaya, N.; Ajo-Franklin, C.; Northen, T.; Jansson, C. Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Yasumoto, K.; Yasumoto-Hirose, M.; Yasumoto, J.; Murata, R.; Sato, S.; Baba, M.; Mori-Yasumoto, K.; Jimbo, M.; Oshima, Y.; Kusumi, T.; et al. Biogenic polyamines capture CO2 and accelerate extracellular bacterial CaCO3 formation. Mar. Biotechnol. 2014, 16, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Riding, R. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic-Cambrian changes in atmospheric composition. Geobiology 2006, 4, 299–316. [Google Scholar] [CrossRef]
- Dittrich, M.; Sibler, S. Calcium carbonate precipitation by cyanobacterial polysaccharides. Geol. Soc. Lond. Spec. Publ. 2010, 336, 51–63. [Google Scholar] [CrossRef]
- Lee, B.D.; Apel, W.A.; Walton, M.R. Calcium carbonate formation by Synechococcus sp. strain PCC 8806 and Synechococcus sp. strain PCC 8807. Bioresour. Technol. 2006, 97, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Wehrli, B.; Dittrich, M. CaCO3 nucleation by cyanobacteria: Laboratory evidence for a passive, surface-induced mechanism. Geobiology 2009, 7, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.Q.; Paulo, C.; Zhu, Y.; Dittrich, M. CaCO3 biomineralization on cyanobacterial surfaces: Insights from experiments with three Synechococcus strains. Colloid Surf. B 2013, 111, 600–608. [Google Scholar] [CrossRef] [PubMed]
- McConnaughey, T.A.; Whelan, J.F. Calcification generates protons for nutrient and bicarbonate uptake. Earth Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Jiang, H.B.; Cheng, H.M.; Gao, K.S.; Qiu, B.S. Inactivation of Ca2+/H+ exchanger in Synechocystis sp. strain PCC 6803 promotes cyanobacterial calcification by upregulating CO2-concentrating mechanisms. Appl. Environ. Microb. 2013, 79, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.; Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. A Greenhouse-scale photosynthetic microbial bioreactor for carbon sequestration in magnesium carbonate minerals. Environ. Sci. Technol. 2014, 48, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, S.; Brinkmann, N.; Reimer, A.; Ionescu, D.; Friedl, T.; de Beer, D.; Arp, G. Effect of variable pCO2 on Ca2+ removal and potential calcification of cyanobacterial biofilms-an experimental microsensor study. Geomicrobiol. J. 2015, 32, 304–315. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; Guyot, F.; Skouri, F.; de Lucca, G.; Heulin, T. Biologically controlled precipitation of calcium phosphate by Ramlibacter tataouinensis. Earth Planet. Sci. Lett. 2004, 228, 439–449. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gérard, E.; Moreira, D.; Bernard, S.; Brown, G.E.; López-García, P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Skouri-Panet, F.; Li, J.H.; Ferard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; et al. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Ragon, M.; Benzerara, K.; Moreira, D.; Tavera, R.; Lopez-Garcia, P. 16S rDNA-based analysis reveals cosmopolitan occurrence but limited diversity of two cyanobacterial lineages with contrasted patterns of intracellular carbonate mineralization. Front. Microbiol. 2014, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Amarouche-Yala, S.; Benouadah, A.; el Ouahab Bentabet, A.; López-García, P. Morphological and phylogenetic diversity of thermophilic cyanobacteria in Algerian hot springs. Extremophiles 2014, 18, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.E.; la Rivière, J.W.; Schmidt, K. Some properties of Achromatium oxaliferum. Antonie Van Leeuwenhoek 1971, 37, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.D. The unique role of intracellular calcification in the genus Achromatium. B. In Inclusions in Prokaryotes; Shively, J.M., Ed.; Springer: Berlin, Germany, 2006; pp. 299–309. [Google Scholar]
- Salman, V.; Yang, T.; Berben, T.; Klein, F.; Angert, E.; Teske, A. Calcite-accumulating large sulfur bacteria of the genus Achromatium in Sippewissett Salt Marsh. ISME J. 2015, 9, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Mansor, M.; Hamilton, T.L.; Fantle, M.S.; Macalady, J. Metabolic diversity and ecological niches of Achromatium populations revealed with single-cell genomic sequencing. Front. Microbiol. 2015, 6, 822. [Google Scholar] [CrossRef] [PubMed]
- Cam, N.; Georgelin, T.; Jaber, M.; Lambert, J.F.; Benzerara, K. In vitro synthesis of amorphous Mg-, Ca-, Sr- and Ba-carbonates: What do we learn about intracellular calcification by cyanobacteria? Geochim. Cosmochim. Acta 2015, 161, 36–49. [Google Scholar] [CrossRef]
- Badger, M.R.; Andrews, T.J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus Sp. Plant Physiol. 1982, 70, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Barrán-Berdón, A.L.; Rodea-Palomares, I.; Leganes, F.; Fernandez-Pinas, F. Free Ca2+ as an early intracellular biomarker of exposure of cyanobacteria to environmental pollution. Anal. Bioanal. Chem. 2011, 400, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- DIGIECT Software. Available online: http://www.digisens3-D.com/fr/logiciel-tomographie/soft/2-3-D_Electron_Tomography_Software.html (accessed on 1 February 2016).
- Nierzwicki-Bauer, S.A.; Balkwill, D.L.; Stevens, S.E., Jr. Use of a computer-aided reconstruction system to examine the three-dimensional architecture of cyanobacteria. J. Ultrastruct. Res. 1983, 84, 73–82. [Google Scholar] [CrossRef]
- aXis2000 Software. Available online: http://unicorn.mcmaster.ca/aXis2000.html (accessed on 1 February 2016).
- Cosmidis, J.; Benzerara, K.; Nassif, N.; Tyliszczak, T.; Bourdelle, F. Characterization of Ca-phosphate biomaterials by scanning transmission X-ray microscopy (STXM) at the Ca L2,3-, P L2,3- and C K-edges. Acta Biomater. 2015, 12, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.F.; Kasrai, M.; Bancroft, G.M.; Tan, K.H.; Feng, X.H. X-ray-absorption spectroscopic studies of sodium polyphosphate glasses. Phys. Rev. B 1995, 51, 742–750. [Google Scholar] [CrossRef]
- Kruse, J.; Leinweber, P.; Eckhardt, K.-U.; Godlinski, F.; Hu, Y.; Zuin, L. Phosphorus L2,3-edge XANES: Overview of reference compounds. J. Synchrotron Radiat. 2009, 16, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, A.; Kalkan, B.; Clark, S.M.; Waychunas, G.A. Pressure-induced polyamorphism and formation of “aragonitic” amorphous calcium carbonate. Angew. Chem. Int. Ed. 2013, 52, 8354–8357. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Pan, Y.X.; Chen, G.J.; Liu, Q.S.; Tian, L.X.; Lin, W. Magnetite magnetosome and fragmental chain formation of Magnetospirillum magneticum AMB-1: Transmission electron microscopy and magnetic observations. Geophys. J. Int. 2009, 177, 33–42. [Google Scholar] [CrossRef]
- Edelmann, L. Freeze-dried and resin-embedded biological material is well suited for ultrastructure research. J. Microsc. 2002, 207, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Porta, D.; Rippka, R.; Hernandez-Marine, M. Unusual ultrastructural features in three strains of Cyanothece (cyanobacteria). Arch. Microbiol. 2000, 173, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Blue, C.R.; Rimstidt, J.D.; Dove, P.M. A mixed flow reactor method to synthesize amorphous calcium carbonate under controlled chemical conditions. In Research Methods in Biomineralization Science; Yoreo, J.J.D., Ed.; Elsevier: San Diego, CA, USA, 2013; Volume 532, pp. 557–568. [Google Scholar]
- Reeder, R.J.; Tang, Y.; Schmidt, M.P.; Schmidt, M.P.; Kubista, L.M.; Cowan, D.F.; Phillips, B.L. Characterization of structure in biogenic amorphous calcium carbonate: Pair distribution function and nuclear magnetic resonance studies of lobster gastrolith. Cryst. Growth Des. 2013, 13, 1905–1914. [Google Scholar] [CrossRef]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates; Wiley: Chichester, UK, 2004; pp. 9–12. [Google Scholar]
- Cartwright, J.H.E.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Keyser, D.; Walter, R. Calcification in Ostracodes. Rev. Esp. Micropaleontol. 2004, 36, 1–11. [Google Scholar]
- Hurbain, I.; Sachse, M. The future is cold: Cryo-preparation methods for transmission electron microscopy of cells. Biol. Cell 2011, 103, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; MacLellan, K.; Boisset, N.; Benzerara, K. Preservation of protein globules and peptidoglycan in the mineralized cell wall of nitrate-reducing iron(II) oxidizing bacteria: A cryo-electron microscopy study. Geobiology 2011, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Komeili, A.; Vali, H.; Beveridge, T.J.; Newman, D.K. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc. Natl. Acad. Sci. USA 2004, 101, 3839–3844. [Google Scholar] [CrossRef] [PubMed]
- Eberl, D.D.; Drits, V.A.; Srodon, J. Deducing growth mechanisms for minerals from the shapes of crystal size distributions. Am. J. Sci. 1998, 298, 499–533. [Google Scholar] [CrossRef]
- Jandacka, P.; Alexa, P.; Pistora, J.; Li, J.H.; Vojtkova, H.; Hendrych, A. Size distributions of nanoparticles from magnetotactic bacteria as signatures of biologically controlled mineralization. Am. Mineral. 2013, 98, 2105–2114. [Google Scholar] [CrossRef]
- Head, I.M.; Gray, N.D.; Babenzien, H.D.; Glockner, F.O. Uncultured giant sulfur bacteria of the genus Achromatium. FEMS Microbiol. Ecol. 2000, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Head, I.M.; Gray, N.D.; Howarth, R.; Pickup, R.W.; Clarke, K.J.; Jones, J.G. Achromatium oxaliferum understanding the unmistakable. Adv. Microb. Ecol. 2000, 16, 1–40. [Google Scholar]
- Gray, N.; Head, I. The Family Achromatiaceae, B. In The Prokaryotes; Rosenberg, E., DeLong, E., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2014; pp. 1–14. [Google Scholar]
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Functions, compositions, and evolution of the two types of carboxysomes: Polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Mol. Biol. Rev. 2013, 77, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Heinhorst, S.; Cannon, G.C.; Shively, J.M. Chapter 4: Carboxysomes and their structural organization in prokaryotes. In Nanomicrobiology: Physiological and Environmental Characteristics; Springer: New York, NY, USA, 2014. [Google Scholar]
- van de Meene, A.M.L.; Hohmann-Marriott, M.F.; Vermaas, W.F.J.; Roberson, R.W. The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 2006, 184, 259–270. [Google Scholar]
- Savage, D.F.; Afonso, B.; Chen, A.H.; Silver, P.A. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science 2010, 327, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Whitehead, L.F.; Forster, B.; Badger, M.R.; Price, G.D. Cyanobacterial carboxysomes: Microcompartments that facilitate CO2 fixation. J. Mol. Microbiol. Biotechnol. 2013, 23, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Dou, Z.; Bernstein, S.L.; Leverenz, R.; Williams, E.; Heinhorst, S.; Shively, J.; Cannon, G.C.; Kerfeld, C.A. Advances in understanding carboxysome assembly in Prochlorococcus and Synechococcus implicate CsoS2 as a critical component. Life 2015, 5, 1141–1171. [Google Scholar] [CrossRef] [PubMed]
- Iancu, C.V.; Morris, D.M.; Dou, Z.; Heinhorst, S.; Cannon, G.C.; Jensen, G.J. Organization, structure, and assembly of α-carboxysomes determined by electron cryotomography of intact cells. J. Mol. Biol. 2010, 396, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Liberton, M.; Austin, J.R., II; Berg, R.H.; Pakrasi, H.B. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2011, 155, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kerfeld, C.A.; Sawaya, M.R.; Cai, F.; Heinhorst, S.; Cannon, G.C.; Yeates, T.O. Atomic-level models of the bacterial carboxysome shell. Science 2008, 319, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.W.; Errington, J. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 2009, 7, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.R.; Beech, P.L. Cell division protein FtsZ: Running rings around bacteria, chloroplasts and mitochondria. Res. Microbiol. 2001, 152, 3–10. [Google Scholar] [CrossRef]
- Mazouni, K.; Domain, F.; Cassier-Chauvat, C.; Chauvat, F. Molecular analysis of the key cytokinetic components of cyanobacteria: FtsZ, ZipN and MinCDE. Mol. Microbiol. 2004, 52, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Chauvat, F. Cell division in cyanobacteria. In The Biology of Cyanobacteria; Flores, E., Herrero, A., Eds.; Caister Academic Press: Norfolk, UK, 2014. [Google Scholar]
- Yu, X.C.; Margolin, W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997, 16, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lutkenhaus, J. Analysis of FtsZ assembly by light scattering and determination of the role of divalent metal cations. J. Bacteriol. 1999, 181, 823–832. [Google Scholar] [PubMed]
- Jaiswal, R.; Panda, D. Differential assembly properties of Escherichia coli FtsZ and Mycobacterium tuberculosis FtsZ: An analysis using divalent calcium. J. Biochem. 2009, 146, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Benzerara, K.; Morin, G.; Kapplerb, A.; Bernardc, S.; Obstd, M.; Férarda, C.; Skouri-Paneta, F.; Guignera, J.; Posth, N.; et al. Iron biomineralization by neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 2009, 73, 696–711. [Google Scholar] [CrossRef]
- Lindner, A.B.; Madden, R.; Demarez, A.; Stewart, E.J.; Taddei, F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. USA 2008, 105, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Margaret Oliver, I.; Cam, N.; Boudier, T.; Blondeau, M.; Leroy, E.; Cosmidis, J.; Skouri-Panet, F.; Guigner, J.-M.; Férard, C.; et al. Biomineralization Patterns of Intracellular Carbonatogenesis in Cyanobacteria: Molecular Hypotheses. Minerals 2016, 6, 10. https://doi.org/10.3390/min6010010
Li J, Margaret Oliver I, Cam N, Boudier T, Blondeau M, Leroy E, Cosmidis J, Skouri-Panet F, Guigner J-M, Férard C, et al. Biomineralization Patterns of Intracellular Carbonatogenesis in Cyanobacteria: Molecular Hypotheses. Minerals. 2016; 6(1):10. https://doi.org/10.3390/min6010010
Chicago/Turabian StyleLi, Jinhua, Isabel Margaret Oliver, Nithavong Cam, Thomas Boudier, Marine Blondeau, Eric Leroy, Julie Cosmidis, Feriel Skouri-Panet, Jean-Michel Guigner, Céline Férard, and et al. 2016. "Biomineralization Patterns of Intracellular Carbonatogenesis in Cyanobacteria: Molecular Hypotheses" Minerals 6, no. 1: 10. https://doi.org/10.3390/min6010010







