Chemical Environment of Unusually Ge- and Pb-Rich Willemite, Tres Marias Mine, Mexico
Abstract
:1. Introduction
2. Analytical Methods
3. Sample Descriptions
4. Results
4.1. X-Ray Mapping and Microprobe Analyses
- (a)
- Pb and Ge are positively correlated (r = 0.77). The Pb and Ge concentrations range from below the detection limits to over 1.5 wt % and 1400 ppm, respectively. Concentrations of both elements increase from core to rim, overprinting tandem oscillations between extreme values.
- (b)
- Pb and Zn are negatively correlated, with a slope near −1.0 (r = −0.94). The slope of this line is similar if data are plotted in wt % or in formula units.
- (c)
- Zn and Ge are also negatively correlated (r = −0.66).
4.2. XAFS and XANES Analyses
5. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
X-Ray Absorption Spectroscopy at the Ge K-Edge

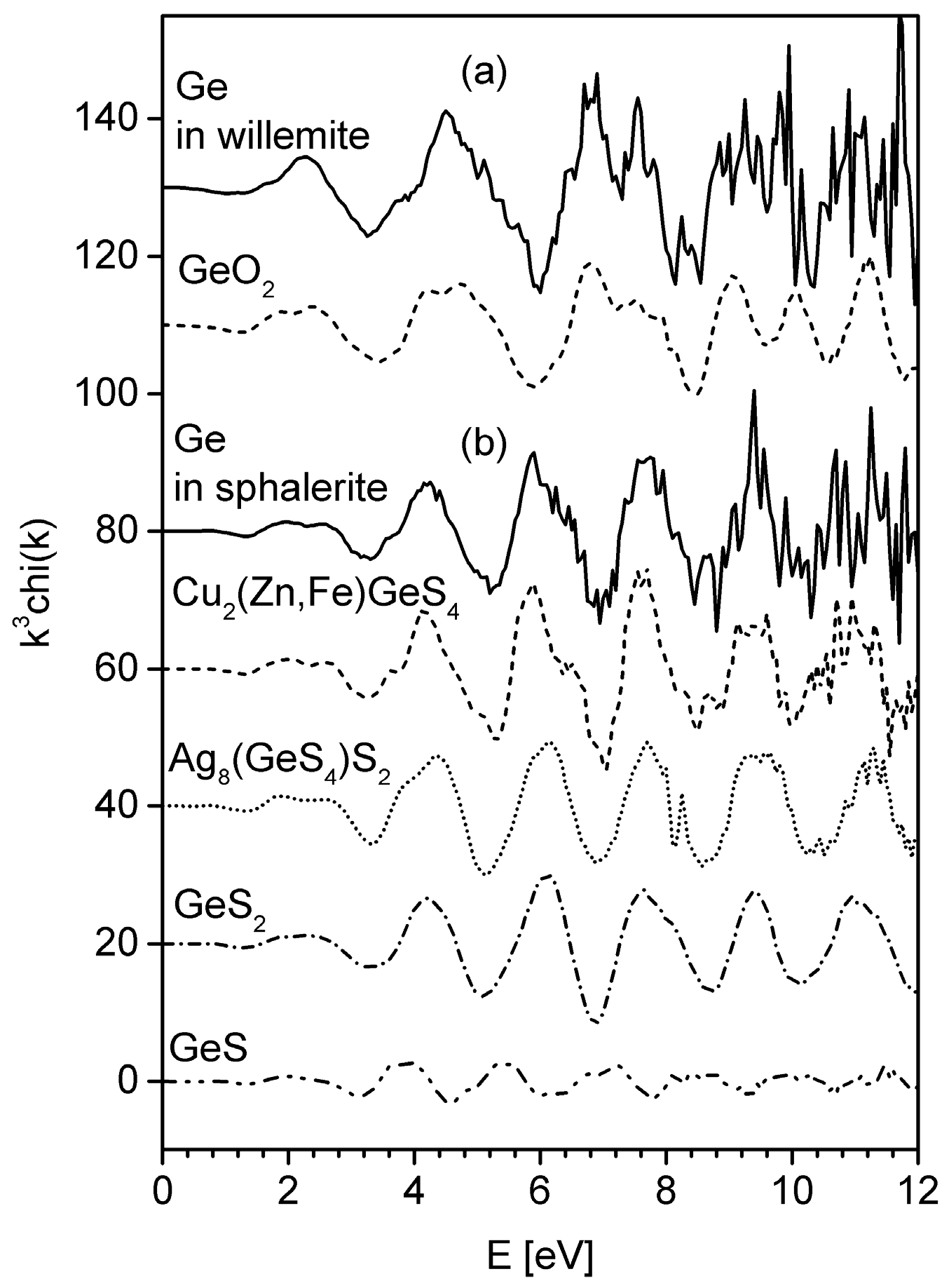
References
- Butterman, W.C.; Jorgenson, J.D. Mineral Commodity Profiles: Germanium; U.S. Geological Survey Open-File Report 2004–1218; U.S. Geological Survey: Reston, CA, USA, 2004.
- Melcher, F.; Buchholz, P. Germanium. In Critical Metals Handbook; Gunn, G., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 177–204. [Google Scholar]
- U.S. Geological Survey. Mineral commodity summaries, 2015. Available online: minerals.usgs.gov/minerals/pubs/mcs/2015/mcs2015.pdf (accessed on 5 November 2015).
- Hitzman, M.W.; Reynolds, N.A.; Sangster, D.F.; Allen, C.R.; Carman, C.E. Classification, genesis and exploration guides for nonsulfide zinc deposits. Econ. Geol. 2003, 98, 685–714. [Google Scholar] [CrossRef]
- Wang, S.F.; Lü, M.K.; Gu, F.; Qi, Y.X.; Xu, D.; Yuan, D.R.; Cao, D.X. Effect of Pb2+ ions on the photoluminescence characteristics of Mn2+-doped Zn2SiO4. Appl. Phys. A 2005, 80, 871–874. [Google Scholar] [CrossRef]
- Yang, P.; Lü, M.K.; Song, C.F.; Liu, S.W.; Gu, F.; Wang, S.F. Photoluminescence of Pb2+ ions in sol-gel derived Zn2SiO4. Inorg. Chem. Commun. 2004, 7, 268–270. [Google Scholar] [CrossRef]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Sheffer, H.W. The occurrence of germanium in willemite. Geochim. Cosmochim. Acta 1966, 30, 837–838. [Google Scholar] [CrossRef]
- Lombaard, A.F.; Günzel, A.; Innes, J.; Krüger, T.L. The Tsumeb lead-copper-zinc-silver deposit, South West Africa/Namibia. In Mineral Deposits of Southern Africa; Anhaeusser, C.R., Maske, S., Eds.; Geological Society of South Africa: Johannesburg, South Africa, 1986; Volume 2, pp. 1761–1782. [Google Scholar]
- Dutrizac, J.E.; Chen, T.T.; Longton, R.J. The mineralogical deportment of germanium in the Clarksville electrolytic zinc plant of Savage Zinc Inc. Metall. Mater. Trans. B 1996, 27, 567–576. [Google Scholar] [CrossRef]
- Groves, I.M.; Carman, C.E.; Dunlap, W.J. Geology of the Beltana willemite deposit, Flinders Range, South Australia. Econ. Geol. 2003, 98, 797–818. [Google Scholar] [CrossRef]
- Choulet, F.; Buatier, M.; Richard, J.; Barbanson, L.; Guéguan, R.; Ennaciri, A. Formation of supergene zinc silicates in carbonates: New advances from the Bou Arhous deposit (Morocco). In Proceedings of the 13th Biennial SGA Meeting, Nancy, France, 24–27 August 2015.
- Choulet, F. Personal communication. 2015. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ericsson, T.; Filippidis, A. Cation ordering in the limited solid solution Fe2SiO4-Zn2SiO4. Am. Mineral. 1986, 71, 1502–1509. [Google Scholar]
- Harper, K.; Griffen, D.T. Crystallographic consequences of Mn substitution in willemite. In Proceedings of the Abstracts, Geological Society of America Annual Meeting, Salt Lake City, UT, USA, 16–19 October 2005; Volume 37, p. 287.
- Gusken, E.; Nunes, C.C.; Dalmon, D.L.; Ono, E.; Santos, J.S.; Suzuki, C.K. Study of GeO2 addition in silica-germania glasses by XAS; Photon Factory Activity Report 2006 #24 Part B, Users’ Report 169, 9A/2006P020; The State University of Campinas, Faculty of Mechanical Engineering, Laboratory of Photonic Materials & Devices: Campinas, Brazil, 2007. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Saini-Eidukat, B.; Melcher, F.; Lodziak, J. Zinc-germanium ores of the Tres Marias Mine, Chihuahua, Mexico. Miner. Depos. 2009, 44, 363–370. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Philippot, E.; Chapon, L.; Hull, S. A neutron diffraction study of the thermal stability of the alpha-quartz-type structure in germanium dioxide. J. Solid State Chem. 2002, 166, 434–441. [Google Scholar] [CrossRef]
- Baur, W.H.; Khan, A.A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Crystallogr. Sect. B 1971, 27, 2133–2139. [Google Scholar] [CrossRef]
- Nitsche, R.; Sargent, D.F.; Wild, P. Crystal growth of quaternary 122464 chalcogenides by iodine vapor transport. J. Cryst. Growth 1967, 1, 52–53. [Google Scholar] [CrossRef]
- Wintenberger, M. Etude de la structure cristallographique et magnetique de Cu2FeGeS4 et remarque sur la structure magnetique de Cu2MnSnS4. Mater. Res. Bull. 1979, 14, 1195–1202. (In French) [Google Scholar] [CrossRef]
- Guen, L.; Glaunsinger, W.S. Electrical, magnetic, and EPR studies of the quaternary chalcogenides Cu2AIIBIVX4 prepared by iodine transport. J. Solid State Chem. 1980, 35, 10–21. [Google Scholar] [CrossRef]
- Eulenberger, G. Die Kristallstruktur der Tieftemperaturmodifikation von Ag8GeS6. Monatshefte Chem. 1977, 108, 901–913. (In German) [Google Scholar] [CrossRef]
- Zachariasen, W.H. The crystal structure of germanium disulphide. J. Chem. Phys. 1936, 4, 618–619. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Young, H.S. Germanium and silicon disulfides: Structure and synthesis. Science 1965, 149, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Klaska, K.H.; Eck, J.C.; Pohl, D. New investigation of willemite. Acta Crystallogr. 1978, B34, 3324–3325. [Google Scholar] [CrossRef]
- Oribe, A.; Tanaka, K.; Morikawa, H.; Marumo, F. Refinement of Crystal Structure of Zn2GeO4; Report of the Research Laboratory on Engineering Materials; Tokyo Institute of Technology: Tokyo, Japan, 1987; pp. 7–12. [Google Scholar]
- Chichagov, A.V.; Belonozhko, A.B.; Lopatin, A.L.; Dokina, T.N.; Samokhvalova, O.L.; Ushakovskaya, T.V.; Shilova, Z.V. Information-calculating system on crystal structure data for minerals (MINCRYST). Kristallografiya 1990, 35, 610–616. [Google Scholar] [CrossRef]
- Brugger, J.; McPhail, D.C.; Wallace, M.; Waters, J. Formation of willemite in hydrothermal environments. Econ. Geol. 2003, 98, 819–835. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Melcher, F. The Otavi Mountain Land in Namibia: Tsumeb, germanium and snowball earth. Mitt. Österreichischen Mineral. Ges. 2003, 148, 413–435. [Google Scholar]
- Dutrizac, J.E.; Jambor, J.L.; Chen, T.T. Host minerals for the gallium-germanium ores of the Apex mine, Utah. Econ. Geol. 1986, 81, 946–950. [Google Scholar] [CrossRef]










| Analyte | TM06-15 | TM06-18 | Method 1 | Analyte | TM06-15 | TM06-18 | Method 1 |
|---|---|---|---|---|---|---|---|
| wt % | wt % | ppm | ppm | ||||
| SiO2 | 16.03 | 28.68 | 1 | Ag | 0.8 | 6.8 | 6 |
| TiO2 | 0.004 | 0.044 | 1 | As | 1200 | 685 | 7 |
| Al2O3 | 0.07 | 16.97 | 1 | Au | <2 | <2 | 7 |
| Fe2O3 | 6.9 | 21.16 | 1 | Ba | 219 | 13 | 1 |
| MnO | 0.007 | 0.007 | 1 | Bi | <12 | 6 | 1 |
| MgO | 0.03 | 0.15 | 1 | Cd | 179 | 80.4 | 5 |
| CaO | 4.03 | 0.34 | 1 | Ce | <3 | 18 | 7 |
| Na2O | <0.01 | <0.01 | 1 | Cr | <8 | 137 | 1 |
| K2O | 0.01 | 0.26 | 1 | Cs | <1 | 7 | 7 |
| P2O5 | 0.028 | 0.137 | 1 | Cu | 32 | 19 | 5 |
| (SO3) | 4.22 | 0.95 | 5 | Ga | 5 | 58 | 6 |
| (F) | <0.10 | <0.10 | 1 | Ge | 1070 | 304 | 6 |
| LOI | 3.2 | 14.7 | 3 | Ge | 1248 | 344 | 1 |
| Pb | 21.4 | 0.728 | 4 | In | 0.2 | 6.5 | 6 |
| Zn | 33.5 | 12.4 | 1 | Mo | 44 | 99 | 7 |
| C org | 0.23 | 0.30 | 2 | Nb | 20 | <5 | 1 |
| C total | 2.03 | 0.70 | 2 | Ni | 6 | <5 | 1 |
| CO2 | 8.99 | 2.00 | diff | Sb | 28 | 22 | 1 |
| Sum | 98.75 | 98.84 | Sc | <2 | 5 | 1 | |
| Sr | 252 | 478 | 1 | ||||
| V | 438 | 168 | 6 | ||||
| W | <30 | <18 | 1 | ||||
| Zr | <9 | 14 | 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini-Eidukat, B.; Melcher, F.; Göttlicher, J.; Steininger, R. Chemical Environment of Unusually Ge- and Pb-Rich Willemite, Tres Marias Mine, Mexico. Minerals 2016, 6, 20. https://doi.org/10.3390/min6010020
Saini-Eidukat B, Melcher F, Göttlicher J, Steininger R. Chemical Environment of Unusually Ge- and Pb-Rich Willemite, Tres Marias Mine, Mexico. Minerals. 2016; 6(1):20. https://doi.org/10.3390/min6010020
Chicago/Turabian StyleSaini-Eidukat, Bernhardt, Frank Melcher, Jörg Göttlicher, and Ralph Steininger. 2016. "Chemical Environment of Unusually Ge- and Pb-Rich Willemite, Tres Marias Mine, Mexico" Minerals 6, no. 1: 20. https://doi.org/10.3390/min6010020
APA StyleSaini-Eidukat, B., Melcher, F., Göttlicher, J., & Steininger, R. (2016). Chemical Environment of Unusually Ge- and Pb-Rich Willemite, Tres Marias Mine, Mexico. Minerals, 6(1), 20. https://doi.org/10.3390/min6010020





