1. Introduction
Graphite is a type of widely used non-metallic material; large, flaky graphite is an irreplaceable raw material in many industries [
1]. Natural graphite is found in three commercial varieties: crystalline flake, microcrystalline or amorphous, and crystalline vein or lump graphite [
2]. Among them, crystalline flake graphite has the best floatability, followed by crystalline graphite; amorphous graphite has the poorest floatability. Due to the strong floatability of flake graphite, flotation is the most commonly used means of graphite beneficiation.
In conventional processing of large flaky graphite ores, a multistage grinding-flotation process is used to prevent large flakes from being destroyed during regrinding in graphite concentrate [
3]. However, high-quality graphite resources that contain large graphite flakes are becoming increasingly scarce; moreover, fractional flaky graphite ores do not contain any large flakes and have relatively finely disseminated grain sizes. To increase the recovery of graphite, these graphite ores must be ground to a much smaller size. However, by doing so, gangue minerals are also ground finely. These fine/ultrafine gangue mineral particles not only affect sub-flotation processes in recovering valuable minerals based on true flotation but also lead to grade reduction through mechanical entrainment. Thus, the quality of final graphite concentrate is often greatly reduced due to the recovery of gangue minerals [
4].
The separation efficiency between the valuable mineral and fully liberated and dispersed gangue is dependent on the degree of entrainment [
5]. Unlike true flotation, which is selective, both gangue and valuable minerals alike can be recovered by entrainment. There are many factors that control the entrainment of hydrophilic gangue such as the properties of gangue, viscosity of the slurry, froth structure [
6], particle mass [
7], and shape [
8]. Because entrainment has a detrimental effect on the grade of the concentrate, a number of studies have been carried out to research entrainment mechanisms, identify factors affecting entrainment and develop models with an objective of predicting entrainment in a flotation cell [
4].
Sericite is one of the main gangue minerals in graphite ore and is also the main contaminant in graphite flotation concentrate. Moreover, thorough entrainment commonly reports to the concentrate in certain base metal ores [
9]. The entrainment behavior of sericite in microcrystalline graphite flotation was studied by Hongqiang Li, who found that the entrainment of sericite leads to poor flotation selectivity of commercial microcrystalline graphite ore [
10].
Although studies on the entrainment mechanism have been conducted for 30 years, no effective method has been found to control entrainment in graphite flotation. Thus far, there are two main methods to control entrainment in flotation [
11]. The first method is ameliorating the flotation machine, such as optimizing the structure of flotation cell or flotation column [
12,
13,
14]. The second method is optimizing the flotation technology and reagent system such as using polymer depressant for hydrophobic flocculation flotation. The use of a polymer depressant that has flocculability in flotation can not only cause the minerals that must be depressed to become hydrophilic but also increase the particle size of these minerals. Hence, bubble entrainment and mechanical entrainment in flotation can be weakened [
15].
During graphite flotation, it is almost impossible to avoid the entrainment of sericite into graphite concentrate. Bartrum introduced the use of nigrosine as a depressant for graphite in lead zinc ore processing technology [
16]. Carboxyl methylated cellulose (CMC) and dextrin can also be used as depressants of graphite [
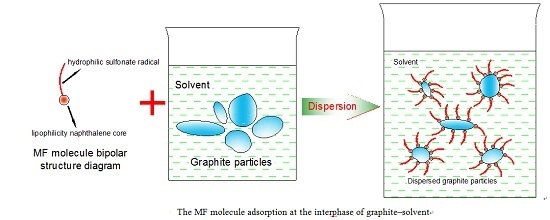
17]. However, few researchers have studied the reverse flotation of graphite in flotation when graphite is the objective mineral. This study used MF (formaldehyde condensate of methyl naphthalene sulfonic sodium salt) as a depressant for graphite during the flotation recovery of sericite. MF is actually a dispersant and a type of surfactant whose full name is formaldehyde condensate of methyl naphthalene sulfonic sodium salt. The formula and name of MF is shown in
Figure 1. It is the formaldehyde condensate of methyl naphthalene sulfonate [
18]. The lipophilicity naphthalene core of MF can adsorb on the nonpolar surface of particles with the sulfonate radical pointing to the solvent. The silfonate radical is hydrophilic; therefore, an energy barrier was formed around the dispersed particles, leading to mutual exclusion between them. The dispersed particles were then dispersed in the solvent uniformly [
19].
To investigate the depressing effect of MF on graphite, batch flotation tests and contact angle measurements of single minerals and artificial mixtures composed of sericite and graphite single mineral and contact angle measurements were carried out. In the end, Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and zeta potential measurements were performed to explain the adsorbing and depressing mechanism of MF.
2. Materials and Methods
2.1. Test Samples and Reagents
The graphite single mineral sample was obtained from Yichang city in Hubei province, China. Large lumps of graphite with high purity were selected first. The lumps were then crushed to −2 mm by a roll crushing mill followed by wet grinding in a conical ball mill with zirconia balls as grinding media; finally, the milled mineral particles were wet screened using 200 and 325 mesh US standard sieves, and the −74 to +45 μm size fraction was collected and used in batch flotation tests. According to the standard method (GB/T 3521-2008) [
20] in China, the fixed carbon content (FC) of graphite single mineral was analyzed. The FC of graphite single mineral was 97.75% with an ash content of 2.25%.
Figure 2a is the X-ray diffraction pattern of graphite single mineral, combined with the result of FC analysis, and indicates that the content of graphite in the sample was higher than 96 wt %.
The sericite single mineral sample was obtained from Xianning city in Hubei province, China. It was processed by the same method as graphite. X-ray fluorescence (XRF) analysis indicated that it contained 9.17 wt % K
2O, 1.09 wt % Na
2O, 37.04 wt % Al
2O
3, and 48.51 wt % SiO
2.
Figure 2b shows the X-ray diffraction pattern of sericite, combined with the results of XRF analysis, and indicates that the content of sericite in the sample was higher than 93 wt %.
Dispersant MF was used as the depressant of graphite in the tests and was purchased from Shandong Xiya Chemical Industry Co., Ltd. in Jinan, China. Dodecylamine acetate salt was used as the collector, and a frother for the experiments was purchased from Sinopharm Chemical Reagent Co., Ltd. in Shanghai, China. All regents were of analytical grade and used without further purification.
2.2. X-ray Powder Diffraction Analysis
The crystalline structure and mineral composition of single mineral samples were determined by X-ray diffraction using a D8 Advance model X-ray powder diffractometer (Bruker Corporation, Stuttgart, Germany), with Cu Kα radiation (λ = 1.5406 Å) operated at a tube current of 40 mA and a voltage of 40 kV. Data of those samples were collected over 2θ values from 3° to 70° at a scan speed of 1°/min.
2.3. FTIR Spectroscopic Measurements and X-ray Photoelectron Spectroscopy (XPS)
For the infrared studies, 2 g of single mineral were suspended in 200 mL of reagent solutions for 25 min at pH 7. The pulp was then centrifuged and washed at least three times with distilled water and dried under natural conditions. The infrared spectra of minerals were recorded using a Model Nicolet IS-10 spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) through KBr disks in the range of 400–4000 cm−1. X-ray photoelectron spectroscopy (XPS) of samples were carried out on photoelectron spectrometer Multilab 2000 (Thermo VG Scientific, East Grinstead, UK)using an Al Kα X-ray source operated at 200 W with 20 eV pass energy. The vacuum pressure ranged from 10−9 to 10−8 Torr, and the takeoff angle was set at 90°.
2.4. Contact Angle Measurement
The assessments of wettability of single minerals were carried out on GBX MiniLab ILMS (GBX Scientific Instruments, Romans, France). The operating principle of the instrument is a washburn technique [
21]. Each value recorded was the average of five separate determinations.
2.5. Zeta Potential Measurement
A suspension solution containing 0.1 wt % graphite single mineral particles that had been grounded to −2.0 μm in an eccentric vibration mill were prepared in a 5 × 10−3 mol/dm3 NaCl solution and dispersed by MF for 10 min. A certain amount of the suspension solution was then taken for zeta potential measurement. The zeta potentials were measured using a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK) equipped with a rectangular electrophoresis cell. The suspension solution pH was adjusted to a desirable value by HCl or NaOH solutions. The conductivity and pH value of the suspension solution were monitored continuously during the measurement, and the environmental temperature was maintained at 25 °C. The results presented were the average of five independent measurements with a typical variation of ±4 mV.
2.6. Batch Flotation Tests of Single Mineral
The flotation test was performed in an XFD flotation cell whose volume was near 140 mL. First of all, the slurry was prepared by adding 2 g of graphite or sericite sample to 120-mL solutions. Second, the depressant MF was added and conditioned for 5 min at an agitation speed of 1000 rpm to allow MF adsorption. Following conditioning the collector, dodecylamine acetate salt was added and conditioned for 3 min at an agitation speed of 1400 rpm. The dosage of dodecylamine acetate salt was 200 mg/L. Flotation was then performed for 3 min. Finally, the floated and unfloated products were collected, and the flotation recovery was calculated based on the solid weight of products.
2.7. Batch Flotation Tests of Artificial Mixture
Sericite is one of the main gangue minerals in graphite ore. To investigate the effect of separating the sericite in graphite by reverse flotation, batch flotation tests of artificial mixture samples composed of sericite and graphite single mineral were carried out, and the mass ratio of graphite to sericite was 4:1. First of all, the slurry was prepared by adding 2.5 g of the artificial mixture sample to 120 mL of solution. Second, the depressant MF was added and conditioned for 5 min at an agitation speed of 1000 rpm to allow MF adsorption. Followed the collector dodecylamine, acetate salt was added and conditioned for 3 min at an agitation speed of 1400 rpm. The pulp pH was adjusted to a desirable pH value by HCl or NaOH solutions before the collector was added. Flotation was then performed for 3 min. Finally, the floated and unfloated products were collected. The recovery of graphite and sericite in floated products can be calculated by Equations (1) and (2).
Rs—the recovery of sericite in floated product (%);
Rg—the recovery of graphite in floated product (%);
m1—the weight of unfloated product (g);
m2—the ash weight of unfloated product (g);
As—the ash content of sericite single mineral (%);
Ag—the ash content of graphite single mineral (%).
3. Results and Discussion
3.1. The Wettability of Graphite and Sericite
Froth flotation is a process of separating valuable minerals from gangues by exploiting differences in their surface wettability. To assess the wettability of sericite and graphite, the contact angles of single minerals were measured. The effect of MF concentration on the wettability of sericite and graphite powder is shown in
Figure 3.
The initial contact angle of sericite powder was 20.0°. This indicated that the surface of sericite was hydrophilic, which agreed with the result of Gao [
22]. The initial contact angle of graphite powder was 60.1°, suggesting that it was a hydrophobic mineral.
From
Figure 3, when the MF concentration was increased from 0 to 2000 mg/L, the contact angle of graphite decreased from 60.1° to 38.9°, suggesting that graphite became moderately hydrophilic. Compared with graphite powder, the contact angle of hydrophilic sericite powder decreased from 20.0° to 9.5° slightly.
3.2. Batch Flotation of Single Mineral
To investigate the depressing effect of MF concentration on sericite and graphite, batch tests of single minerals were conducted, and the test results are shown in
Figure 4.
Sericite recovery decreased sharply with an increasing MF concentration from 50 to 800 mg/L, and the sericite recovery decreased from 91.6% to 18.2%. The sericite recovery continuously decreased slightly to 10.99% as the MF concentration further increased to 2000 mg/L.
The graphite recovery sharply decreased from 78.2% to 11.0% with an increasing MF concentration from 50 to 200 mg/L and then leveled off above 200 mg/L. As the MF concentration further increased to 2000 mg/L, the recovery of graphite changed little and was approximately 10%.
The foregoing results showed that the recovery of both graphite and sericite decreased as MF concentration increased. However, the recovery of graphite decreased more sharply than that of sericite. When the concentrate of MF increased to 200 mg/L, the sericite recovery decreased to 81.3%, whereas the recovery of graphite had already decreased to 11.0%. With a continued increase in the MF concentration, the graphite recovery remained at approximately 10%; however, the recovery of sericite continuously decreased. The difference in recovery between graphite and sericite was due to the difference in floatability between graphite and sericite after interaction with MF. This difference creates opportunities for separating the sericite from graphite.
3.3. Batch Flotation of Artificial Mixtures
To determine the effect of MF concentration and pulp pH value on the separation of sericite and graphite, batch flotation tests of artificial mixtures were carried out.
3.3.1. Effect of MF Concentration
From the test results of single minerals, sericite and graphite were both depressed by MF. However, the effect of the depression was affected by the concentration of MF. The recovery of graphite and sericite showed a general declining trend as MF concentration increased. To fully separate sericite from graphite, the difference in recovery between graphite and sericite should be sufficiently large. When the MF concentration was 50–400 mg/L, the recovery of sericite was 62.3%–91.6%, whereas the recovery of graphite was 11.5%–78.2%; in this range of MF concentration, the difference in recovery between graphite and sericite was significant. Hence, tests were conducted at different MF concentrations ranging from 50 to 600 mg/L with a pulp pH of 7. The results were as shown in
Figure 5.
It can be seen from
Figure 5 that the recovery of both graphite and sericite decreased as MF concentration increased, whereas the difference in the recovery between graphite and sericite increased firstly and then began to decrease above 250 mg/L. As MF concentration increased, graphite recovery showed a sharper decline than did sericite. When the MF concentration increased to 250 mg/L, the difference in recovery between graphite and sericite reached a maximum difference of 70.4%. Simultaneously, the recovery of graphite and sericite was 10.6% and 81.0%, respectively. When the concentration of MF was further increased, the graphite recovery did not change, and the sericite recovery continued to decrease to 31.0%. The difference in recovery between graphite and sericite began to decrease with a further increase of MF concentration above 250 mg/L. Hence, to separate sericite from graphite more effectively, an MF concentration of 250 mg/L was selected as optimum for the separation.
3.3.2. Effect of pH
The pulp pH affected the surface potential of mineral particle surface, thereby affecting the adsorption of depressant MF on the surface of mineral particles. Hence, the depressing effect on the mineral is affected by the pulp pH. The effects of pulp pH on the separation of sericite from graphite were studied by artificial mixture flotation tests. The pulp pH varied from 2 to 12 with a 250 mg/L MF concentration. The tests results were as shown in
Figure 6.
From
Figure 6, the graphite recovery decreased sharply with increasing pulp pH value from 2 to 8 and then increased strongly above pH 8. The sericite recovery increased with increasing pulp pH from 2 to 8 and then varied slightly above pH 8. The difference in recovery between graphite and sericite increased sharply with an increasing pulp pH value from 2 to 8 and then decreased slightly above pH 8. The separation of sericite from graphite was optimal at a pulp pH of 8 with the recovery of graphite and sericite of 11.3% and 79.0%, respectively.
3.4. FTIR Spectra of Minerals Before and After Interaction with MF
To study the mechanism of MF in the flotation separation of sericite from graphite, the IR (infrared) spectra of these two minerals before and after interacting with reagents were measured, and the results are shown in
Figure 7 and
Figure 8.
After interaction with MF, the stretching bands of –CH
3 and –CH
2 groups in MF molecules appeared at approximately 2922 and 2852 cm
−1 on sericite surfaces and 2926 and 2854 cm
−1 on graphite surfaces, respectively [
23]. The stretching bands of =CH
2– groups on naphthalene nucleus in MF molecules appeared at approximately 3055 cm
−1 on sericite surfaces and 3053 cm
−1 on graphite surfaces, respectively. New bands appeared at 895 and 833 cm
−1 on sericite surfaces and at 831 and 752 cm
−1 on graphite surfaces due to the bending vibrations of solitary hydrogen groups on the naphthalene nucleus in MF molecules [
24]. Moreover, the vibration bands of the naphthalene nucleus skeleton in MF molecules appeared at approximately 1599 cm
−1 on sericite surfaces and 1605 cm
−1 on graphite surfaces. The bands that appeared at 1184, 1136 and 1035 cm
−1 on the graphite surfaces were the adsorption bands of –SO
3 groups in MF molecules [
24]. The results of FTIR spectra demonstrated that, after MF treatment, no new adsorption peaks appeared on sericite and graphite surfaces except for MF’s adsorption bands, which implied that MF might adsorb onto the two minerals without the formation of new complexes.
As an alternative to FTIR, the XPS analysis of samples was carried out. The XPS survey spectra of graphite and sericite after interaction with MF was shown in
Figure 9. It can be seen from
Table 1 that S and Na elements appear on the surface of graphite and sericite. Meanwhile, the changes in electron binding energy (ΔE
B) of C, O and S elements after treatment with MF were relatively small, which is less than the error value of experiment equipment. That means the interaction between MF and graphite or sericite was physical adsorption without the formation of new complexes [
25]. XPS spectra agree well with FTIR results and confirm physical adsorption occurred on graphite and sericite surface.
The mechanism of graphite reaction with MF is shown in
Figure 10. The figure illustrates that the hydrophobic naphthalene core could adsorb onto the surface of graphite particles preferentially with the sulfonate radical pointing to the water. The surface tension of particles decreases due to the association between the sulfonate radical of the MF molecule and the hydroxyl in the water [
26], so hydrophobic graphite particles become hydrophilic. The contact angle of graphite decreased after the reaction with MF. In flotation, the depressing effect of MF on graphite was achieved by transforming the surface of graphite particles from hydrophobic to hydrophilic, and then the graphite cannot float. The adsorption of MF onto sericite surface also improves its hydrophilicity, and the pre-adsorption of MF interferes with the adsorption of dodecylamine onto sericite surface [
27]. This is the mechanism of MF’s depressing effect on sericite in the flotation.
In flotation, the sericite and graphite did not have sufficient adsorption of MF on their surfaces when the MF concentration was comparatively small. Hence, the recovery of graphite and sericite did not have a significant decline. As MF concentration increased, due to the sufficient adsorption and selective adsorption of MF on the surface, graphite was intensely depressed and the graphite recovery sharply decreased. Meanwhile, the adsorption of MF on the surface was not adequate enough to lead to the intensely depressing effect on sericite. Therefore, the recovery of sericite was much higher than that of graphite. With the further increase in MF concentration, sericite floated worse due to interference with the adsorption of dodecylamine from the sufficient adsorption of MF on the surface of sericite. Hence, the recovery of sericite similarly had a sharp decrease.
3.5. Zeta Potential Measurement
Zeta potentials of minerals before and after interaction with MF are shown in
Figure 11 and
Figure 12.
The surface of graphite and sericite varied from positively charged to negatively charged as pH value increased. The isoelectric points (IEPs) corresponded to pH values of approximately pH 2.0 for sericite and pH 4.9 for graphite. The surface of graphite was slightly positive due to the adsorption of H
+, OH
−, or some other ions between pH 2 and 5. The zeta potential of graphite and sericite showed a pronounced shift towards more negative zeta potentials in the presence of MF, indicating that the anionic Gemini molecules adsorbed onto graphite and sericite [
28]. Moreover, after interacting with MF, the zeta potential of graphite was much more negative than that of sericite, demonstrating that MF was preferred to adsorb on graphite surfaces. The FTIR results implied that MF might adsorb onto the two minerals without the formation of new complexes. The negatively charged MF adsorb on the negatively charged graphite byhydrophobic/van der Waals interaction between the benzene rings of MF and the graphite hydrocarbon rings at pH > IEP [
29]. However, the negatively charged MF adsorb on the negatively charged sericite by hydrogen bond at pH > IEP [
30,
31]. Obviously, the hydrophobic/van der Waals interaction energy between the nonpolar benzene rings of MF and the nonpolar graphite hydrocarbon rings are much stronger than the hydrogen bond energy between MF and sericite. Therefore, the MF appears to be preferentially selective to the graphite surface relative to the sericite surface.This can explain why the recovery of graphite decreased more sharply than did sericite as MF concentration increased.
4. Conclusions
Batch flotation experiments of single mineral and artificial mixtures of flaky graphite–sericite and contact angle measurements were conducted to verify the feasibility of reverse flotation separation of sericite from graphite by using a Gemini surfactant: MF. FTIR spectra and zeta potential measurements were conducted to confirm the mechanism of MF reaction with sericite and graphite.
The results of contact angle measurements indicate that MF can improve the hydrophilicity of graphite and sericite. The flotation results of single minerals indicated that MF displayed a depression of both graphite and sericite, but the recovery of graphite declined more sharply than sericite as MF concentration increased. The results of artificially mixed mineral flotation demonstrated the preferential flotation separation of sericite from graphite with a pulp pH at 8 with an MF concentration of 250 mg/L; the recovery rates of sericite and graphite were 89.7% and 11.3%, respectively. Sericite can be separated from graphite by reverse flotation separation.
The interaction of MF with graphite and sericite surfaces was dominantly hydrophobic/van der Waals interaction and hydrogen bond, which was confirmed by FTIR spectra, X-ray photoelectron spectroscopy. MF was preferred to adsorb onto the surface of graphite, decreasing its zeta potentials and improving its hydrophilicity relative to sericite. This was confirmed by zeta potential measurement.
The reverse flotation separation of sericite from graphite can dramatically reduce the infusion of sericite in the flotation concentrate. However, in this paper, all test samples were single minerals. Tests should be conducted with practical ore samples to evaluate the effectiveness of the MF reagent.