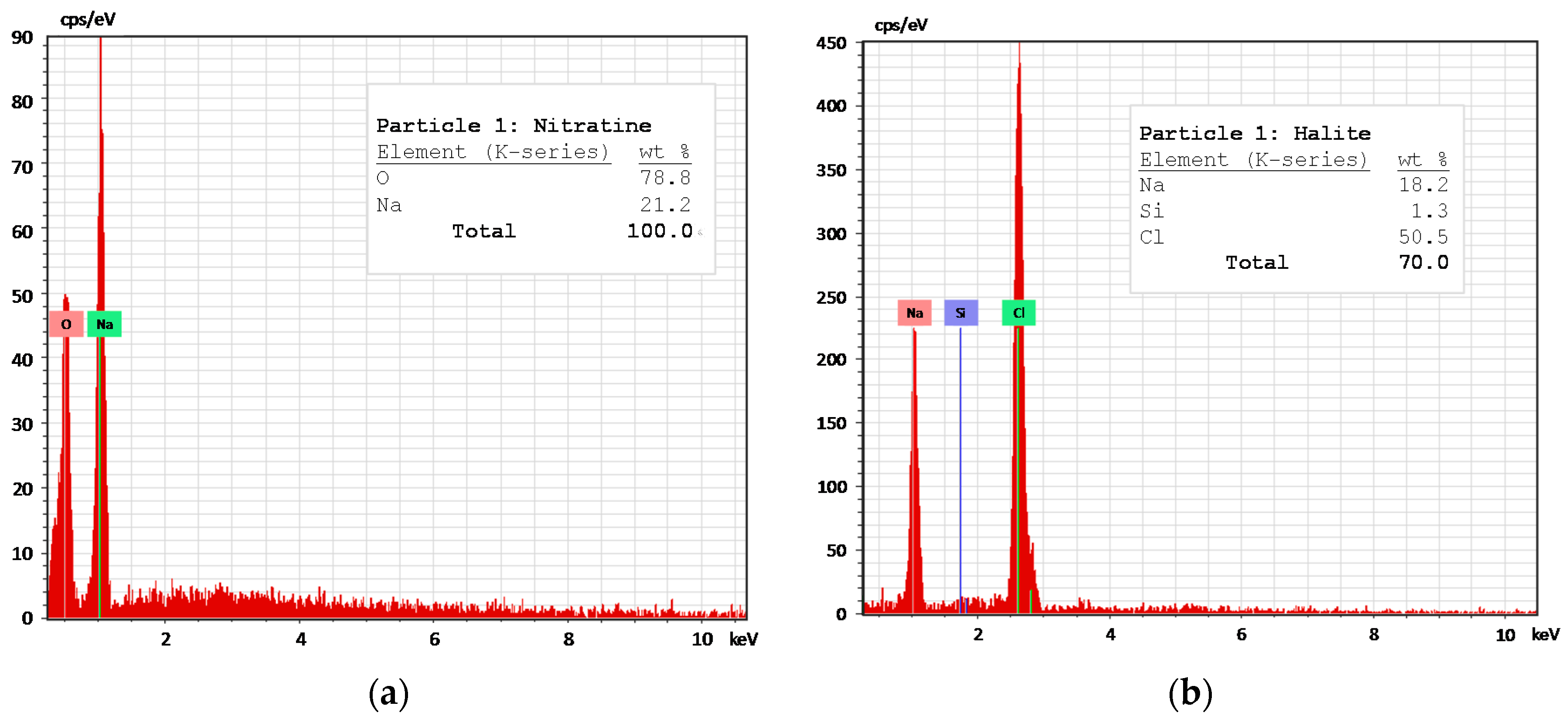
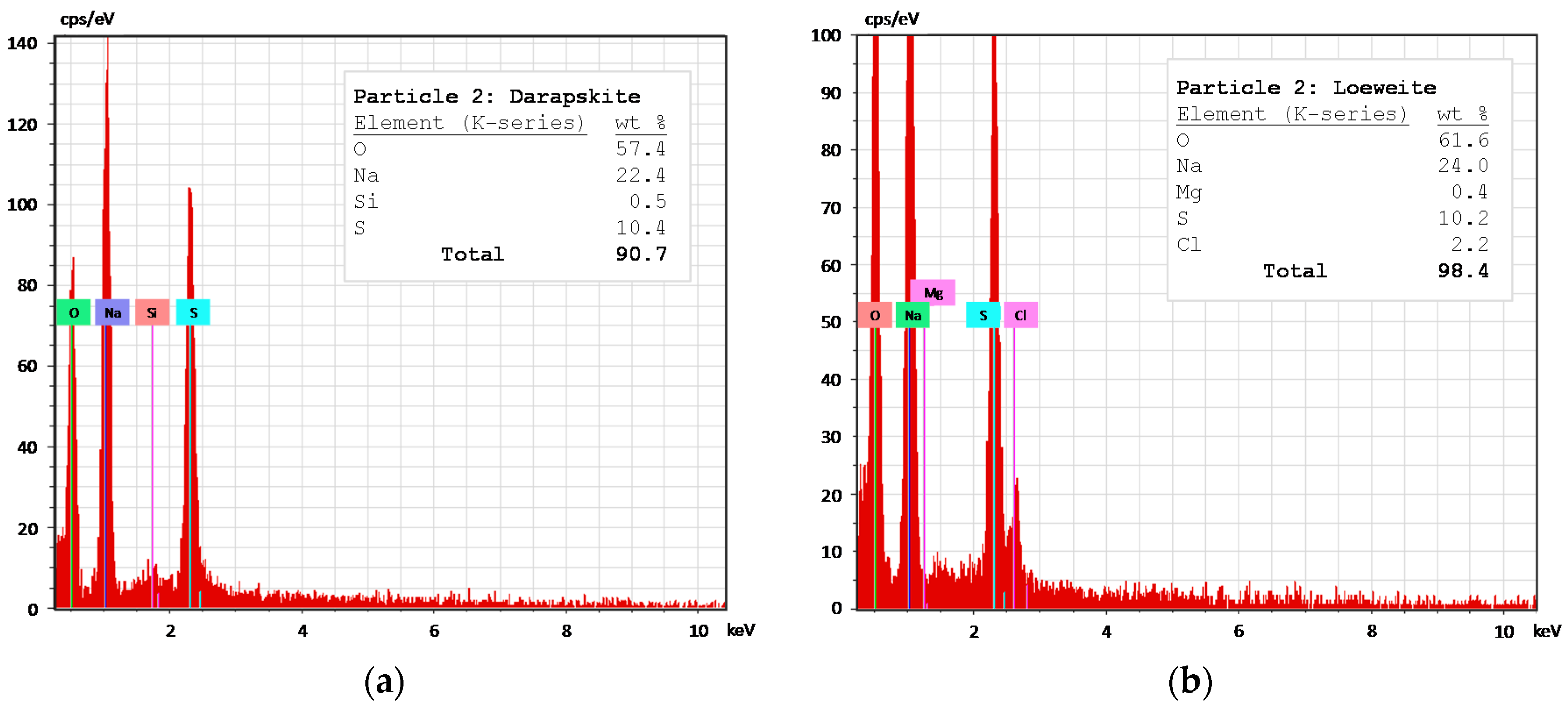
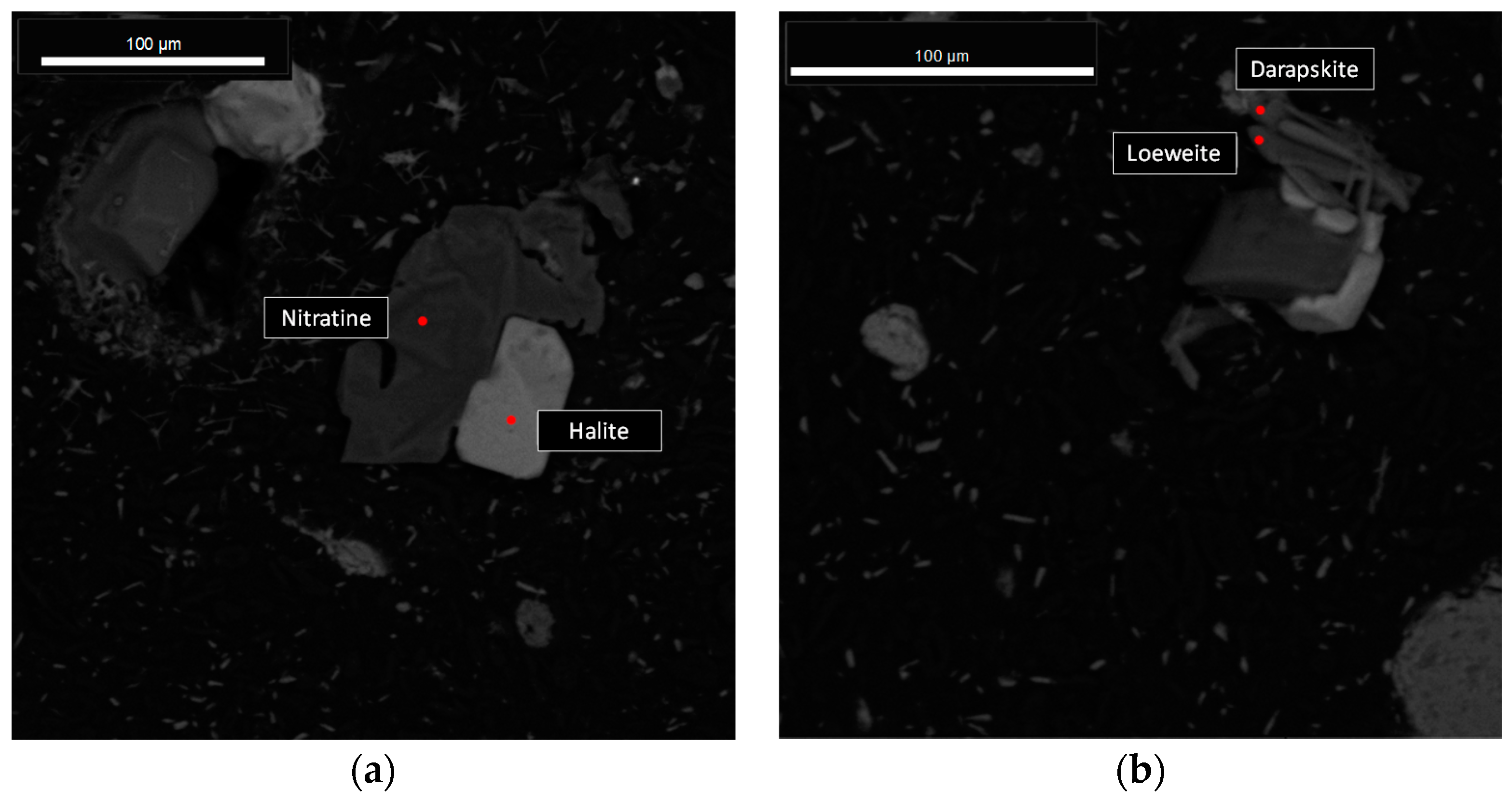
The results were arranged in three subsections: (i) column leaching results, which addresses the dissolution behavior of the most soluble species and contributes with experimental data for the the next subsection; and (ii) model simulation and verification, where the experimental outlet concentrations are compared with the simulations, obtaining fitted kinetic parameters. Finally, (iii) comparison between the use of particle size distribution (PSD) or some average particle radius in the model performance.
3.1. Column Leaching Experiment
The outlet concentration of different soluble species was followed during the leaching period, which was represented as the cumulative volume of percolate that was received at the bottom of the column during a given time. This interval was initially 12 h and later was increased to 24 h. However, the number of samples analyzed was different for the different species. The larger number of analyzed samples was for Nitrate and Magnesium; for the other species, the analysis was scarcer.
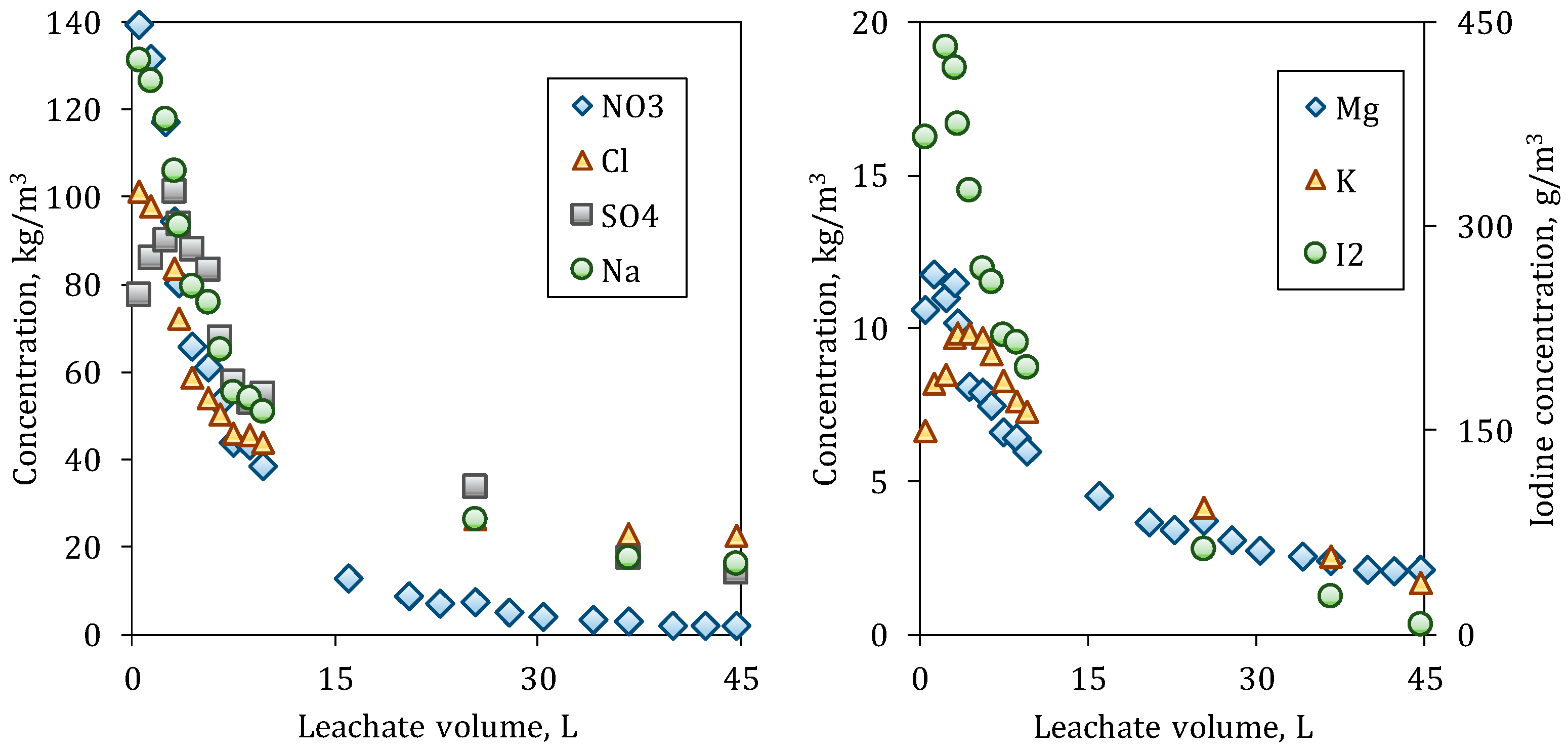
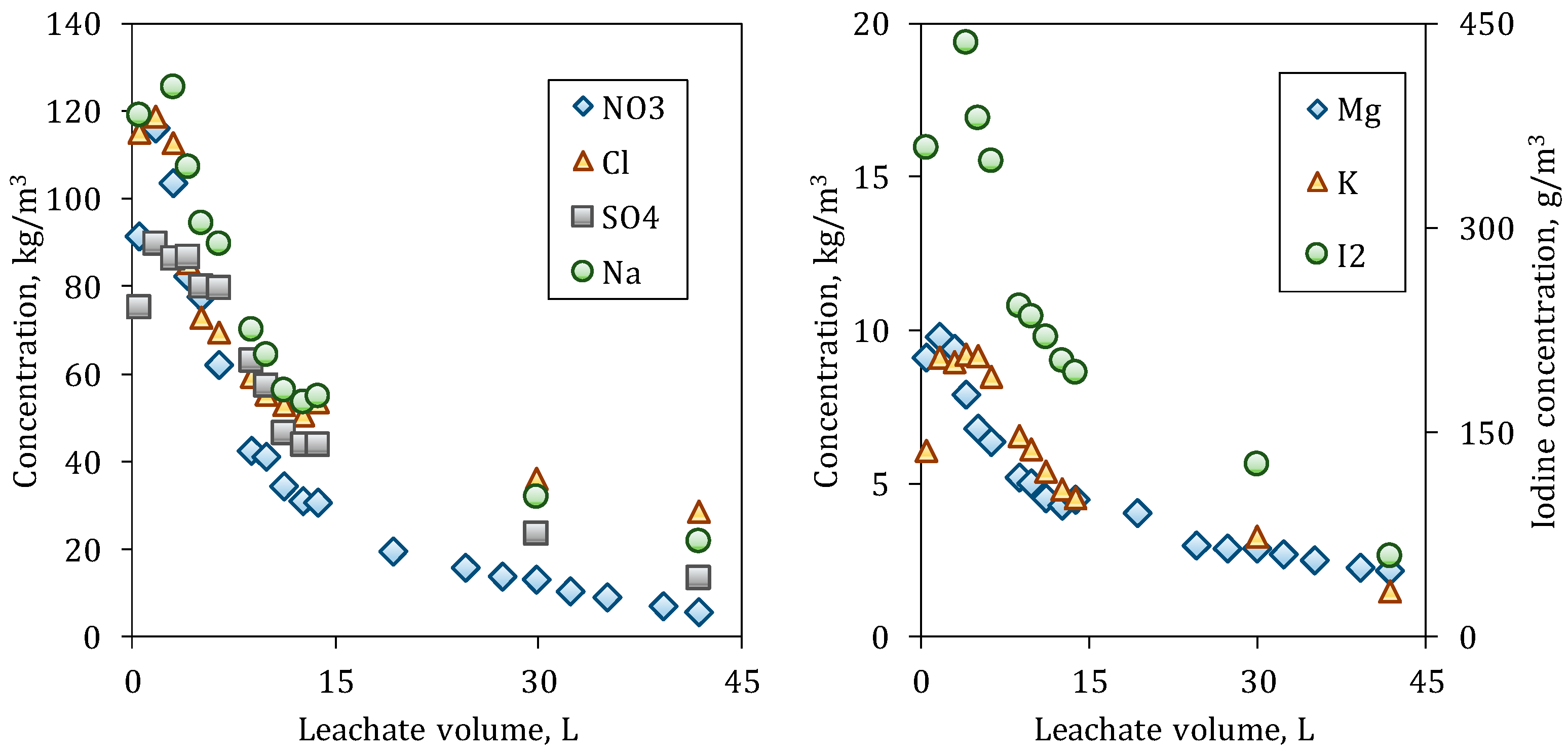
As can be observed in
Figure 5 and
Figure 6, the species with the largest dissolution are Nitrate and Sodium. The initial outlet concentration for all the species from the C80 column is less than those values from C20. During the dissolution of the species at C20, a more abrupt drop in concentrations is observed than at C80. This occurs because of the particle size distribution of the bed, since C20 has initially a larger particle surface caused by the greater number of fine particles, which are dissolved/depleted at short times.
Most species in C20 reach the levels of the seawater (leachant) at the end of the experiment, while, in C80, the ion concentrations are still decreasing, even Nitrate, which is the most soluble species. For Sulfate, the outlet concentrations are similar at the beginning in both columns, since it is less soluble and saturation may exist in the interior of the columns; therefore, the particle size does have a smaller influence.
The outlet concentrations for Potassium and Magnesium are close along leaching and at the beginning around the 10 kg/m
3. Iodine, which is present as Iodate in the caliche ore is in low quantity, and the
Figure 5 and
Figure 6 represent it in another scale.
3.2. Model Verification
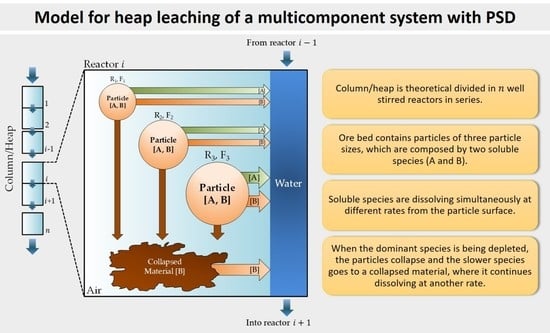
To obtain simulated data from the model, the bed was divided into 11 small and equal reactors, which are initially loaded with the same mass of caliche and leached with seawater, whose composition is shown in
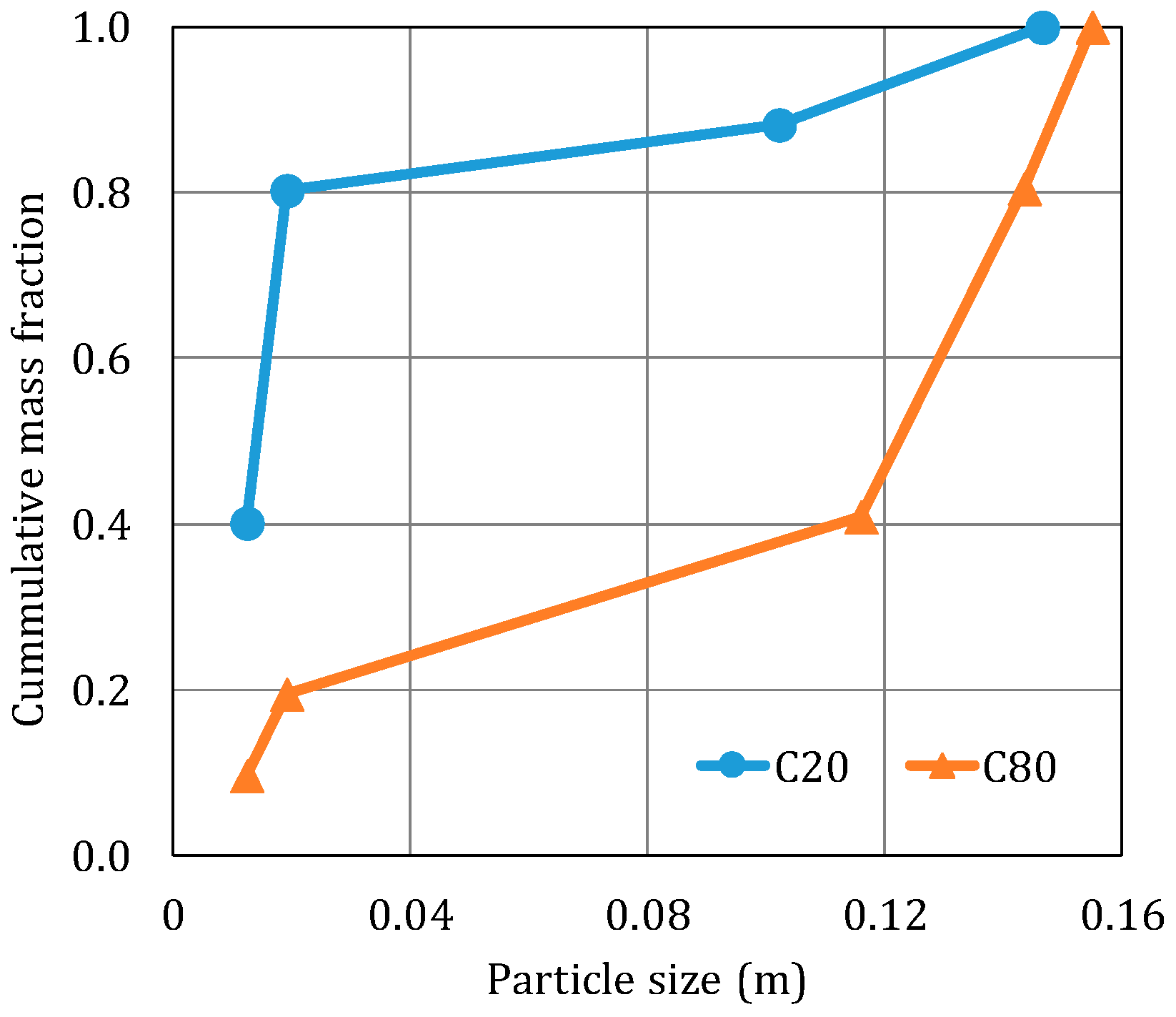
Table 2. In order to simulate the leaching of the A- and the B-species in a wide scenario, the granulometries (particle sizes and mass fractions) were the same as those used in the experiments (
Figure 3). Other input parameters, such as the irrigation rate, initial column height and solubilities, used in the simulations are listed in
Table 4.
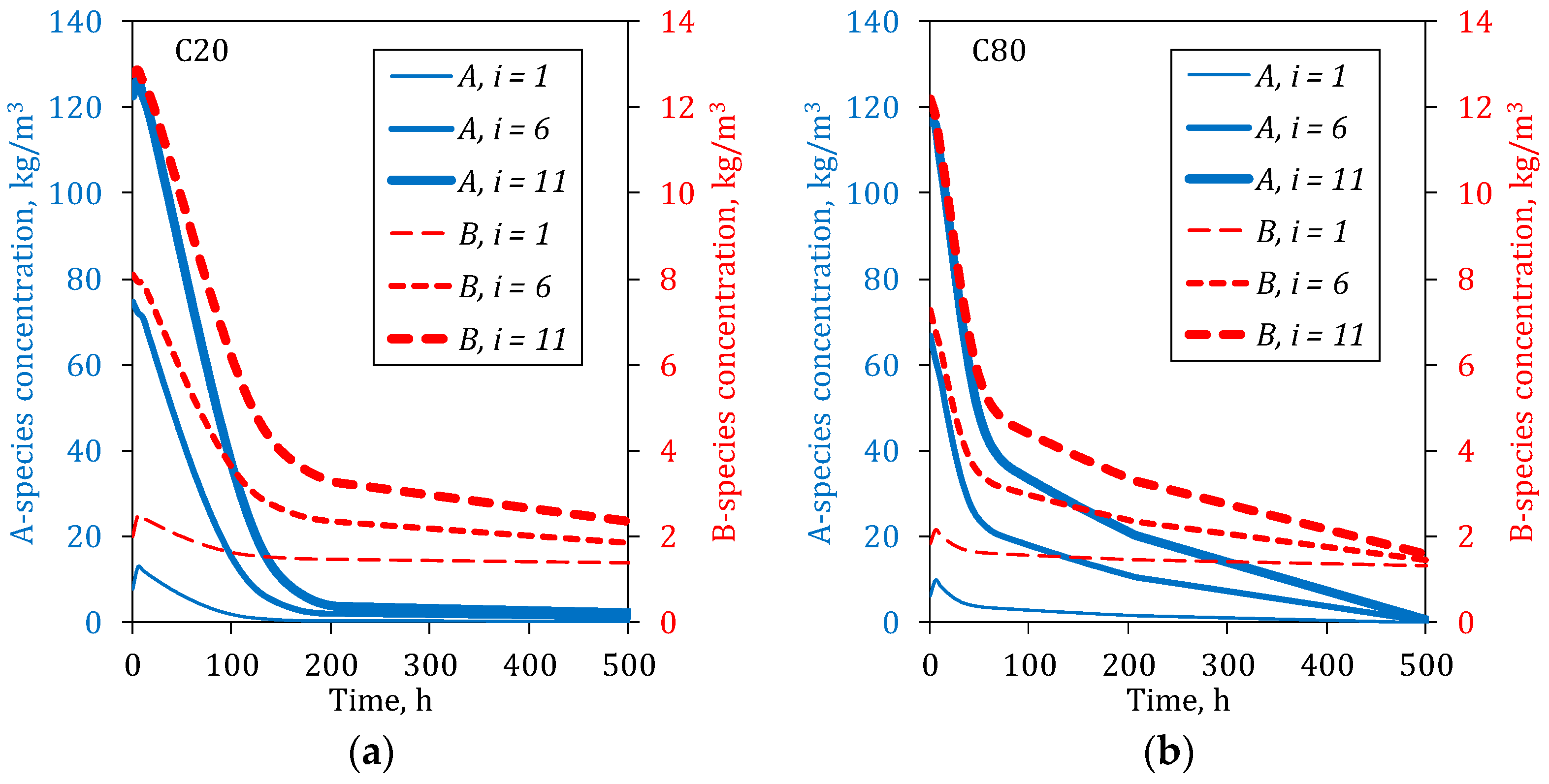
The outlet concentrations of A- and B-species at three different depths into the column, , were considered, where the output of the eleventh reactor corresponds to the bottom of the column. In real situations, the viability of tracking species at different depths of the heap is difficult and usually depends on the use of high-tech devices. However, by using a validated expression, it is easier to give an idea about the leaching progress, considering also that the particle radius of each particle type, the column height and the mass of collapsed material can be also theoretically followed. The model is a useful tool to understand the processes that are taking place within the heap.
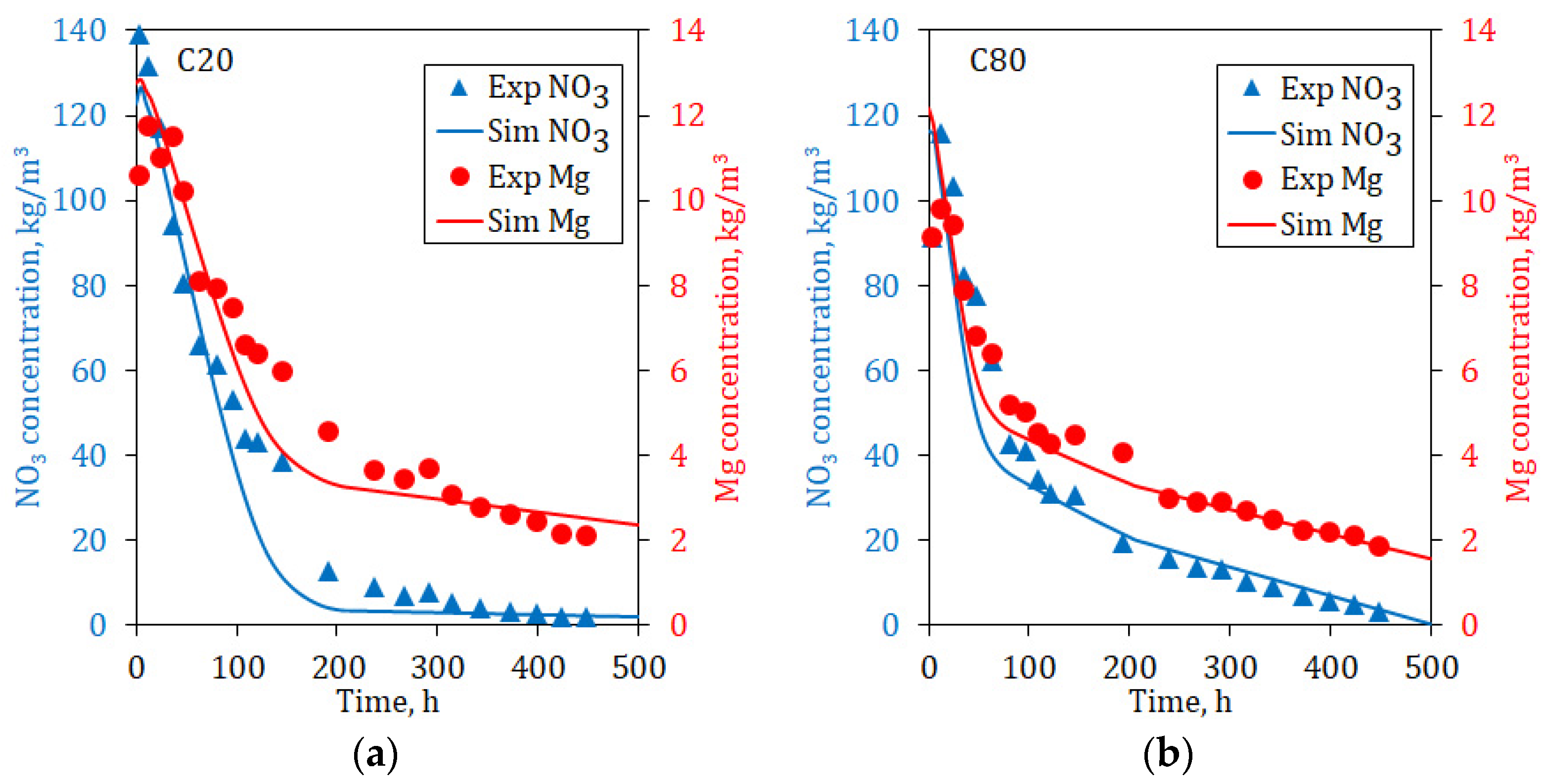
As shown in
Figure 7, if the interior of the column is considered, the concentrations of both species at the top are higher because the dissolution progresses from top to bottom, limited by the dissolution rate of each species. The solubility of the species is not by itself important for Nitrate and Magnesium, due to that both species are highly soluble. In the first reactor, the depletion grade is at the maximum, and increasingly less important in the subsequent reactors; the differences between the A- and B-species are notorious.
Due to the dissolution of the A-species being carried out exclusively on the particle surface, its concentration at the bottom of the column is high in the first part of the leaching, since the A material is always available to be leached. The concentration decreases relatively fast given its high solubility, especially in the upper part of the column, where the levels fall at the seawater concentration before 150 h for a fine material bed and 450 h for a coarser bed. In the other way, the dissolution behavior of the B-species is notoriously different than for the A-species, its dissolution rate being slower than A.
Although the granulometry apparently has an important effect on the dissolution behavior, it is different for each species. Thus, the dissolution of the B species is less sensitive to the particle size than the A-species; this may be attributed to the fact that B-species is dissolved simultaneously from the particle but also from the collapsed material.
The model considers three adjustable parameters that are related to the dissolution kinetics of each species from the particle surface and from collapsed material (in case of B-species); these kinetic parameters are: , and . In the simulations, values of the , and were fitted by the least squares method, minimizing the difference between the experimental concentrations observed for Nitrate and Magnesium in the samples of each column and the simulated concentrations obtained by the integration of Equations (2) and (3). The abundance of the species in the loaded caliche and a factor of scale that is related to the liquid transport into the columns were also considered.
Table 5 shows the kinetic constants adjusted to the experimental data of this work, which are related to properties of the fluid and the solid such as viscosity, diffusivity, irrigation rate and particle size. Comparing the values of the C20 column with those obtained in [
23], which are operated under comparable conditions and similar granulometry, the orders of magnitude resulted similarly, despite the fact that the reference model did not include the PSD component. Differences between the values are expected, due to the heterogeneous characteristics of the mineral and the experimental fluctuations that happen in pilot experiments.
On the other hand, by increasing the mean particle size of 1.4 cm for C20 to 12.2 cm in C80, it can be seen in the fitting of the kinetic constants that both and increase almost three times, which would indirectly be realized for the abundance of the species in the particles of the mineral. On the other hand, does not behave in the same way, decreasing 10 times for the column loaded with more coarse particles. This difference can be attributed to a compensatory effect of increasing the and values, which are the most influential constants of the model, so, if the rate of dissolution of the species increases from the particle, less material would be available to be leached from the collapsed material.
To validate the model and obtain the fitted value of the kinetic parameters, the general species A- and B- were assigned to Nitrate and Magnesium, respectively. The simulated outlet concentrations from the reactors for both ions were then compared with their experimental results at the bottom column. In general, the fitting process was acceptable, and only small differences were observed. In the case of Nitrate, the concentration drop is well captured at the initial times (
Figure 8a); however, for Magnesium, the fitting is less adequate (
Figure 8b). It can be explained by the multiple mineralogical sources of Magnesium, which have dissimilar solubilities. The model cannot address this situation since it supposes a single source of each ion. Moreover, the dissolution of B-species is determined by two sources: from the particle surface and from the collapsed material.
Due to the central focus of the model being the determination of concentrations of soluble species, the assumptions for the calculation of the column height were very simplified (Equation (5)). For this, its approximation by the model could only be observed at the end of the test and not during its course. One of the empirical observations that makes the monitoring of height in the column tests difficult is the non-traceable variation of the bed porosity, since voids formed upon dissolution of the particles are not immediately filled by the collapsed material or by smaller particles, due to a temporary entrapment effect on the bed; a large void is present at the initial times. On the other hand, both and cannot be measured experimentally, and, due to an actual leaching, the particles do not keep their structures and collapse when a part of the soluble species has been depleted. No particles can be clearly observed after a moderate progress of the leaching. Therefore, the quantification of the collapsed material is impossible, since no differentiation between early-destroyed particles and collapsed material is feasible to achieve. For this reason, we highlighted that the model requires an idealization of the system.
3.3. Comparison of Actual PSD and Average Radii
In this section, we try to find a unique average radius that could adequately represent the dissolution process. Two different average radii were tested: (a) the Sauter radius, based on the surface area of the particles (R32), which increases the contribution of the small particles since they have a high specific area and (b) the De Broukere radius, based on the volume of the particles (R43), which increases the contribution of the large particles since they have a large volume. The values used in these simulations are shown in
Table 6.
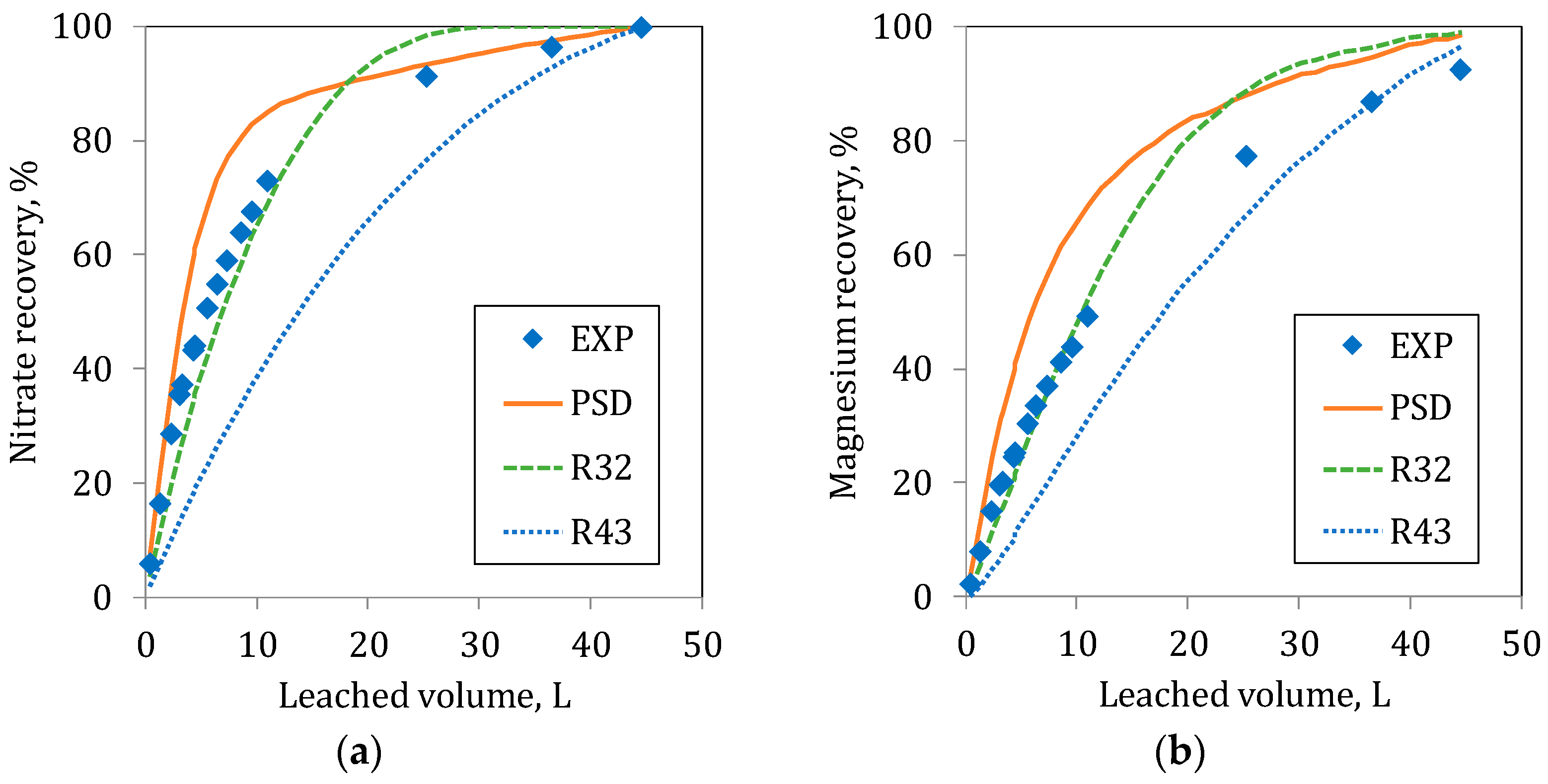
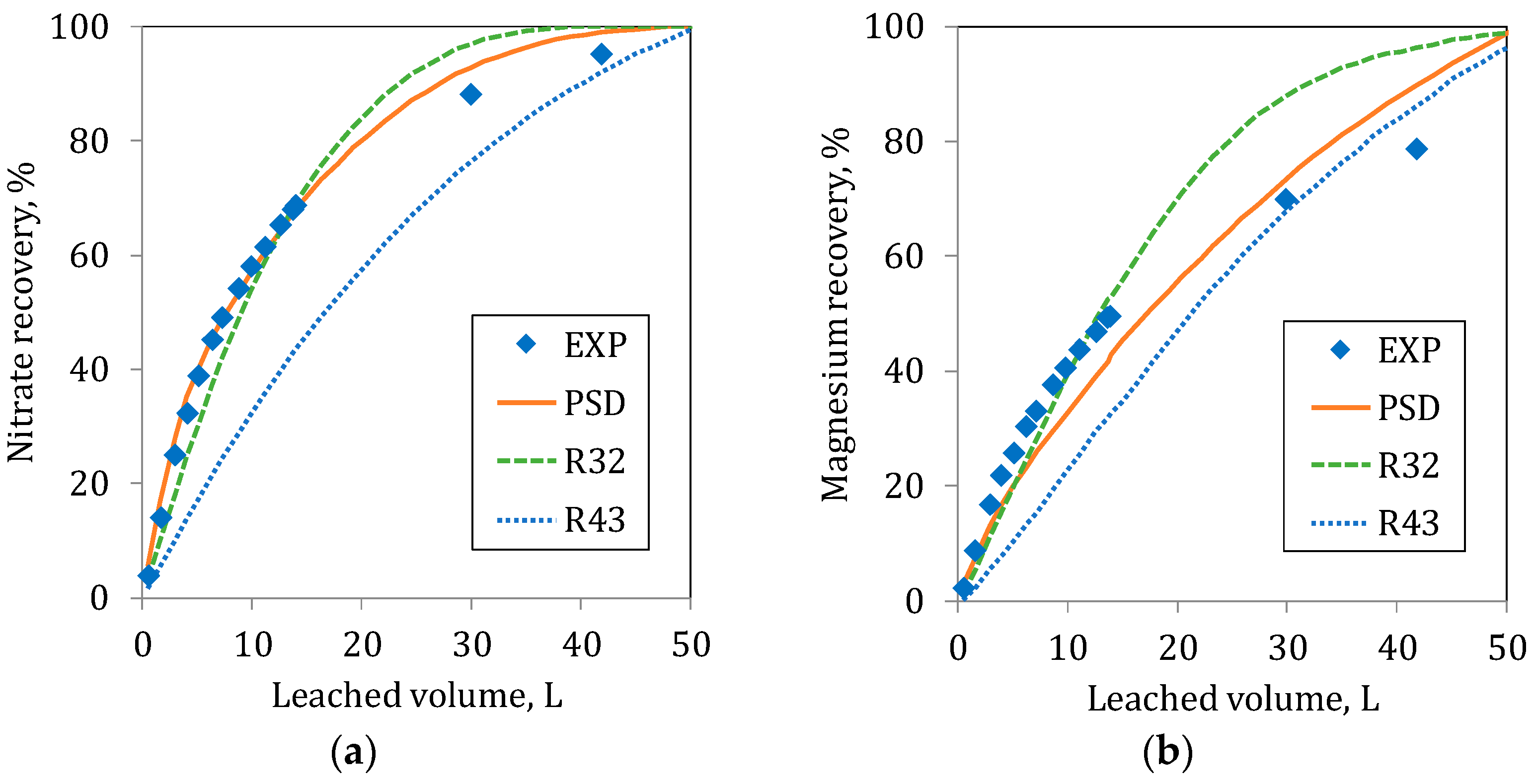
At the initial part of the leaching in C20 and C80, the estimated recoveries with the Sauter radius (R32) represent the experimental responses for both ions (
Figure 9 and
Figure 10) accurately. Moreover, in C80 in the initial step of the leaching of Nitrate, the simulations using the PSD and the R32 were similar to the experimental values. For Magnesium, the agreement between experimental data and R32 prediction is good. In the long term, a rather good agreement is observed between R43 and the experimental results. In general terms, the use of the Sauter radius, (R32) is adequate for the estimation of the recoveries at the initial period of the leaching process. This is due to the fact that at the initial time, the dissolution is controlled by the particle surface; with time, the small particle despair and the process starts to be controlled by the particle volume, i.e., represented by De Broukere mean radius (R43).
The results show that, at the beginning, the leaching follows the paths determined by the small particles (the curve R32), but when the leaching progresses and the importance of the fine particles decreases due to the diminution of its surface area, leaching is controlled by the larger particles when the smaller particles are depleted. The recovery at long periods goes closer to the path determined by the largest particle (the curve R43).
The effect of the particle radius is easily observed in the Nitrate recovery, where the leaching occurs exclusively on the particle surface. However, as mentioned before, for the leaching of Magnesium, the effect is more difficult to observe, since it depends on a double kinetic (particles and collapsed material).
As observed in the figures, none of the mean particle radii can represent the overall process for both ions adequately. However, the better responses to the experimental results are obtained when the PSD curve compared. The PSD describes in detail the particle size distribution really found in the respective columns.