Hydrometallurgical Process and Kinetics of Leaching Manganese from Semi-Oxidized Manganese Ores with Sucrose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Procedure
2.3. Analytical Methods
2.4. Ore Characterization
3. Results and Discussion
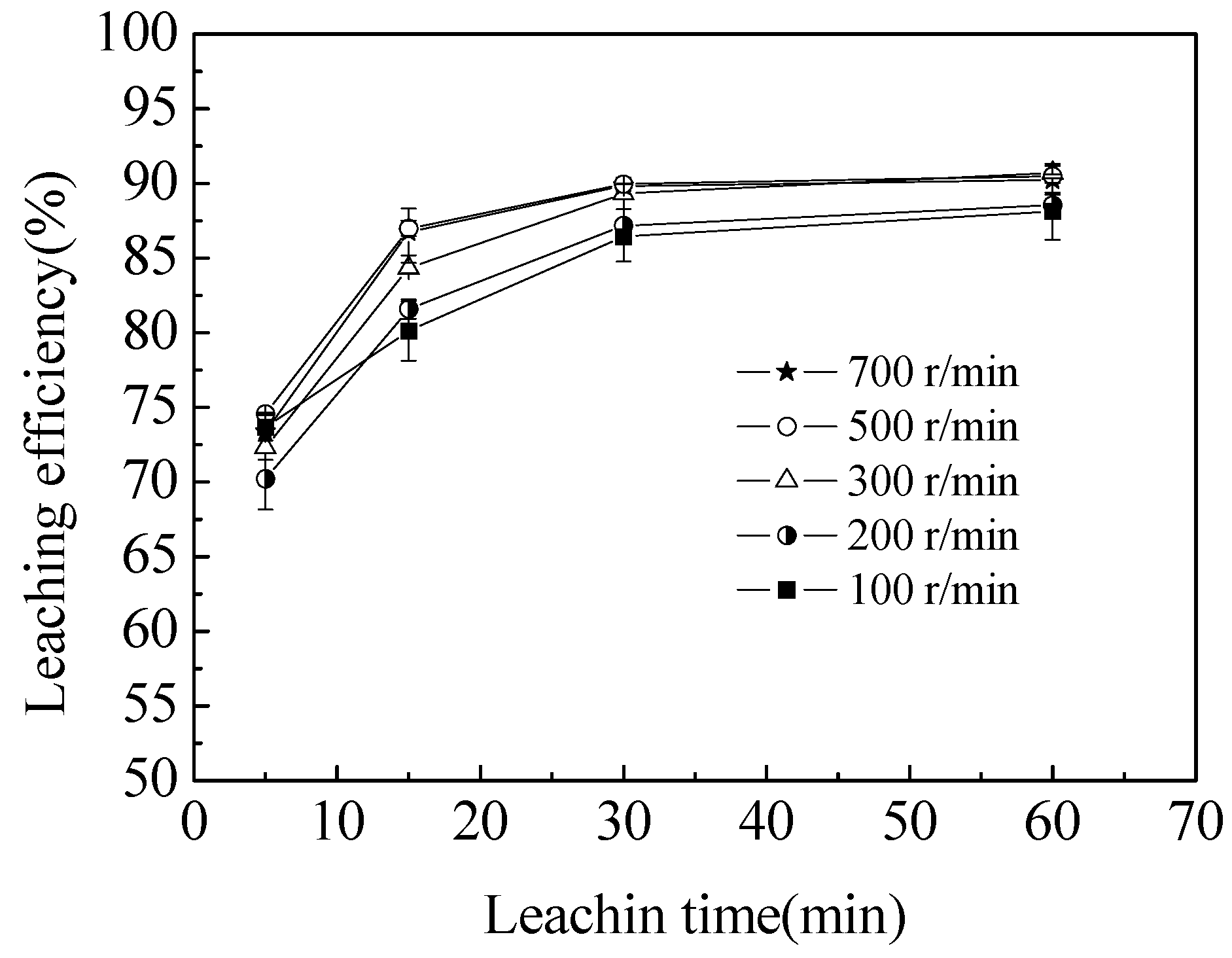
3.1. Effect of Agitation Speed
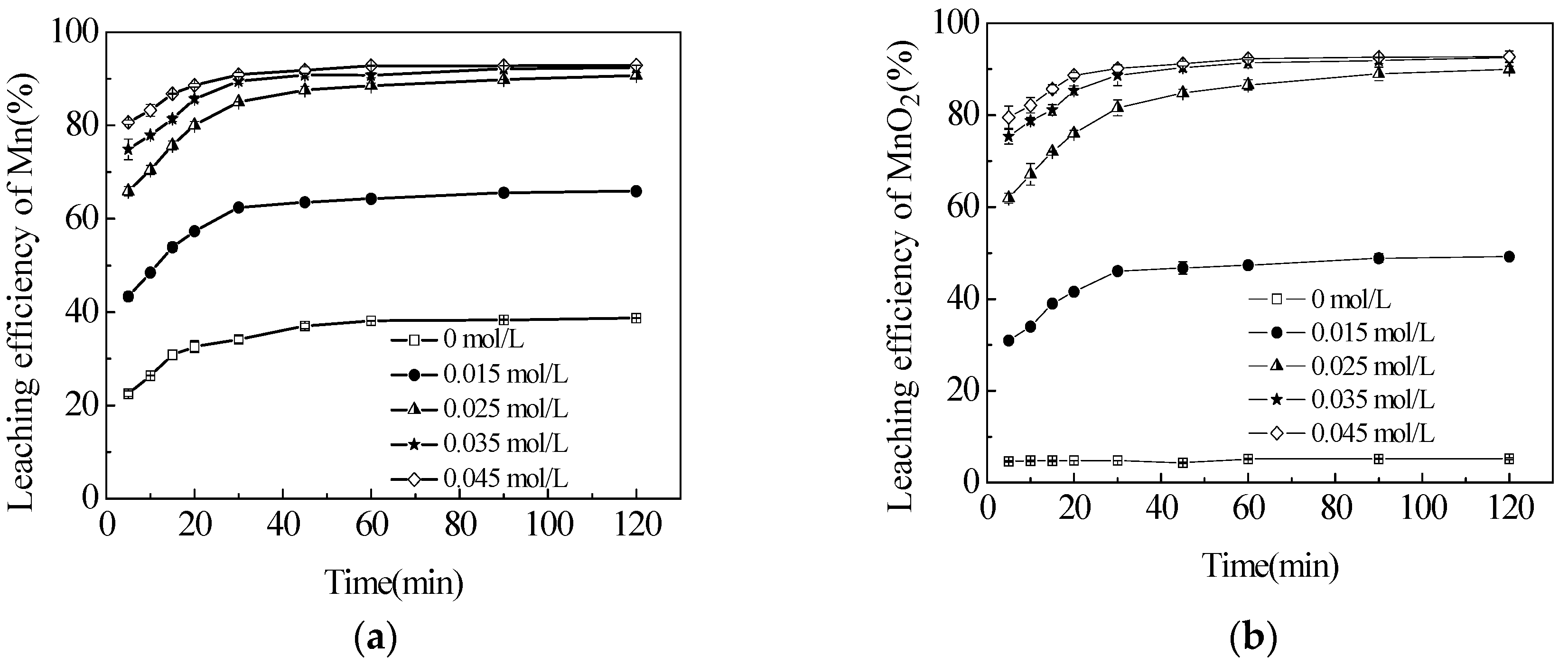
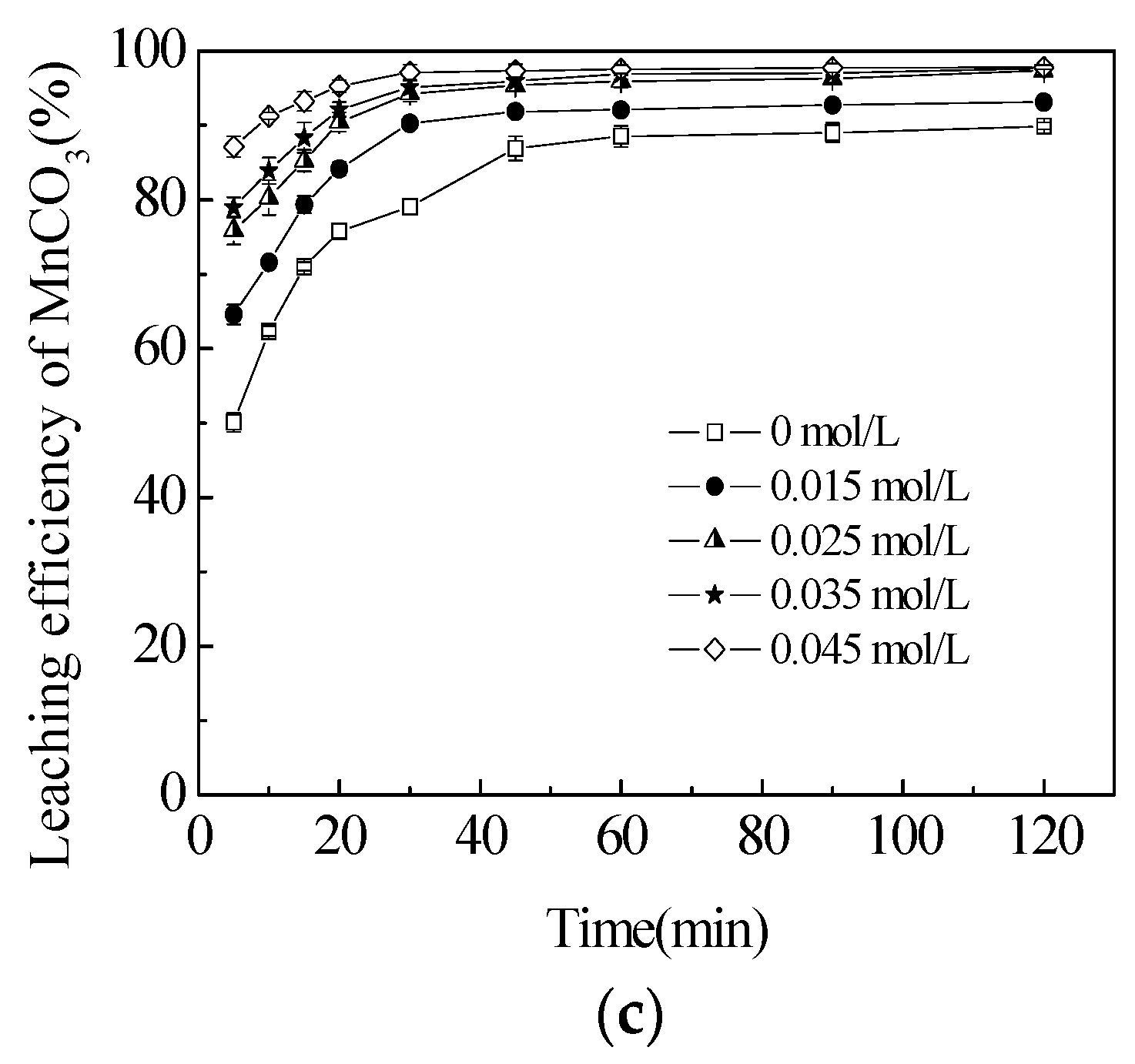
3.2. Effect of Sucrose Concentration on the Leaching Efficiencies of TMn, MnCO3 and MnO2
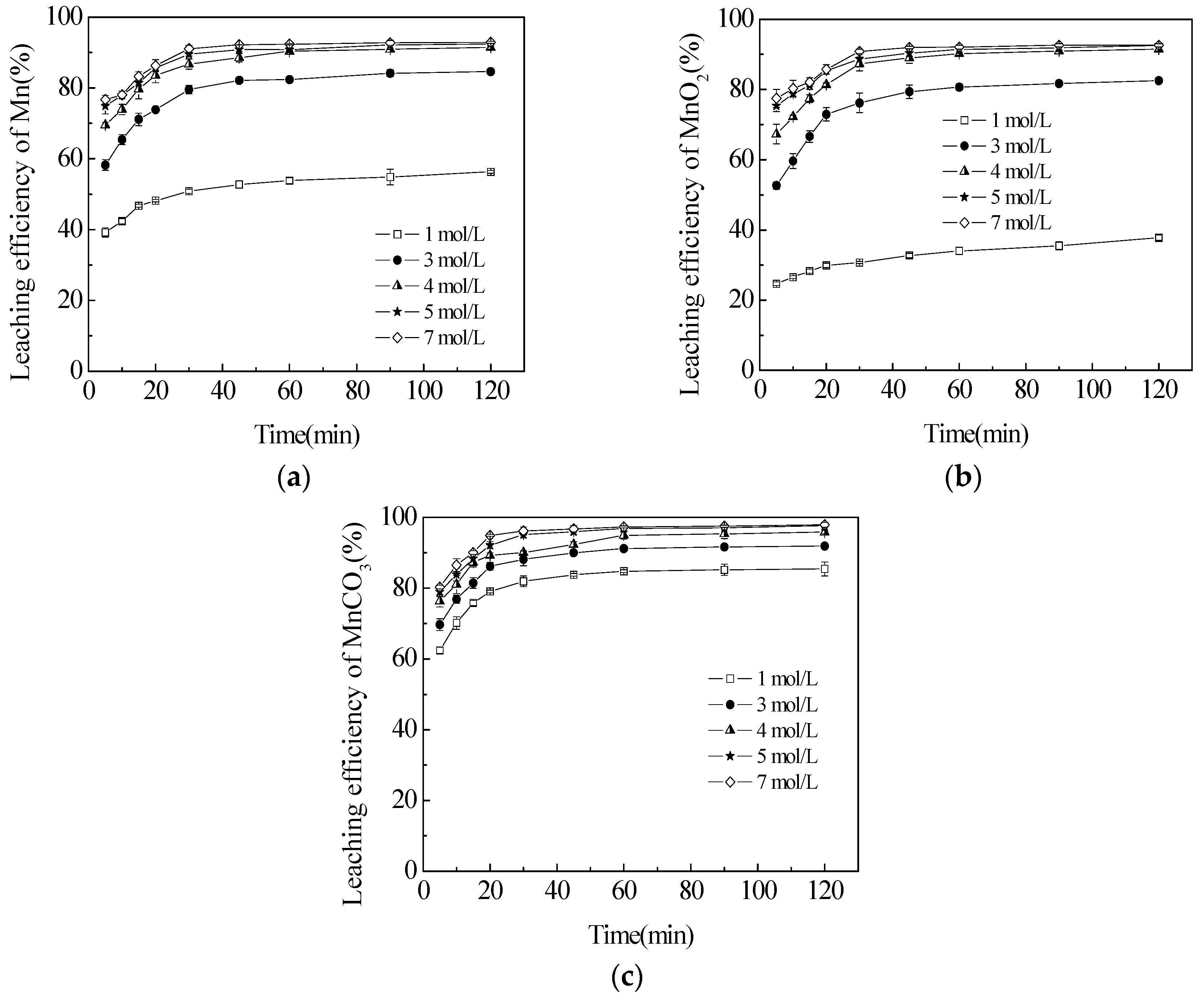
3.3. Effect of Sulphuric Acid Concentration on the Leaching Efficiencies of TMn, MnCO3 and MnO2
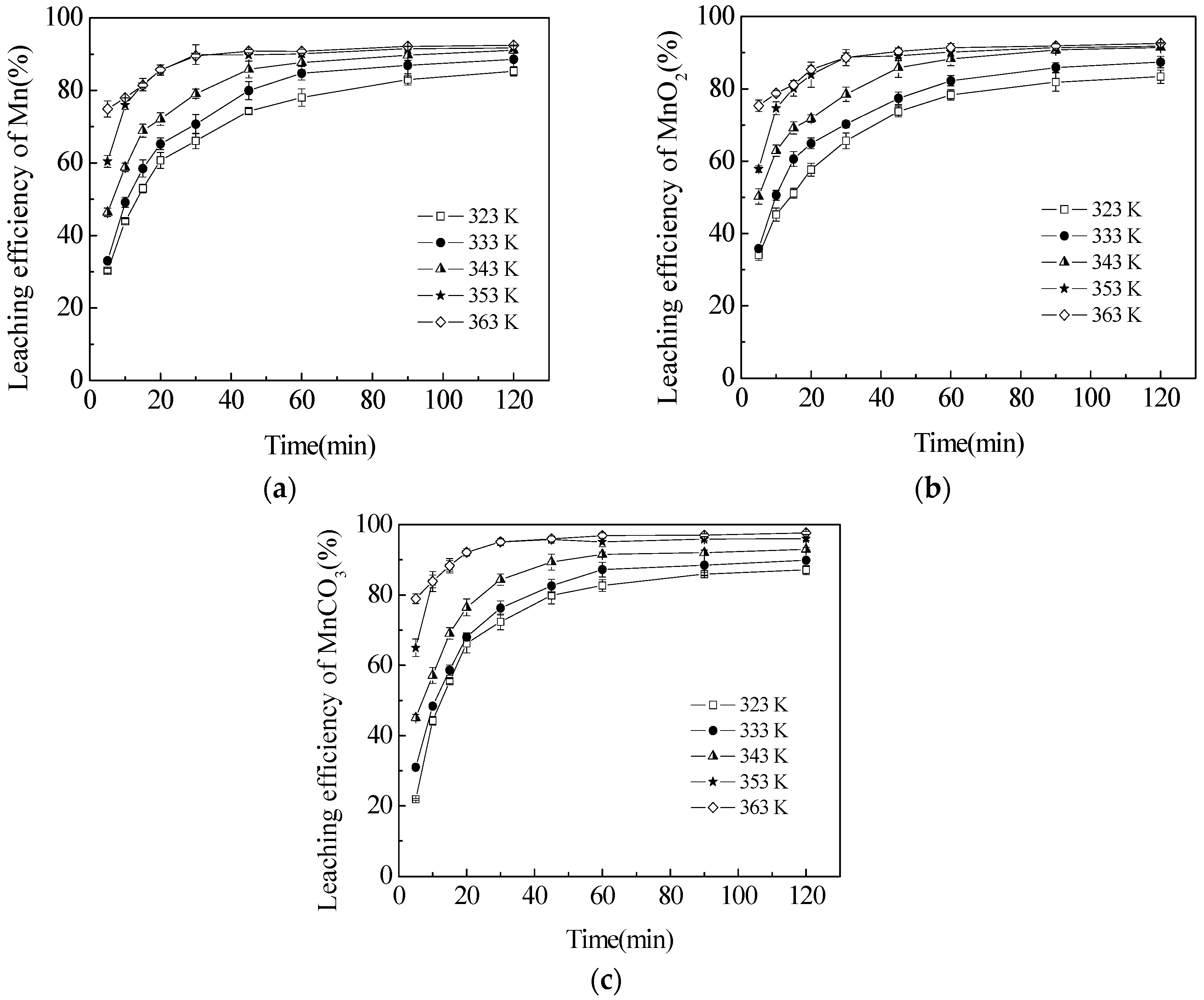
3.4. Effect of Temperature on the Leaching Efficiencies of TMn, MnCO3 and MnO2
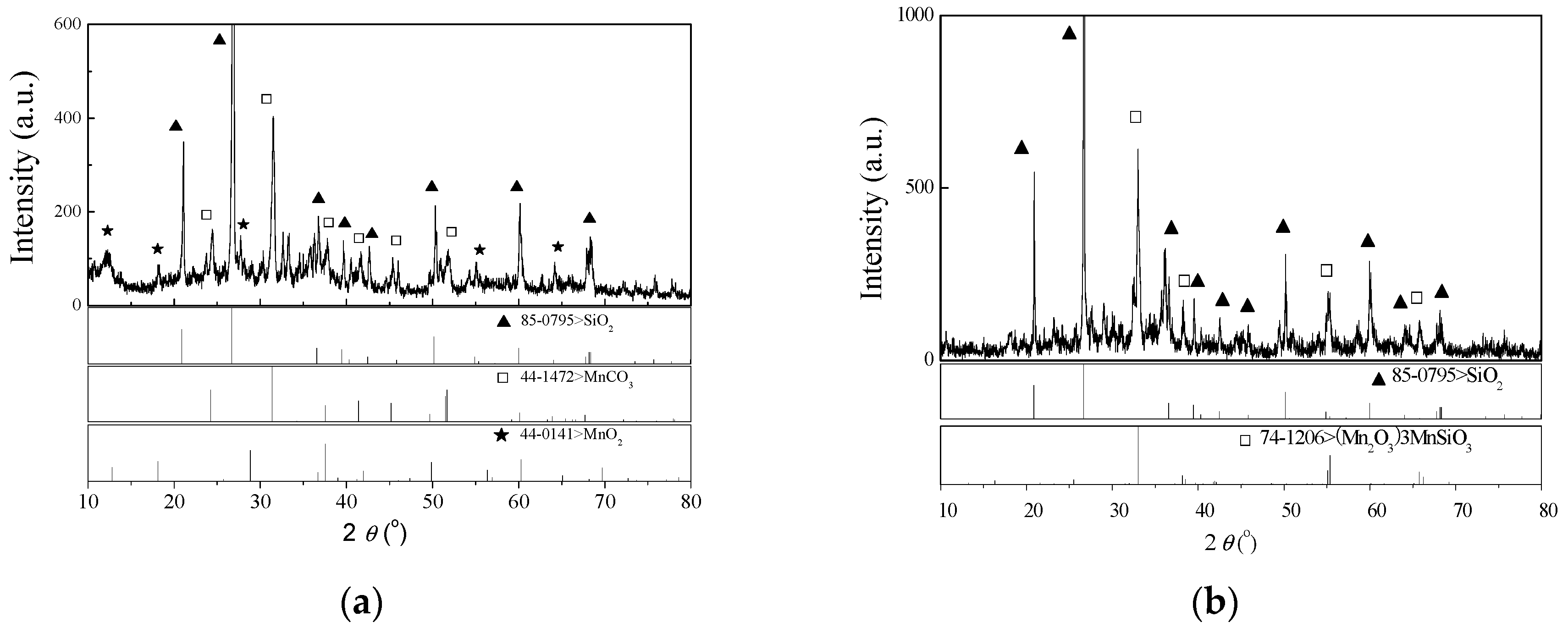
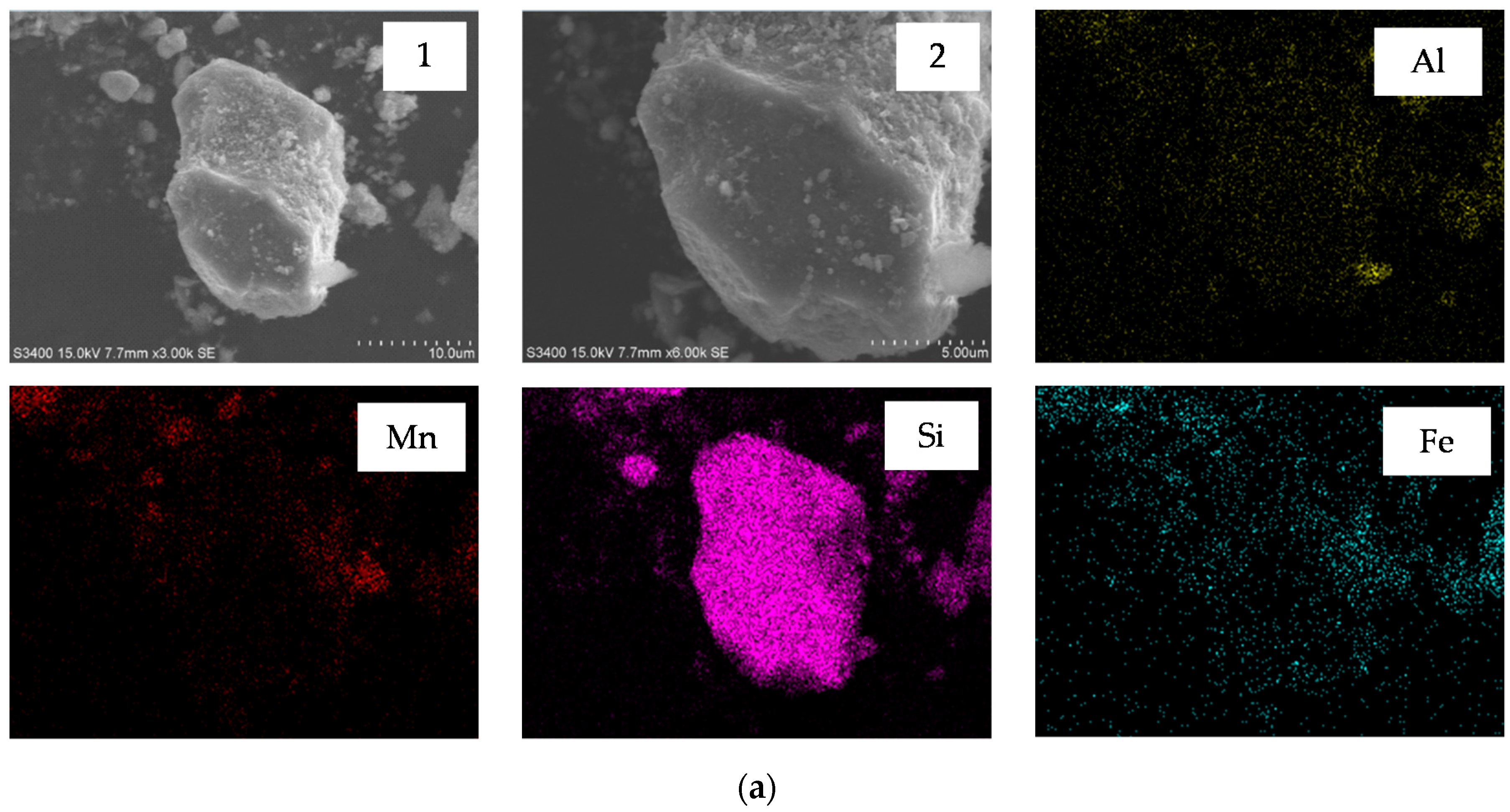
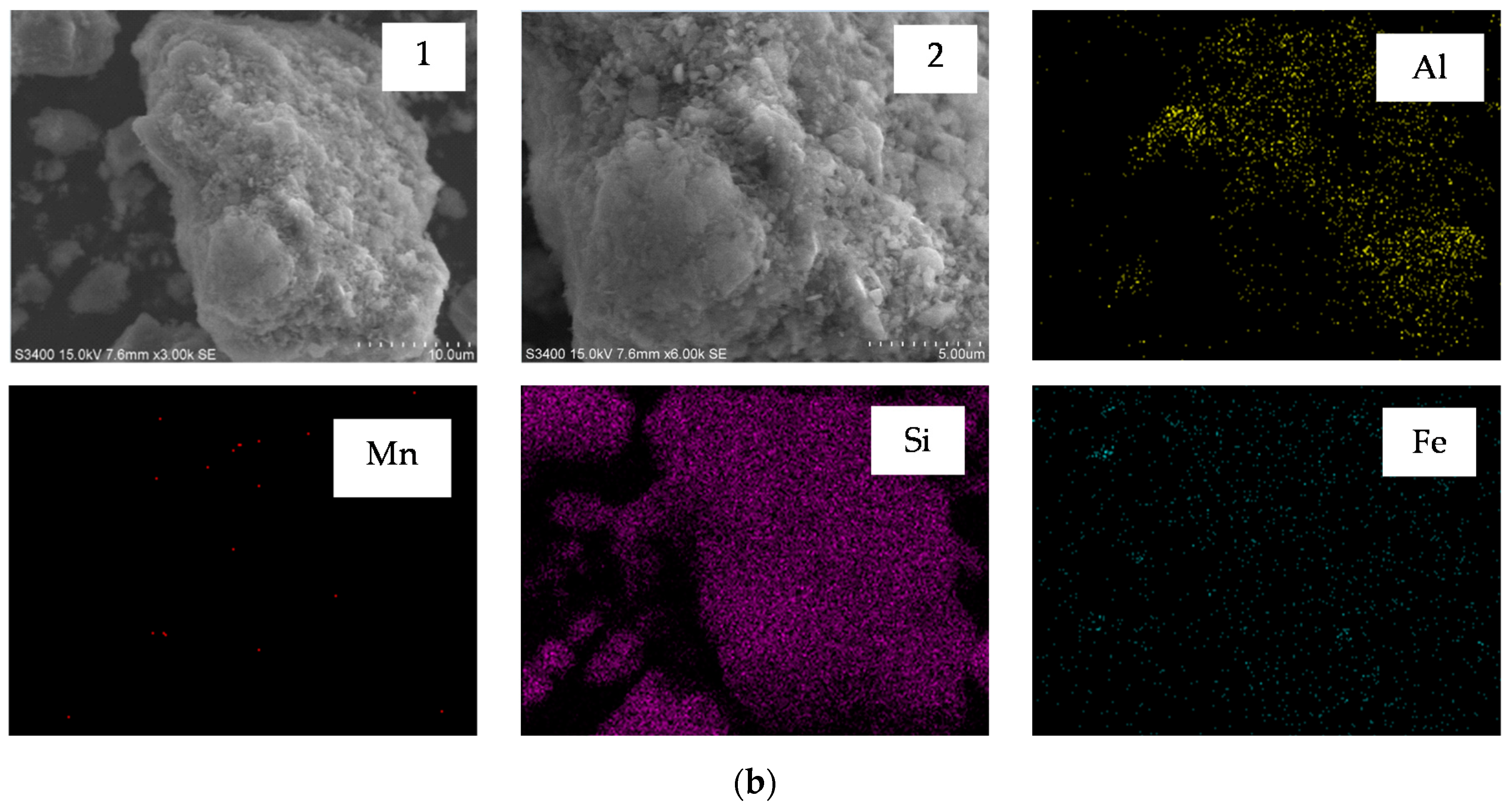
3.5. Characterization of Semi-Oxidized Manganese Ore and Leaching Residue
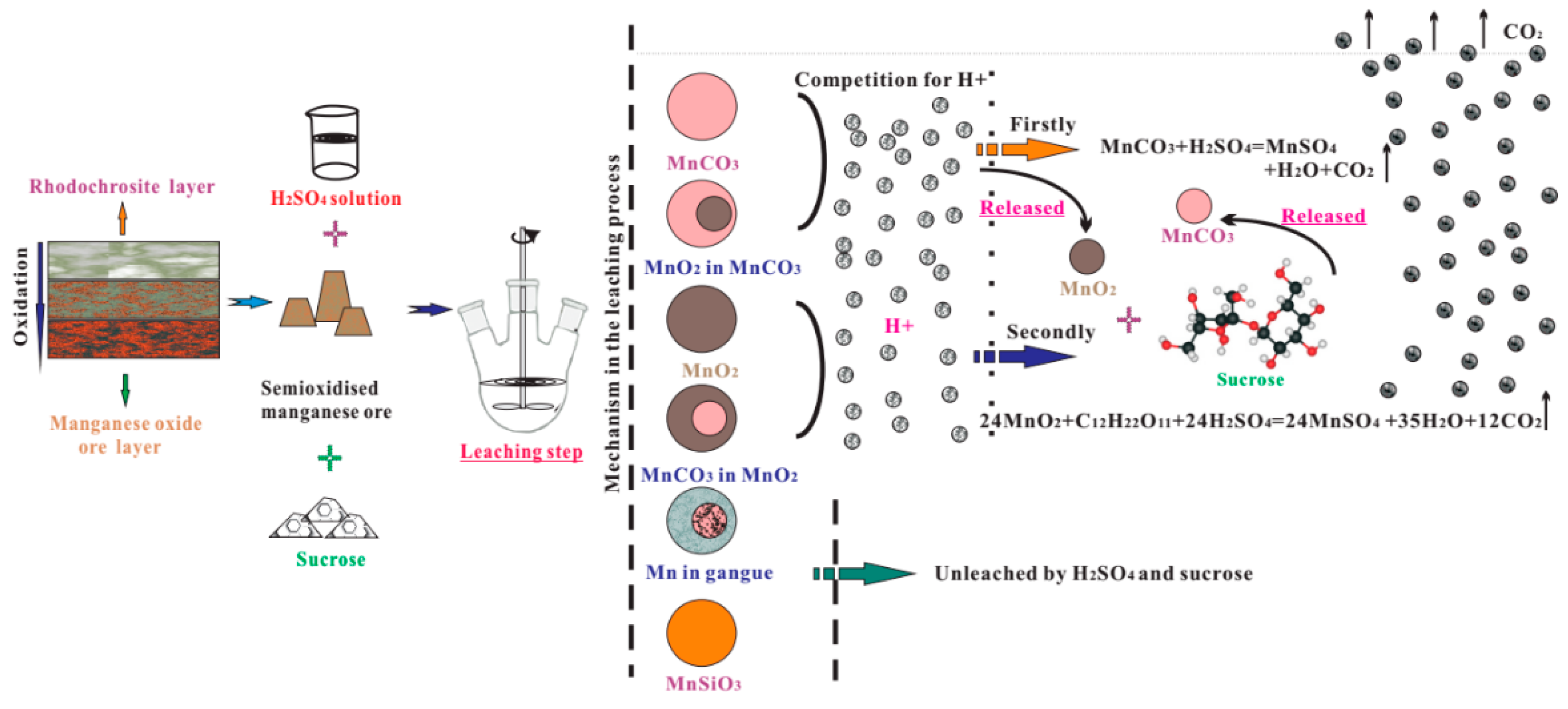
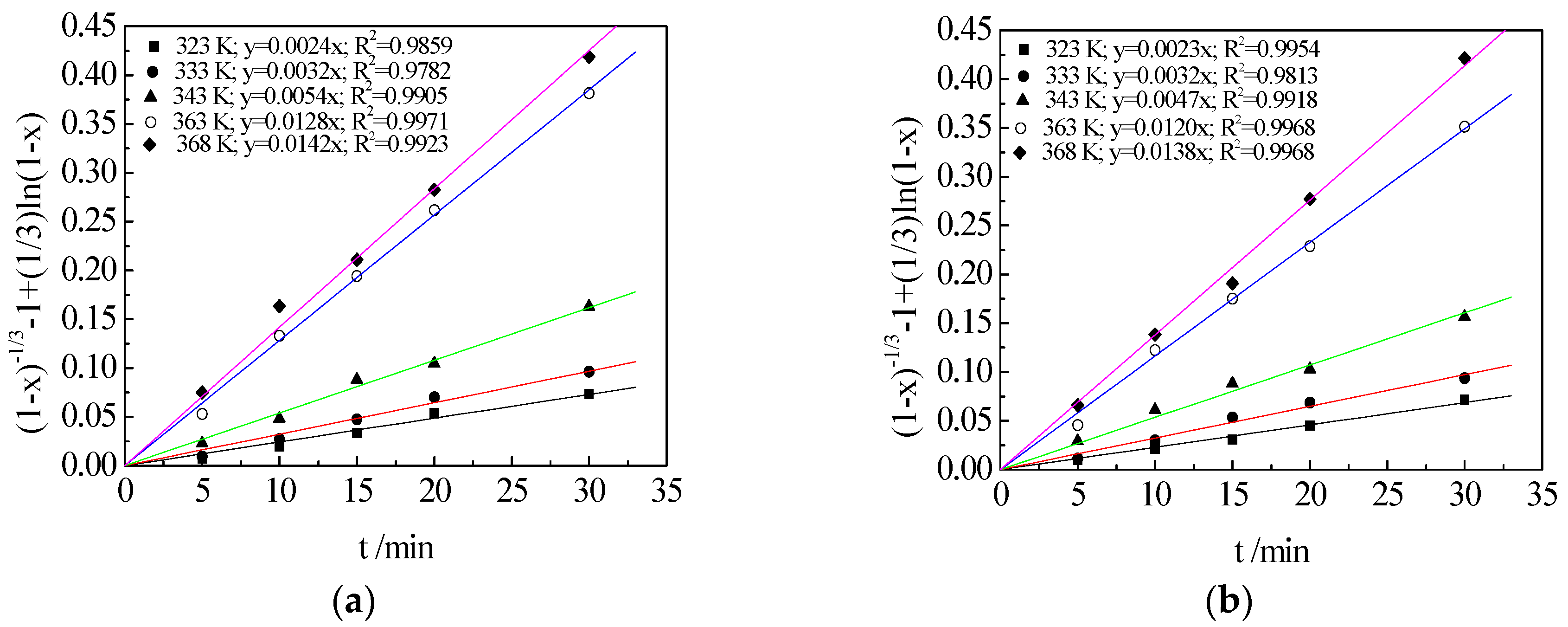
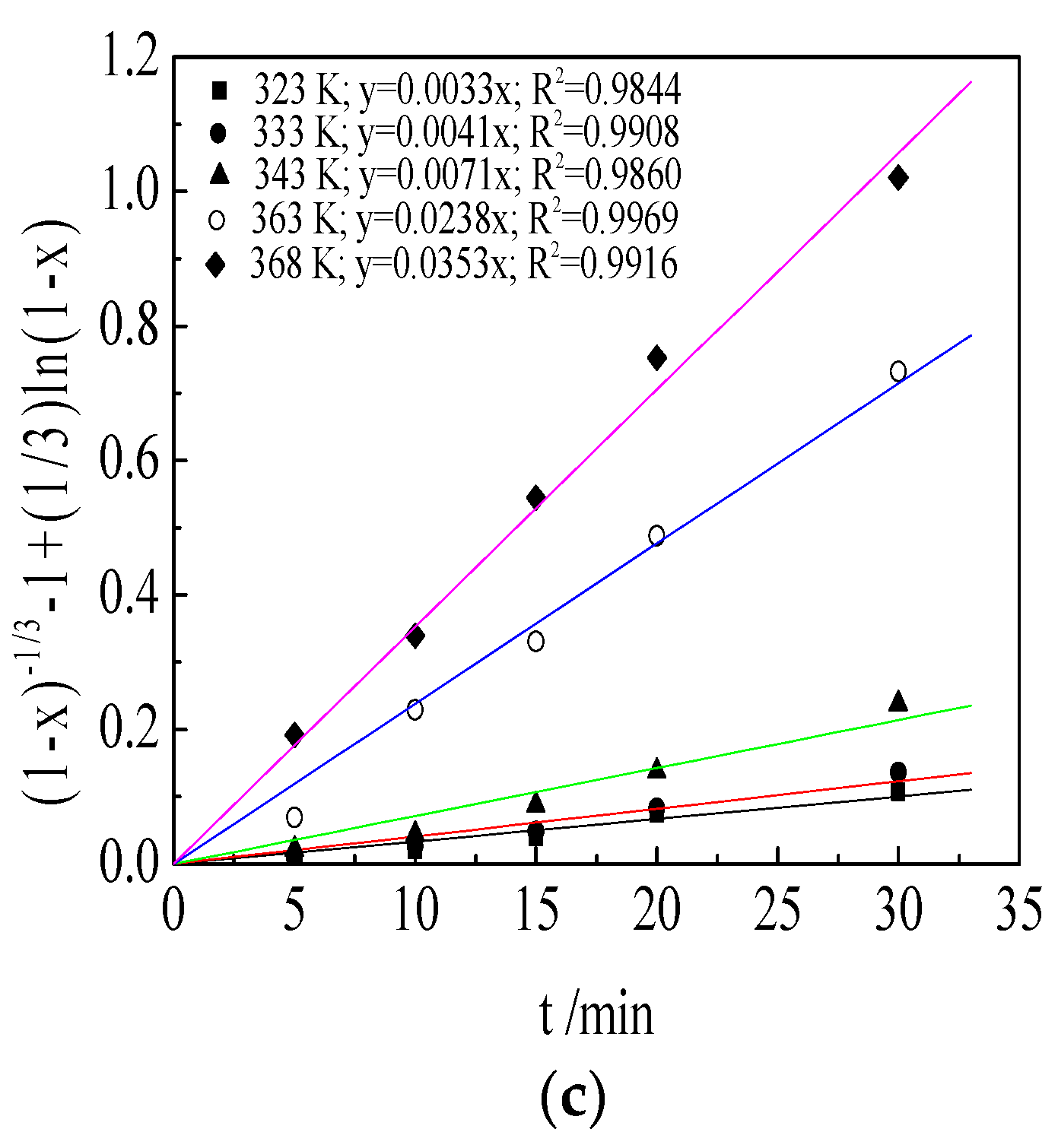
3.6. Kinetic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, J.; Dreisinger, D.; Glück, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Yan, H.; Zhao, Y.; Li, T.; Feng, X. Reduction of low-grade manganese dioxide ore pellets by biomass wheat stalk. Acta Metall. Sin. Engl. Lett. 2013, 26, 167–172. [Google Scholar] [CrossRef]
- Xie, C.; Xu, L.; Peng, T.; Chen, K.; Zhao, J. Leaching process and kinetics of manganese in low-grade manganese ore. Chin. J. Geochem. 2013, 32, 222–226. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Li, G.; Jiang, T. Manganese extraction by sulfur-based reduction roasting-acid leaching from low-grade manganese oxide ores. Hydrometallurgy 2013, 133, 126–132. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Du, Z.; Liu, X. Co-recovery of manganese from low-grade pyrolusite and vanadium from stone coal using fluidized roasting coupling technology. Hydrometallurgy 2013, 131–132, 40–45. [Google Scholar] [CrossRef]
- Yang, K.D.; Ye, X.J.; Su, J. Response surface optimization of process parameters for reduction roasting of low-grade pyrolusite by bagasse. Trans. Nonferrous Met. Soc. China 2013, 23, 548–555. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, G.; Cheng, Z. Thermal analysis and kinetic modeling of manganese oxide ore reduction using biomass straw as reductant. Hydrometallurgy 2010, 105, 96–102. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Wang, S.; Li, J.Z.; Liu, G.Y. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans. Nonferrous Met. Soc. China 2014, 24, 861–867. [Google Scholar] [CrossRef]
- Tian, X.K.; Wen, X.X.; Yang, C.; Liang, Y.J.; Pi, Z.B.; Wang, Y.X. Reductive leaching of manganese from low-grade manganese dioxide ores using corncob as reductant in sulfuric acid solution. Hydrometallurgy 2010, 100, 157–160. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, G.C.; Zhao, Y.L. Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant. Hydrometallurgy 2009, 96, 176–179. [Google Scholar] [CrossRef]
- Su, H.F.; Wen, Y.X.; Wang, F.; Sun, Y.Y.; Tong, Z.F. Reductive leaching of manganese from low-grade manganese ore in H2SO4 using cane molasses as reductant. Hydrometallurgy 2008, 93, 136–139. [Google Scholar] [CrossRef]
- Hariprasad, D.; Dash, B.; Ghosh, M.K.; Anand, S. Leaching of manganese ores using sawdust as a reductant. Miner. Eng. 2007, 20, 1293–1295. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Furlani, G.; Valentini, P.; Vegliò, F.; Toro, L. Leaching of low-grade manganese ores by using nitric acid and glucose: Optimization of the operating conditions. Hydrometallurgy 2004, 75, 157–167. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- El Hazek, M.N.; Lasheen, T.A.; Helal, A.S. Reductive leaching of manganese from low grade Sinai ore in HCl using H2O2 as reductant. Hydrometallurgy 2006, 84, 187–191. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4·H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Process. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Sun, W.Y.; Su, S.J.; Wang, Q.Y.; Ding, S.L. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2. Hydrometallurgy 2013, 133, 118–125. [Google Scholar] [CrossRef]
- Lan, Y.Z. Laboratory study: Simultaneous leaching silver-bearing low-grade manganese ore and sphalerite concentrate. Miner. Eng. 2004, 17, 1053–1056. [Google Scholar]
- Xin, B.P.; Li, T.; Li, X.; Dan, Z.G.; Xu, F.Y.; Duan, N.; Zhang, Y.T.; Zhang, H.Y. Reductive dissolution of manganese from manganese dioxide ore by autotrophic mixed culture under aerobic conditions. J. Clean. Prod. 2015, 92, 54–64. [Google Scholar] [CrossRef]
- Vegliò, F.; Toro, L. Fractional factorial experiments in the development of manganese dioxide leaching by sucrose in sulphuric acid solutions. Hydrometallurgy 1994, 36, 215–230. [Google Scholar] [CrossRef]
- Beolchini, F.; Peterangelpapini, M.; Toro, L.; Trifoni, M.; Vegliò, F. Acid leaching of manganiferous by sucrose: Kinetic modeling and related statistical analysis. Miner. Eng. 2001, 14, 175–184. [Google Scholar] [CrossRef]
- Su, H.; Wen, Y.; Wang, F.; Li, X.; Tomg, Z. Leaching of pyrolusite using molasses alcohol wastewater as a reductant. Miner. Eng. 2009, 22, 207–209. [Google Scholar] [CrossRef]
- Su, H.; Liu, H.; Wang, F.; Lü, X.; Wen, Y. Kinetics of reductive leaching of low-grade pyrolusite with molasses alcohol wastewater in H2SO4. Chin. J. Chem. Eng. 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, Q.Q.; Li, L.F.; Fu, J.G.; Zhu, Z.S.; Wang, C.Q.; Qian, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. Int. J. Min. Sci. Technol. 2014, 24, 567–571. [Google Scholar] [CrossRef]
- Das, S.C.; Sahoo, P.K.; Rao, P.K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching. Hydrometallurgy 1994, 8, 35–47. [Google Scholar] [CrossRef]
- Wu, F.; Zhong, H.; Wang, S.; Lai, S. Kinetic of reductive leaching of manganese oxide ore using cellulose as reductant. J. Cent. South Univ. 2014, 21, 1763–1770. [Google Scholar] [CrossRef]
- Xue, J.; Zhong, H.; Wang, S.; Li, C.; Li, J.; Wu, F. Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution. J. Saudi Chem. Soc. 2014, 9, 1–6. [Google Scholar] [CrossRef]

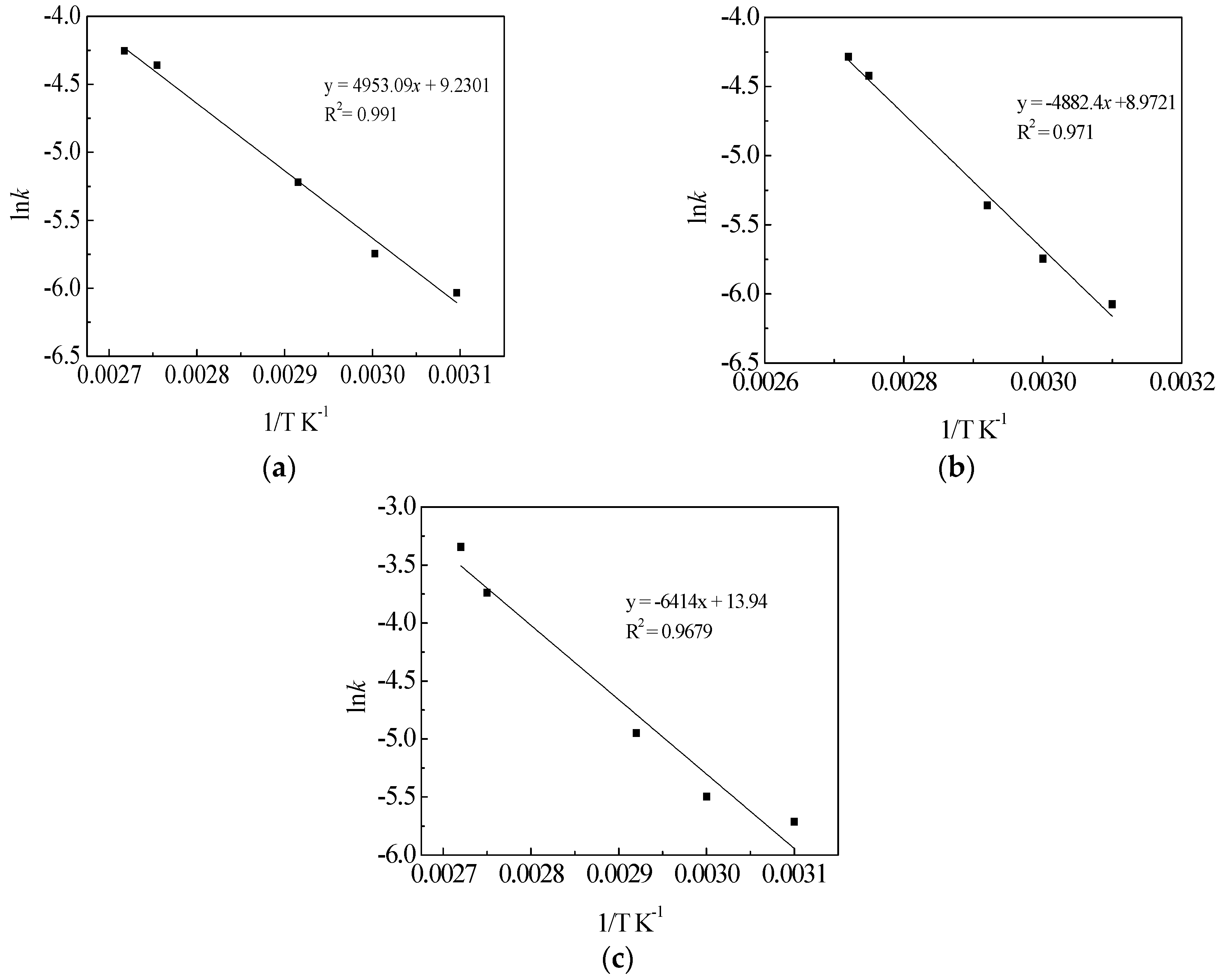
| Component | Total Mn | Mn (MnCO3) | Mn (MnO2) | Mn (MnSiO3) | Al2O3 | CaO |
| Content (%, ω) | 25.61 | 10.02 | 14.82 | 0.36 | 3.49 | 1.72 |
| Component | S | MgO | P | SiO2 | Fe | |
| Content (%, ω) | 0.049 | 1.74 | 0.084 | 37.70 | 5.46 |
| Component | TMn | Mn (MnO2) | Mn (MnCO3) | Mn (MnSiO3) | Mn (Other) |
|---|---|---|---|---|---|
| Content (g) | 0.199 | 0.113 | 0.026 | 0.036 | 0.025 |
| Residual content/original content (%) | 7.77 | 7.62 | 2.59 | 100 | 59.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jin, S.; Lv, Y.; Zhang, Y.; Su, H. Hydrometallurgical Process and Kinetics of Leaching Manganese from Semi-Oxidized Manganese Ores with Sucrose. Minerals 2017, 7, 27. https://doi.org/10.3390/min7020027
Wang Y, Jin S, Lv Y, Zhang Y, Su H. Hydrometallurgical Process and Kinetics of Leaching Manganese from Semi-Oxidized Manganese Ores with Sucrose. Minerals. 2017; 7(2):27. https://doi.org/10.3390/min7020027
Chicago/Turabian StyleWang, Yuhong, Shenglong Jin, Yan Lv, Yanjuan Zhang, and Haifeng Su. 2017. "Hydrometallurgical Process and Kinetics of Leaching Manganese from Semi-Oxidized Manganese Ores with Sucrose" Minerals 7, no. 2: 27. https://doi.org/10.3390/min7020027
APA StyleWang, Y., Jin, S., Lv, Y., Zhang, Y., & Su, H. (2017). Hydrometallurgical Process and Kinetics of Leaching Manganese from Semi-Oxidized Manganese Ores with Sucrose. Minerals, 7(2), 27. https://doi.org/10.3390/min7020027






