2.1. Materials
Phosphate ore was obtained from Yichang, Hubei Province, China. The ore was crushed to a grain size of 0~3 mm with a jaw crusher (model XPC-60 × 100) (Wuhan Boshan Machinery Co. Ltd., Wuhan, China) and a double-roll crusher (model HLXPS-Φ250 × 150) (Wuhan Exploring Machinery Factory, Wuhan, China), then wet ground to −0.074 mm accounting for 70% in a laboratory ball mill (HLXMQ-Φ 240 × 90) (Wuhan Hengle Mineral Engineering Equipment Co. Ltd., Wuhan, China) at 50 wt % solids. The ground ore is referred to from here on as “separation feed”.
The chemical composition of the raw ore was analyzed by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry) performed on an IRIS Advantage ER/S instrument (Thermo Elemental, MA, USA). The results were shown in
Table 1. The contents of P
2O
5, SiO
2, and MgO were 23.98%, 22.14%, and 2.11%, respectively; sesquioxide (Al
2O
3 and Fe
2O
3) content was 6.40%.
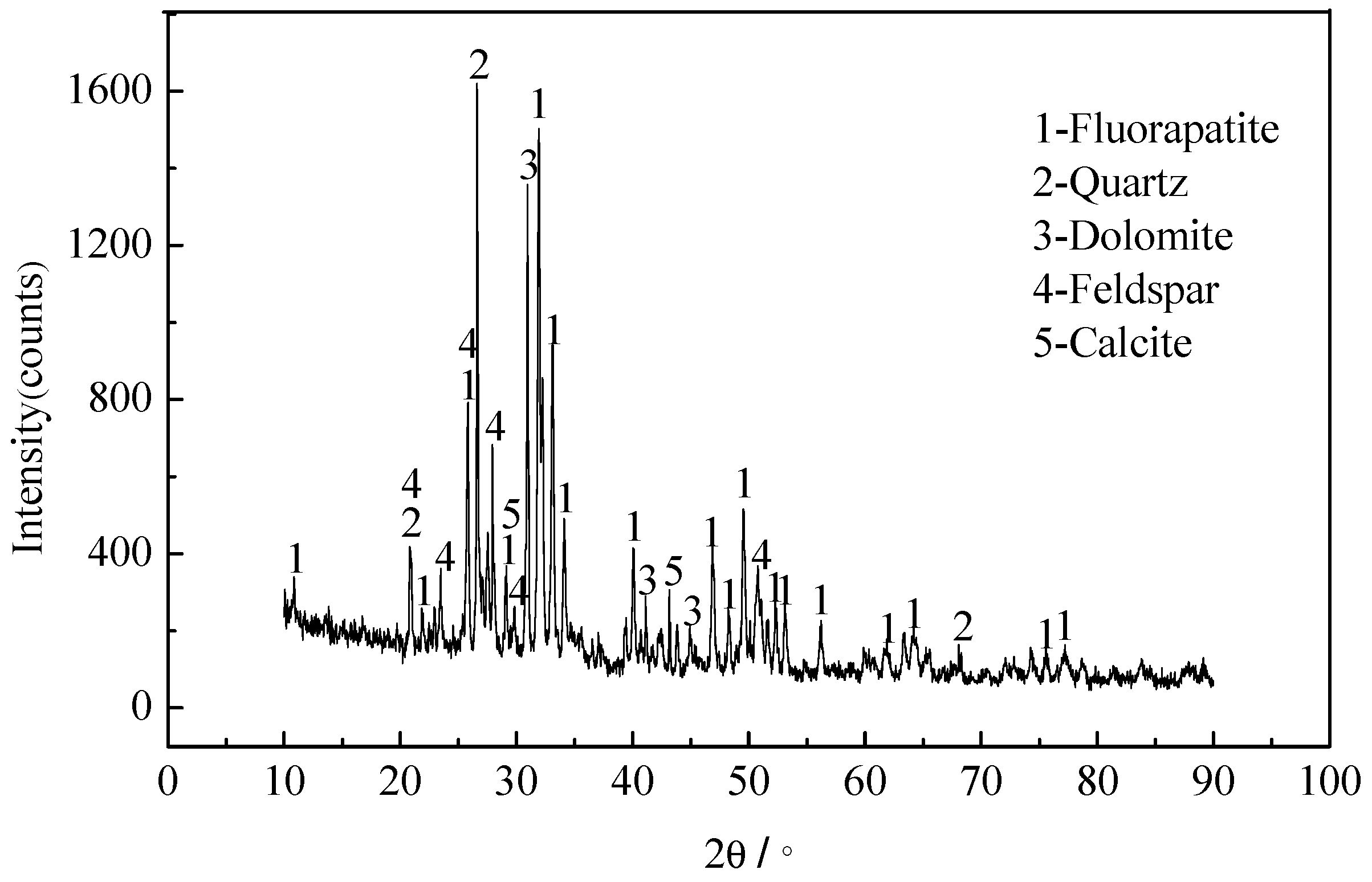
The mineral composition of the raw ore was determined via X-ray diffraction (XRD) and microscopy analyses. The XRD analyses were conducted using a Rigaku D/MAX-RB X-ray diffraction (Rigaku, Akishima City, Japen) using Cu Kα radiation. The results are shown in
Figure 1 and
Table 2.
The raw ore mainly contained collophane, dolomite, quartz, feldspar, and clay (56.5%, 9.5%, 15.3%, 3.6%, and 12.8% of the total content, respectively) plus some calcite and pyrite. The collophane was mainly fluorapatite.
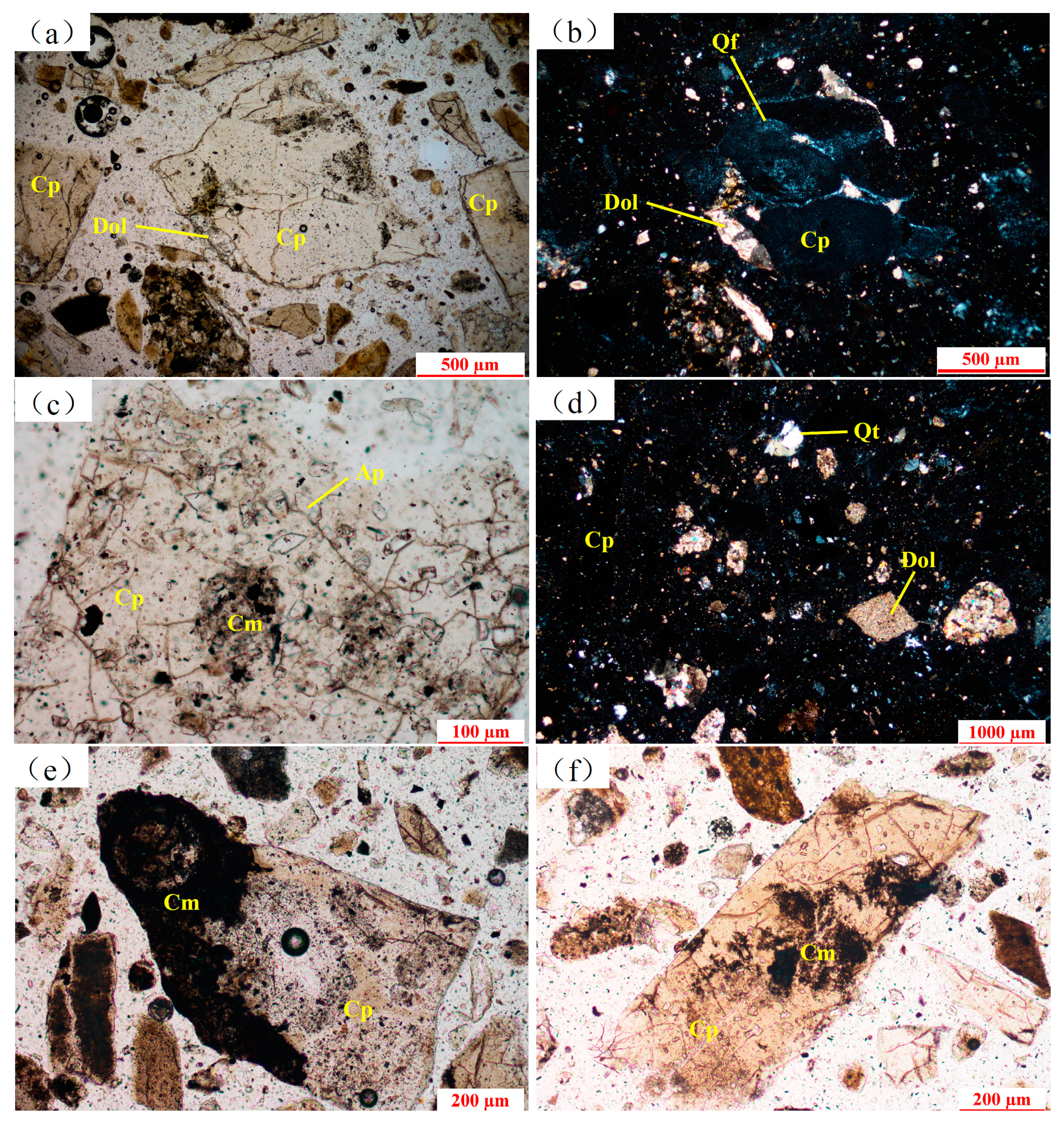
Optical microscopy was also performed to determine the main mineral dissemination characteristics. Collophane is characterized by “water chestnut”, polygonal, plate-like, and scattered states. The particles and certain edge portions are mixed with a subtle silica, quartz, and dolomite mixture (
Figure 2a,b) and the individual particle is nearly entirely replaced by siliceous rock. Collophane also contains apatite (
Figure 2c) embedded in the dolomite fraction, siliceous, and argillaceous pieces, as well as fine-grained pyrite. The particle size of collophane is 0.03–1 mm.
Dolomite has half-automorphic granules, and its aggregates appear to be irregular briquette granules. Most are individually scattered (
Figure 2d), while some crystallize or parcel in the collophane and some are distributed as dust-like or fine granules in the collophane and argillaceous fractions. The particle size of dolomite is 0.03–0.15 mm.
Quartz is fine granular, mostly scattered in collophane and argillaceous fractions, and a small fraction are individual scattered states. The particle size of quartz is 0.03–0.4 mm.
Argillaceous fractions are water chestnut or long plates in scattered distribution mainly consisting of illite, sericite, and kaolinite. Some fractions contain quartz powder sand and some appear to be in independent distribution. A small portion appear to have crystallized (
Figure 2e) or embedded in the collophane (
Figure 2f). The particle size of argillaceous fractions is 0.1–1 mm.
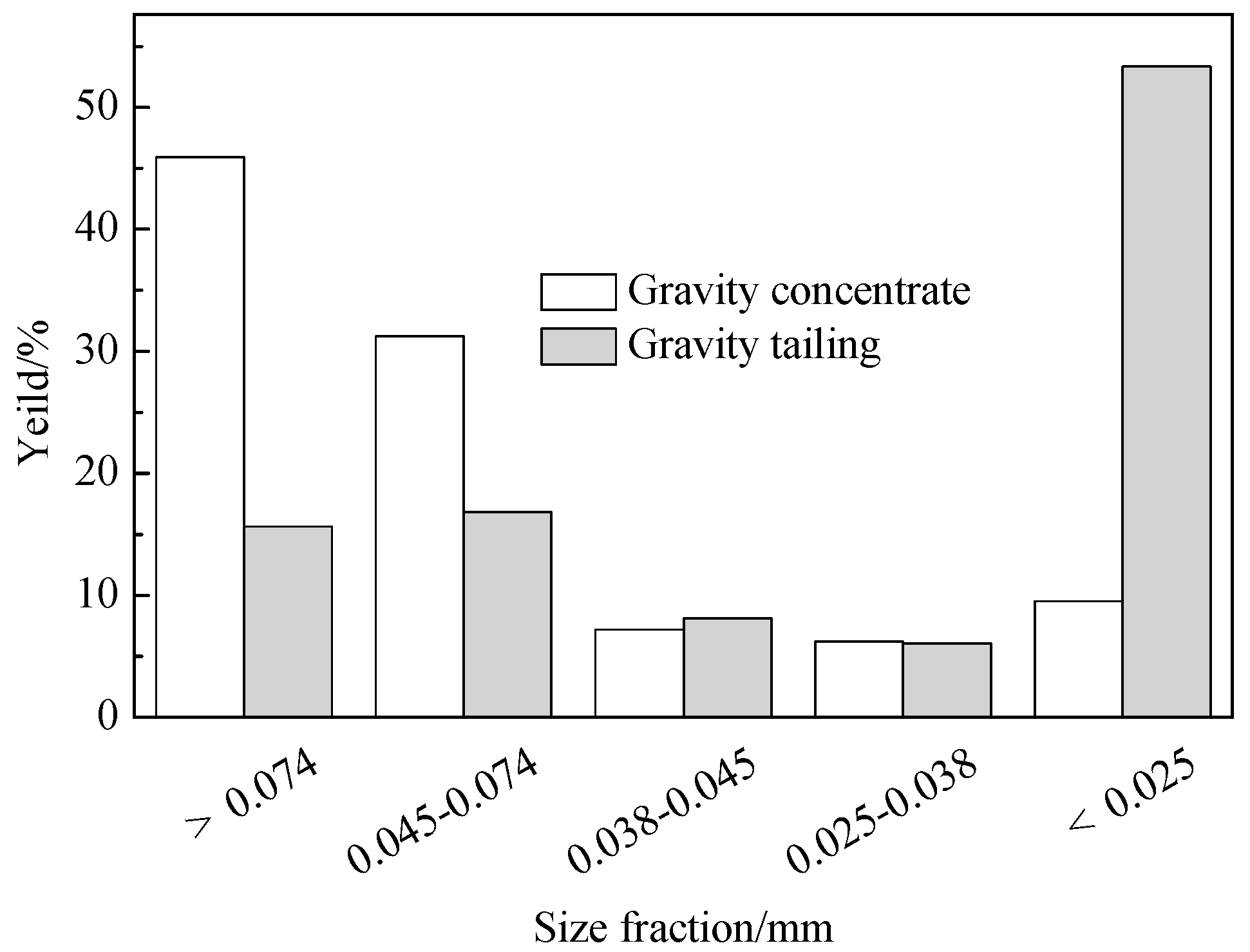
Grain analyses were performed to further observe the characteristics of the separation feed (e.g., differences among size fractions).
The results in
Table 3 showed that phosphorus was concentrated in coarse size fractions, which might be related to mineral properties: (1) The particle size of collophane is larger than dolomite and quartz; and (2) Argillaceous fractions are easy to grind. Meanwhile, the density of collophane is slightly greater than dolomite, quartz, and clay [
20], so the ore can indeed be concentrated by gravity separation (and, thus, separated by a gravity-flotation combination process).
2.2. Methods
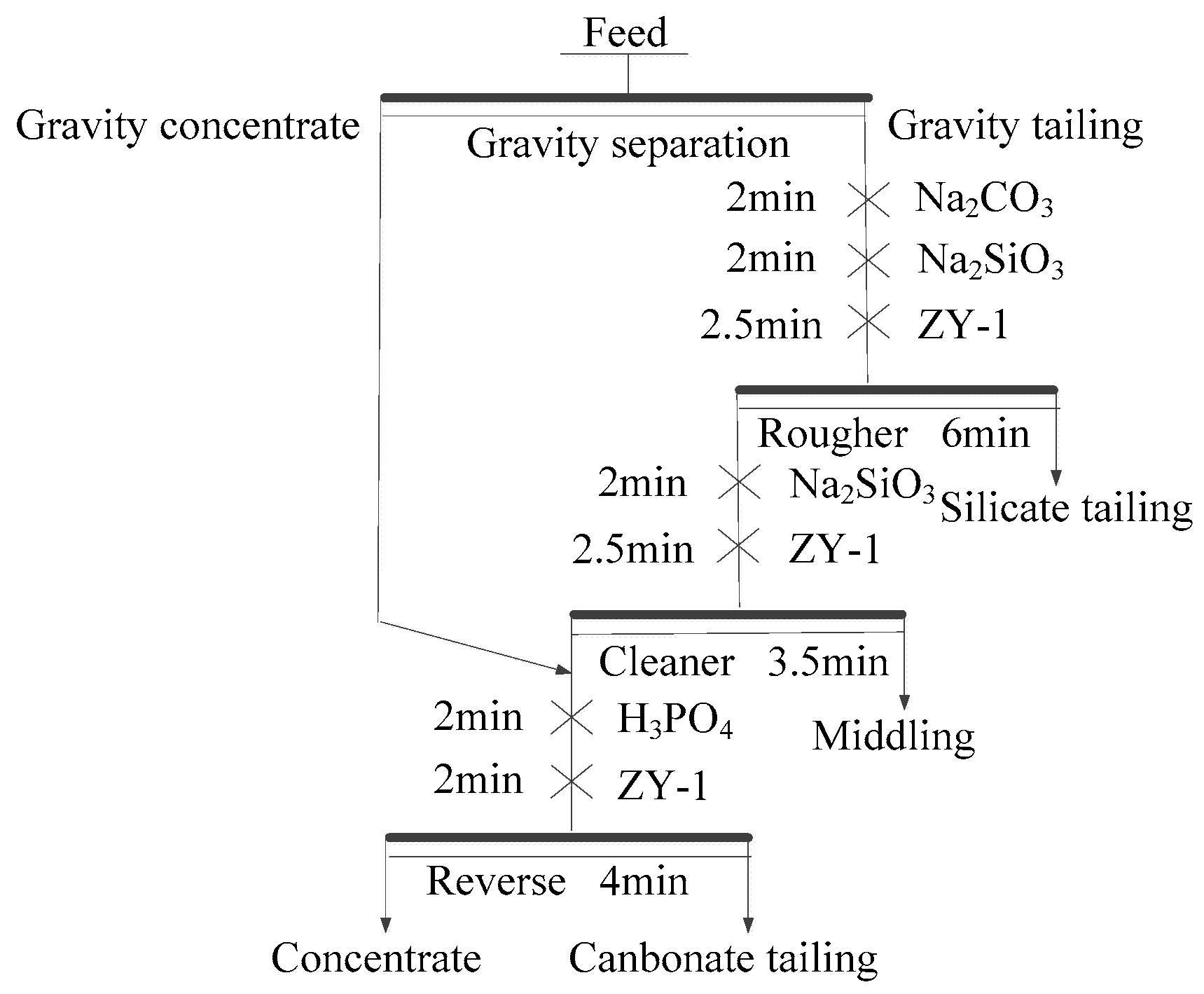
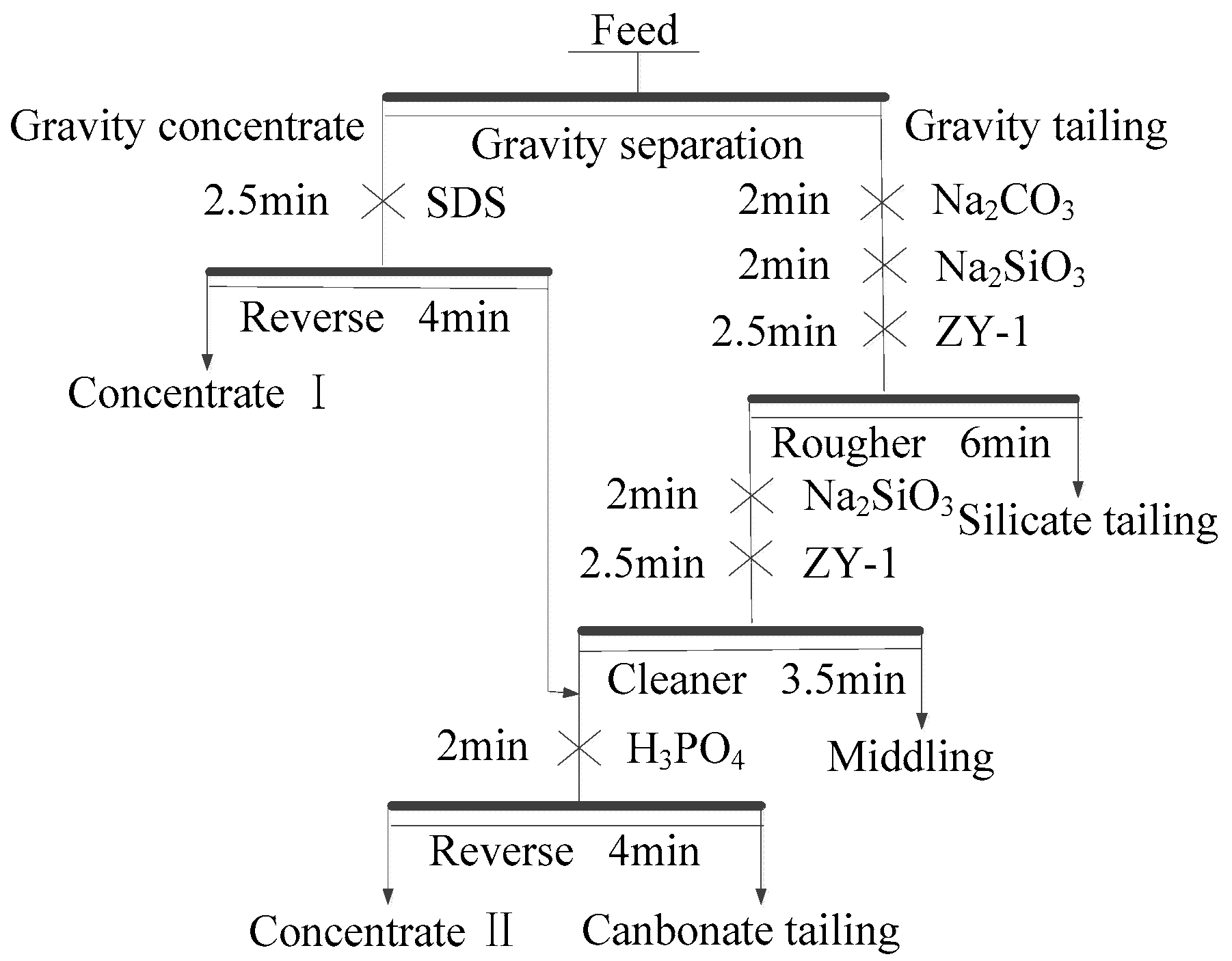
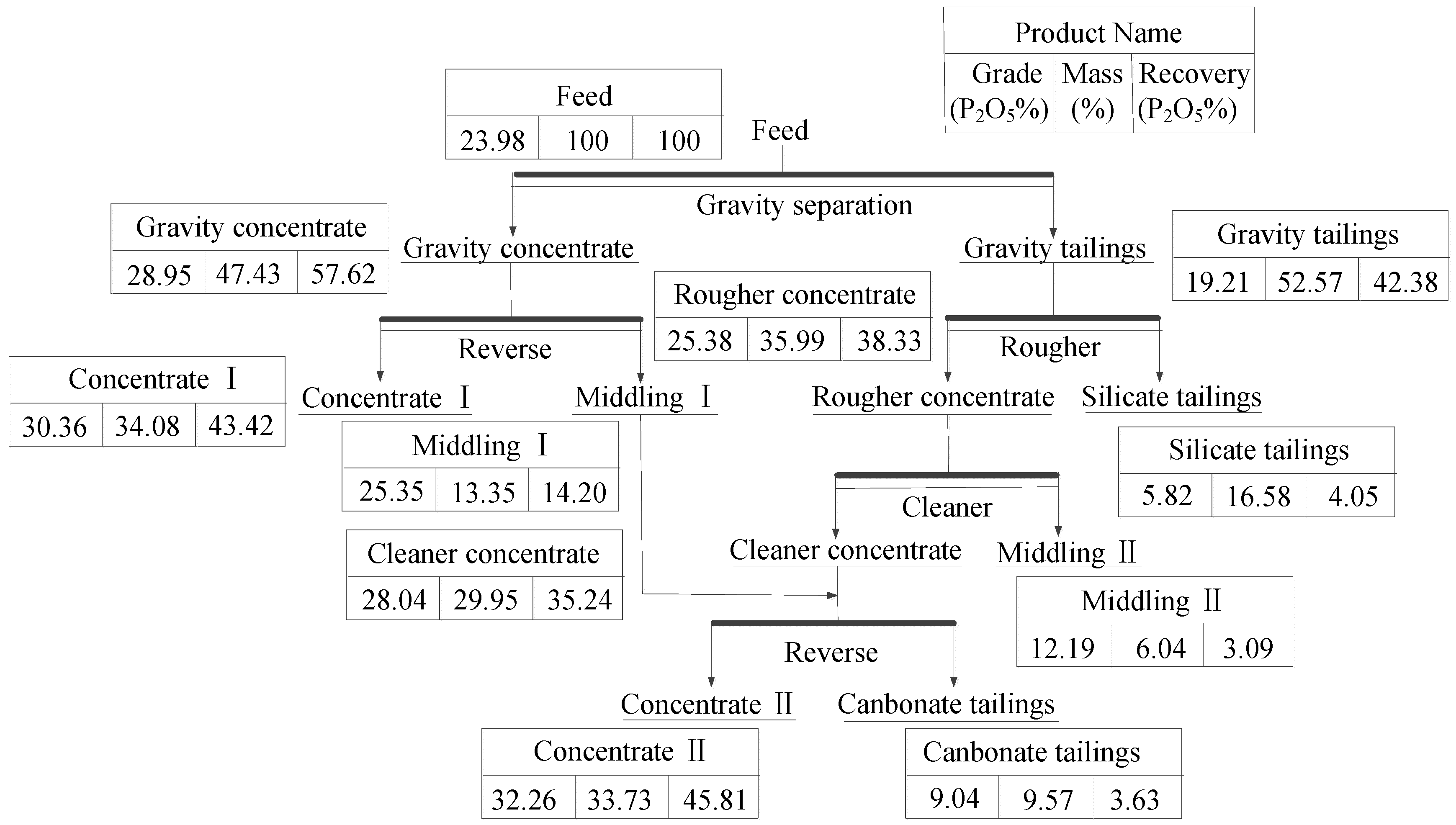
2.2.1. Gravity Separation Tests
Owing to the small density differences among the minerals in the raw ore and fine-grained separation feed, a spiral chute (P/D = 0.36) was selected for separation [
20,
26]; 3 kg of separation feed was processed with a five-turn laboratory spiral chute at a pulp density of 18 wt % solids. Tests were conducted under the condition of 215 L/h feed rate and no wash water. The products of gravity separation included concentrate and tailing.
2.2.2. Flotation Separation Tests
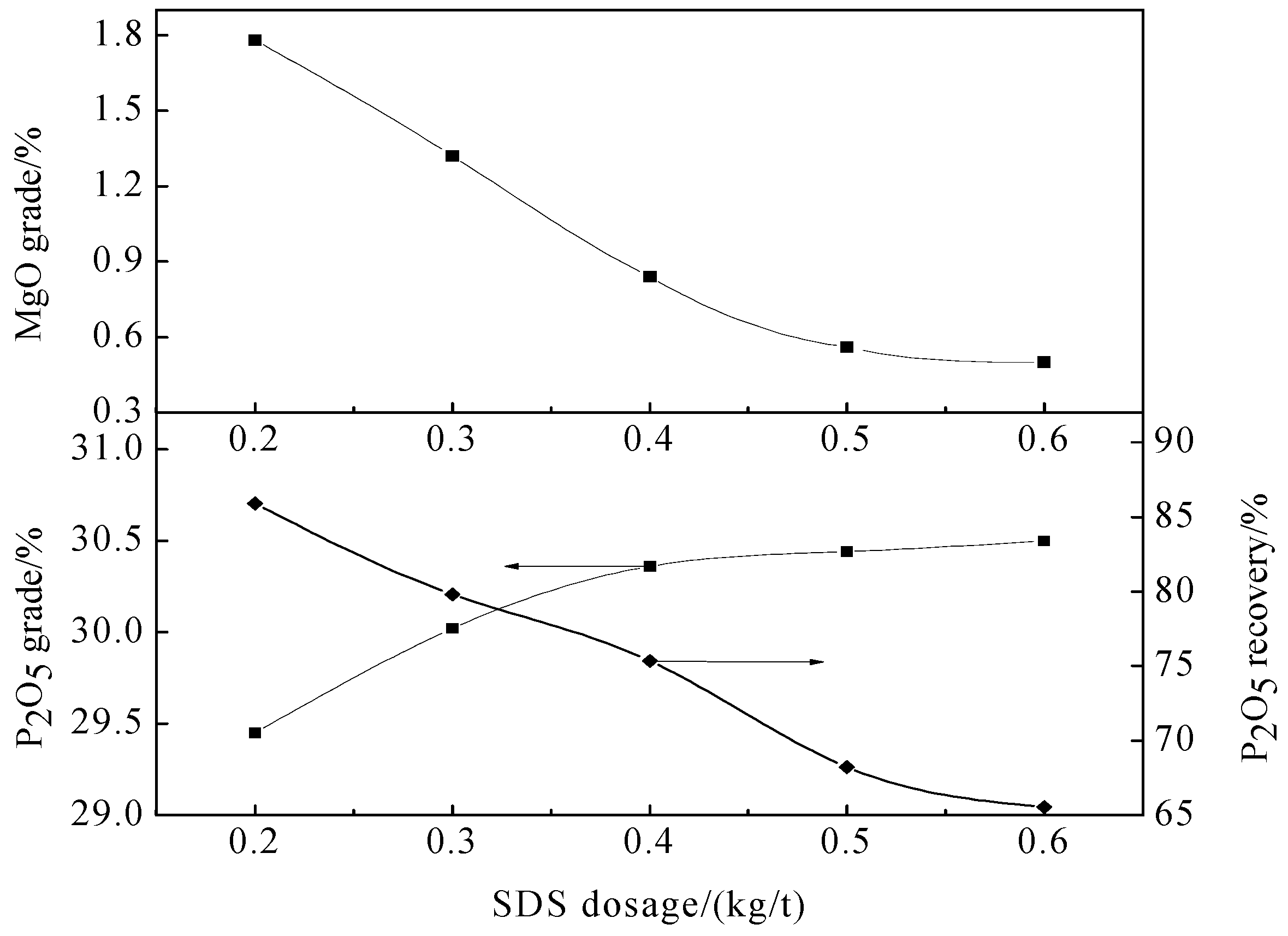
Flotation tests were carried out with a 0.5/0.75L XED-IV single cell flotation machine (Jilin Prospecting Machinery Factory, Changchun, China). Tests were conducted at a solid content of 33%, pulp temperature of 298 K, and impeller speed of 1725 rpm. Chemical reagents were added to the cell at different time points. Sodium carbonate, water glass, and ZY-1 (fatty acid) served as regulator, depressant, and collector, respectively, during the direct flotation tests, and phosphoric acid served as regulator and depressant during reverse flotation. As discussed above, SDS served as a dolomite collector during reverse flotation.
2.2.3. Zeta Potential Analysis Tests
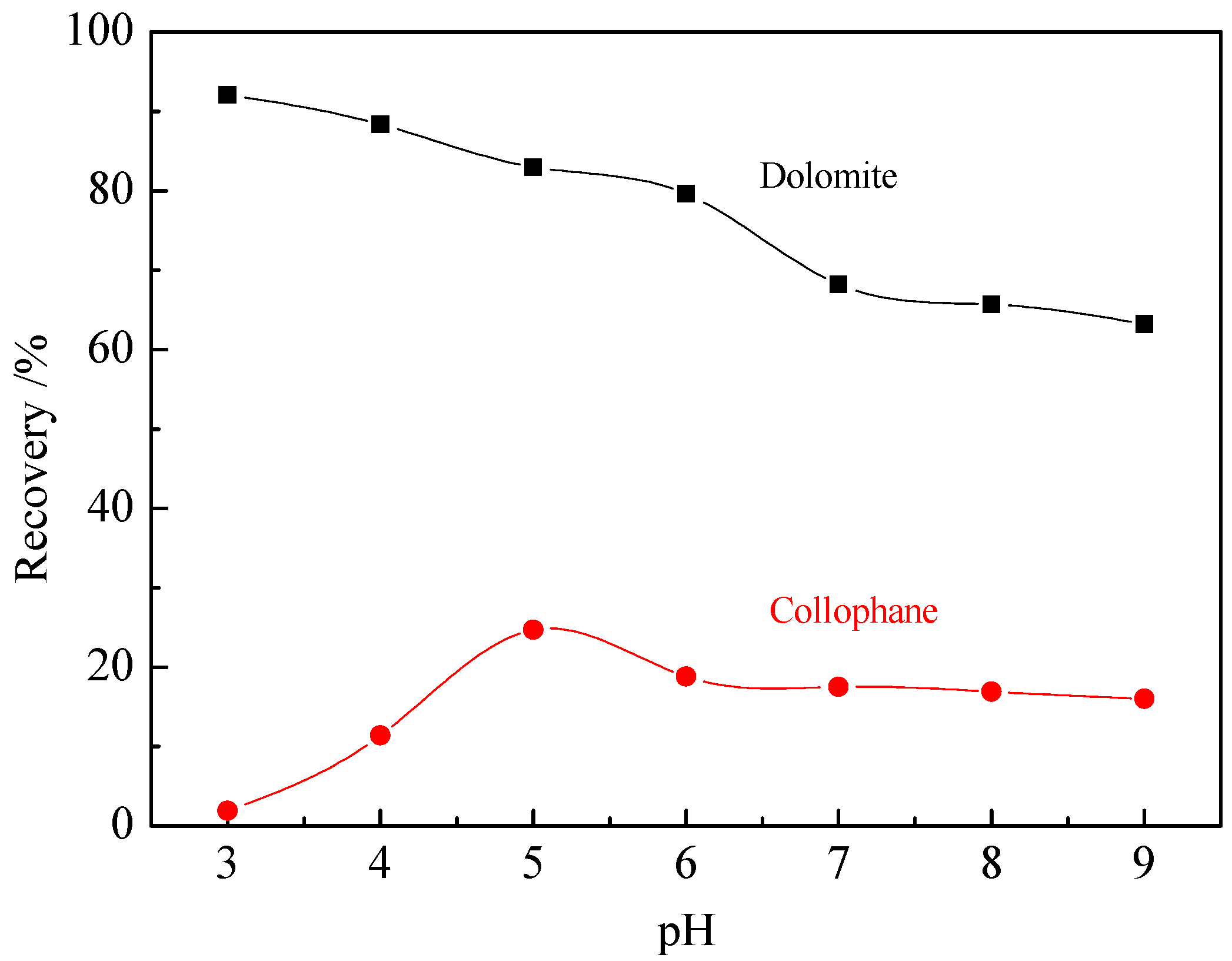
A suspension containing 0.01 wt % single apatite or dolomite particles ground to −2 mm in an agate mortar was prepared in KCl solution (10−3 M). The pH value of the suspension was adjusted to the desired value using HCl or NaOH solution. The suspension was stirred with magnetic stirrer for 5 min to prepare samples for analysis. The samples were placed into a zeta analyzer (Malvern Instruments Ltd., Malvern, UK) to measure the zeta potential of the mineral surfaces at 298 K. The tests were run in three replicates and the average is reported as the final value.
2.2.4. Single Mineral Flotation Tests
Single mineral flotation tests were carried out in a XFGC II flotation machine (Wuhan Hengle Mineral Engineering Equipment Co. Ltd., Wuhan, China) with a 40 mL flotation cell. Four grams of single collophane or dolomite (particle size 0.038–0.074 mm) and 40 mL of distilled water were added to the flotation cell, then HCl or NaOH was added to adjust the pH and the pulp was stirred for 1 min. A moderate amount of the collector was then added to the flotation cell followed by stirring for another 2.5 min, then the pH values were measured with a precise pH meter (PHS-3C). Tests were conducted at a pulp temperature of 298 K and impeller speed of 1500 rpm. The flotation time was 5 min and the froth was manually scraped. The froths obtained were dried and weighed to calculate the yield, which was reported here as the sample recovery.
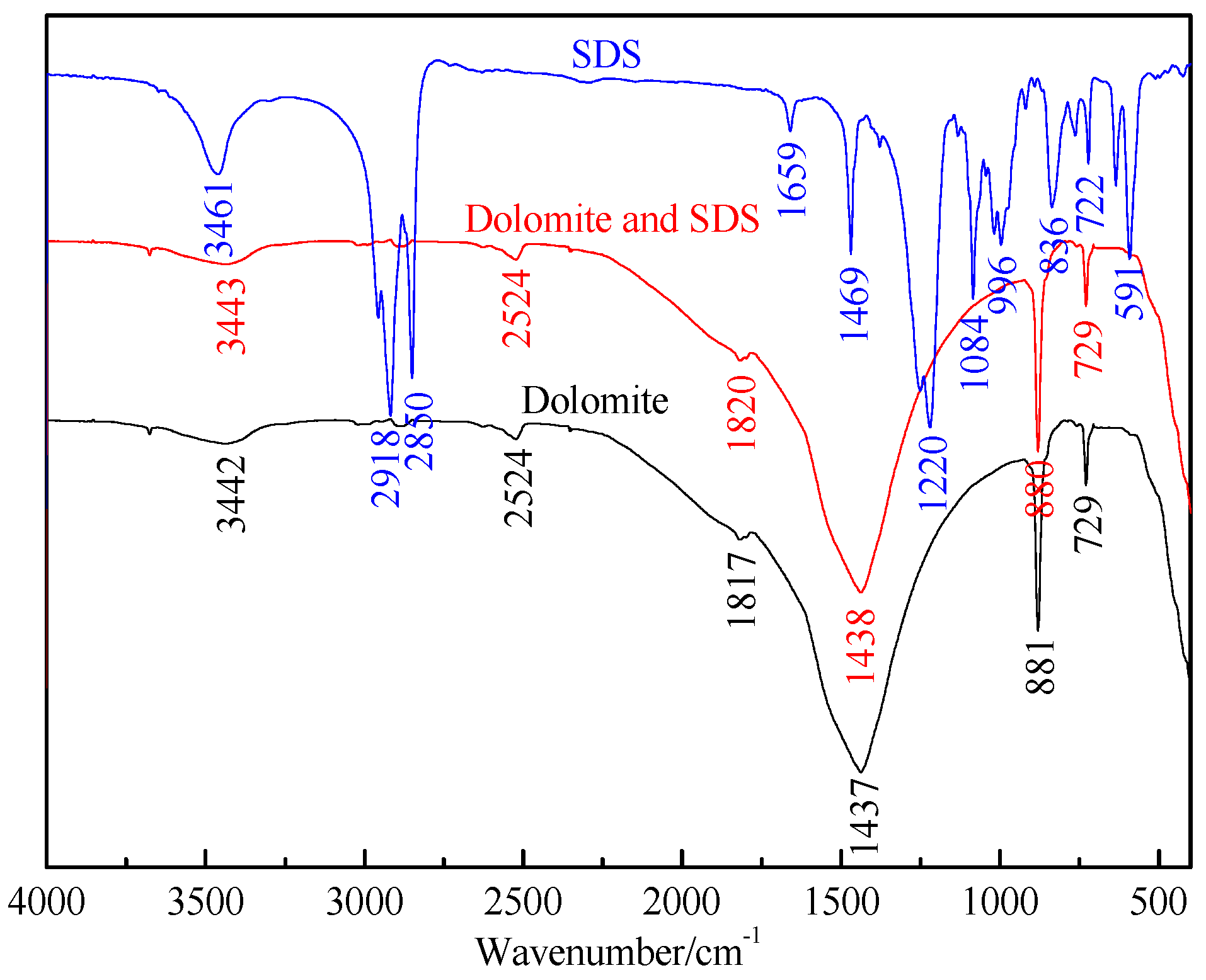
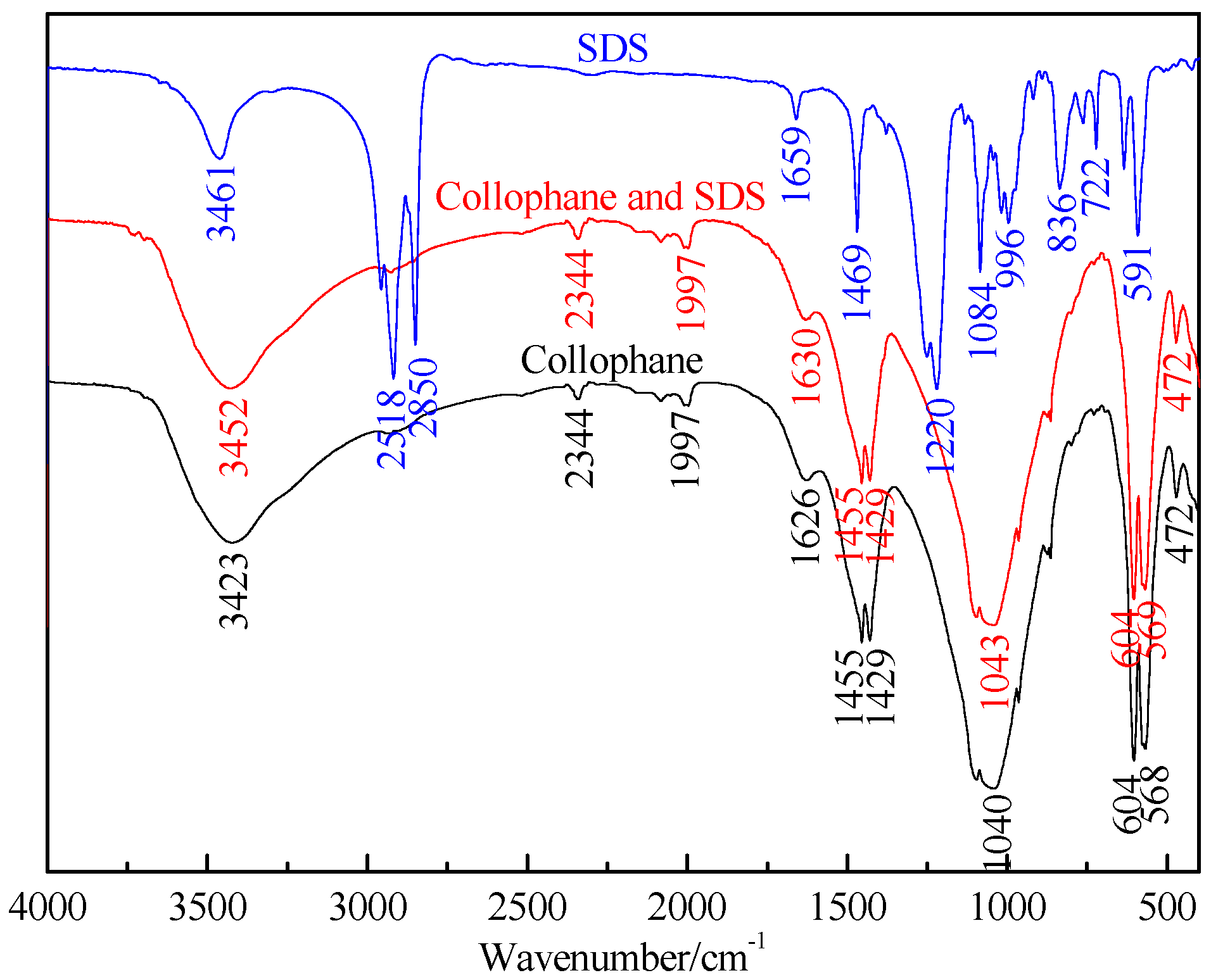
2.2.5. FTIR Spectrum
The infrared spectra were obtained on a NEXUS670 spectrometer (Thermo Nicolet, MA, USA). The FTIR (Fourier Transform Infrared Spectroscopy) spectra of samples were recorded in the range from 400 to 4000 cm−1. Collophane or dolomite (0.15 g, −0.045 mm) was equilibrated with 100 mL of the SDS solution at a concentration of 20 mg/L and pH of 7.5. The solution was stirred at 250 rpm for 6 h, then gently washed three times with deionized water and air-dried after conditioning. Fifty milligrams of the sample was mixed with 100 mg of KBr powder in an agate mortar, then the mixture was ground to further reduce the particle size and complete the mixing process. The powdered mixture was then pressed into a thin plate for FTIR analysis.