Dielectric Properties of Zinc Sulfide Concentrate during the Roasting at Microwave Frequencies
Abstract
:1. Introduction
2. Experimental
2.1. Material and Preparation
2.2. Measurement of Dielectric Properties
2.3. Thermogravimetric Analysis-Differential Scanning Calorimetry Analysis of Zinc Sulfide Concentrate
2.4. Dielectric Properties Modeling
3. Results and Discussion
3.1. Dielectric Properties of Zinc Sulfide Concentrate
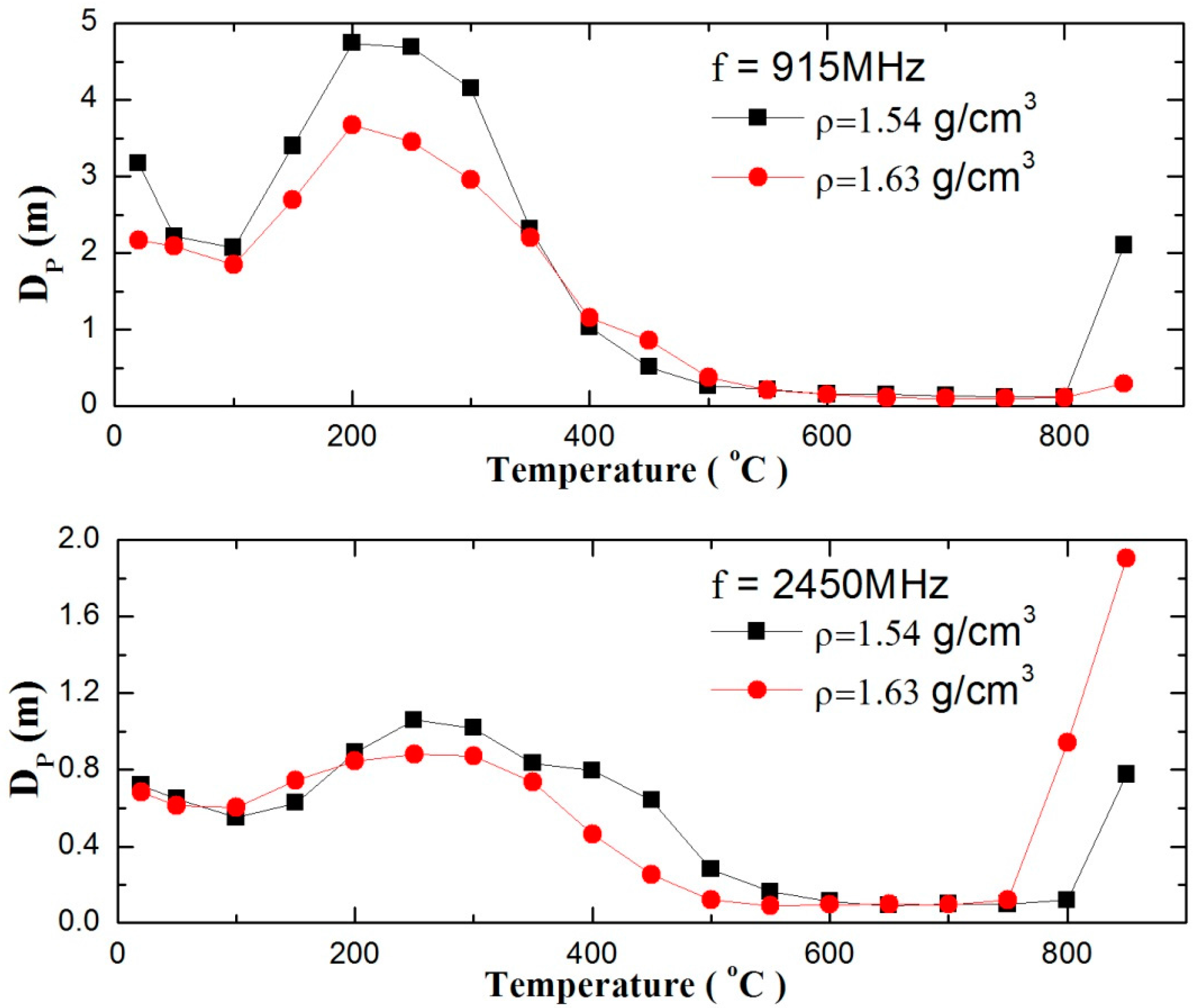
3.2. Effect of Temperature on Penetrate Depth of Zinc Sulfide Concentrate
3.3. Heating Experimental Results in the Microwave Oven
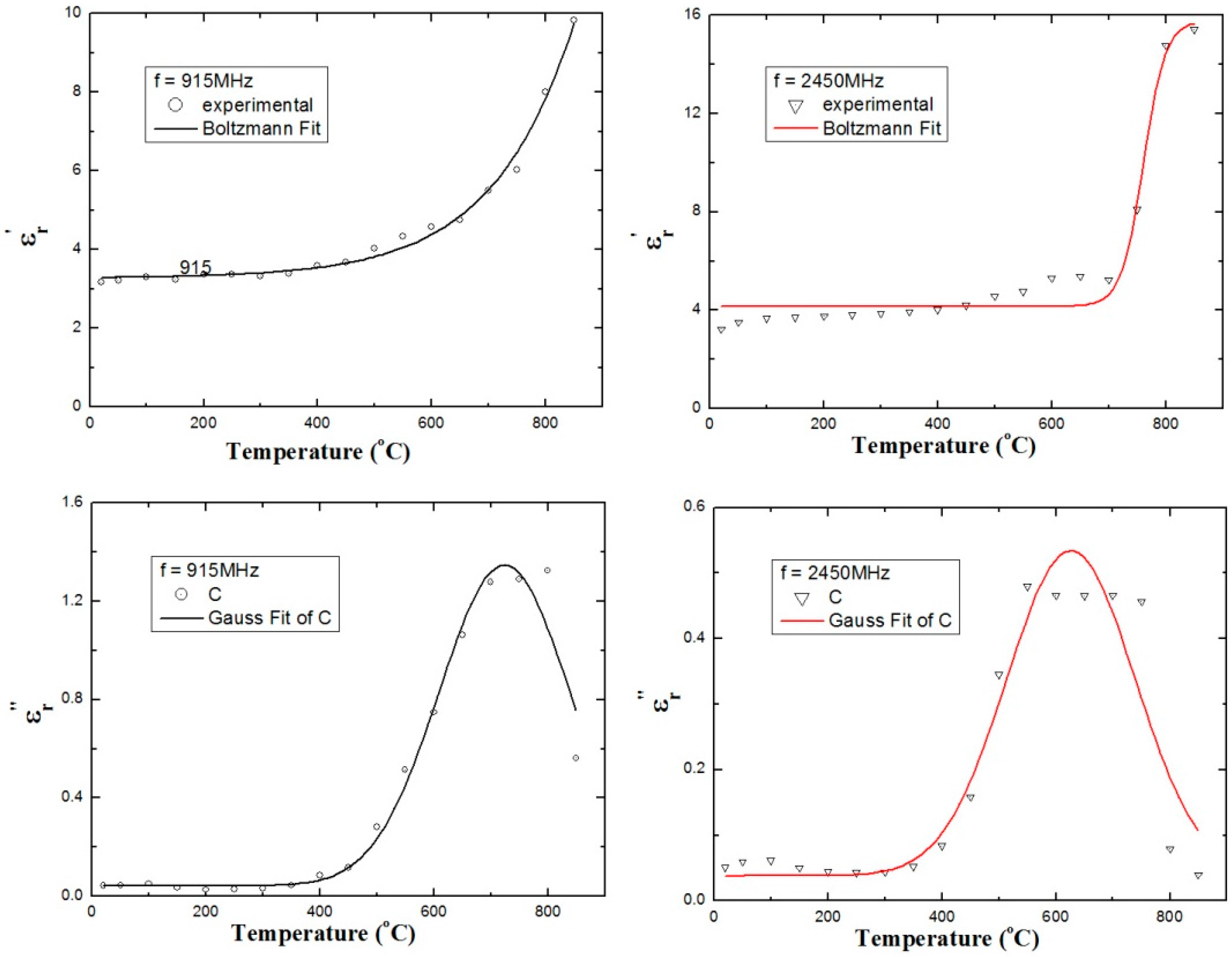
3.4. Modeling the Dielectric Constant
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gregurek, D.; Peng, Z.W.; Wenzl, C. Lead and zinc metallurgy. JOM 2015, 67, 1986–1987. [Google Scholar] [CrossRef]
- Al-harahsheh, M.; Kingman, S.; Bradshaw, S. The reality of non-thermal effects in microwave assisted leaching systems? Hydrometallurgy 2006, 84, 1–13. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Haque, K.E. Microwave energy for mineral treatment process—A brief review. Int. J. Miner. Process. 1999, 57, 1–24. [Google Scholar] [CrossRef]
- Tang, J.M. Unlocking potentials of microwaves for food safety and quality. J. Food Sci. 2015, 80, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Hwang, J.Y.; Kim, B.G.; Mouris, J.; Hutcheon, R. Microwave absorption capability of high volatile bituminous coal during pyrolysis. Energy Fuels 2012, 26, 5146–5151. [Google Scholar] [CrossRef]
- Laybourn, A.; Katrib, J.; Palade, P.A.; Easun, T.L.; Champness, N.R.; Schroder, M.; Kingman, S.W. Understanding the electromagnetic interaction of metal organic framework reactants in aqueous solution at microwave frequencies. Phys. Chem. Chem. Phys. 2016, 18, 5419–5431. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Sahu, J.N.; Ganesan, P.; Dey, T.K. Effect of temperature on dielectric properties and penetration depth of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS. Fuel 2015, 153, 257–266. [Google Scholar] [CrossRef]
- Beatriz, G.B.; Jose, C.C.; Felipe, P.F.; Pedro, P.G.; Gabriel, L.V. In situ monitoring of materials at high temperatures through dielectric properties measurement. Materials 2016, 9, 349. [Google Scholar]
- Chen, W.H.; Zhang, L.B.; Peng, J.H.; Yin, S.H.; Ma, A.Y.; Yang, K.; Li, S.W.; Xie, F. Effects of roasting pretreatment on zinc leaching from complicated zinc ores. Green Process. Synth. 2016, 5, 41–47. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.W.; Zhang, L.B.; Peng, J.H.; Chen, W.H.; Xie, F.; Ma, A.Y. Microwave roasting and leaching of an oxide-sulfide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Makul, N.; Rattanadecho, P.; Agrawal, D.K. Applications of microwave energy in cement and concrete—A review. Renew. Sustain. Energy Rev. 2014, 37, 715–733. [Google Scholar] [CrossRef]
- Beberoso, D.; Albero-ortiz, A.; Monzo-cabrera, J.; Diza-morcillo, A.; Arenills, A.; Menendez, J.A. Dielectric characterization of biodegradable wastes during pyrolysis. Fuel 2016, 172, 146–152. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T.; Salema, A.A.; Mouris, J.; Hutcheon, R.M. Microwave dielectric characterization of switchgrass for bioenergy and biofuel. Fuel 2014, 124, 151–157. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T.; Salema, A.A. Microwave dielectric characterization of hay during pyrolysis. Ind. Crop. Prod. 2014, 61, 492–498. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.L.; Yue, Q.Y. Review on microwave-matter interaction fundaments and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef]
- Muley, P.D.; Boldor, D. Investigation of microwave dielectric properties of biodiesel components. Bioresour. Technol. 2013, 127, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Julrat, S.; Chongcheawchamnan, M.; Robertson, L.D. Characterization of the dielectric properties of rubber latex from 0.5 to 33 GHz. Biosyst. Eng. 2014, 125, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yu, X.J.; Li, X.B. Zinc recovery from franklinite by sulphation roasting. Hydrometallurgy 2011, 109, 211–214. [Google Scholar] [CrossRef]
- Boyanov, B.; Peltekov, A.; Petkova, V. Thermal behavior of zinc sulfide concentrates with different iron content at oxidative roasting. Thermochim. Acta 2014, 586, 9–16. [Google Scholar] [CrossRef]
- Peng, Z.W.; Hwang, J.Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Franco, A.P.; Yamamoto, L.Y.; Tadini, C.C.; Gut, J.A. Dielectric properties of green coconut water relevant to microwave processing: Effect of temperature and field frequency. J. Food Eng. 2015, 155, 69–78. [Google Scholar] [CrossRef]
- Peng, Z.W.; Lin, X.L.; Wu, X.J.; Hwang, J.Y.; Kim, B.G.; Zhang, Y.B. Microwave absorption characteristics of anthracite during pyrolysis. Fuel Process. Technol. 2016, 150, 58–63. [Google Scholar] [CrossRef]
- Nowak, D.; Granat, K.; Opyd, B. Examination and analysis of influence of compaction degree on dielectric properties of moulding sand components. Metalurgija 2015, 54, 353–356. [Google Scholar]
- Zhang, L.B.; Ma, A.Y.; Liu, C.H.; Qu, W.W.; Peng, J.H.; Luo, Y.G.; Zuo, Y.G. Dielectric properties and temperature increase characteristics of zinc oxide dust from fuming furnace. Trans. Nonferr. Met. Soc. China 2014, 24, 4004–4011. [Google Scholar] [CrossRef]
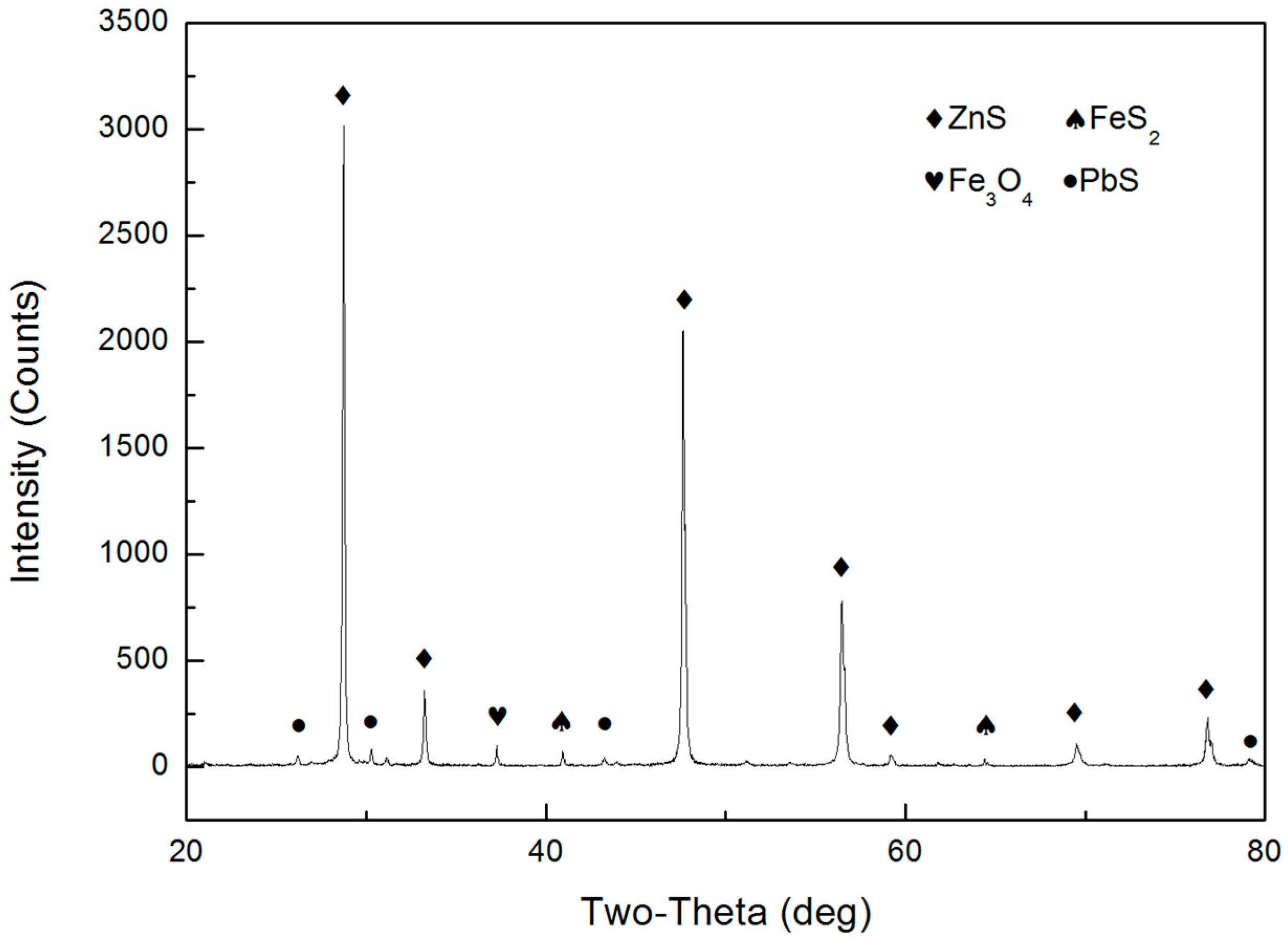
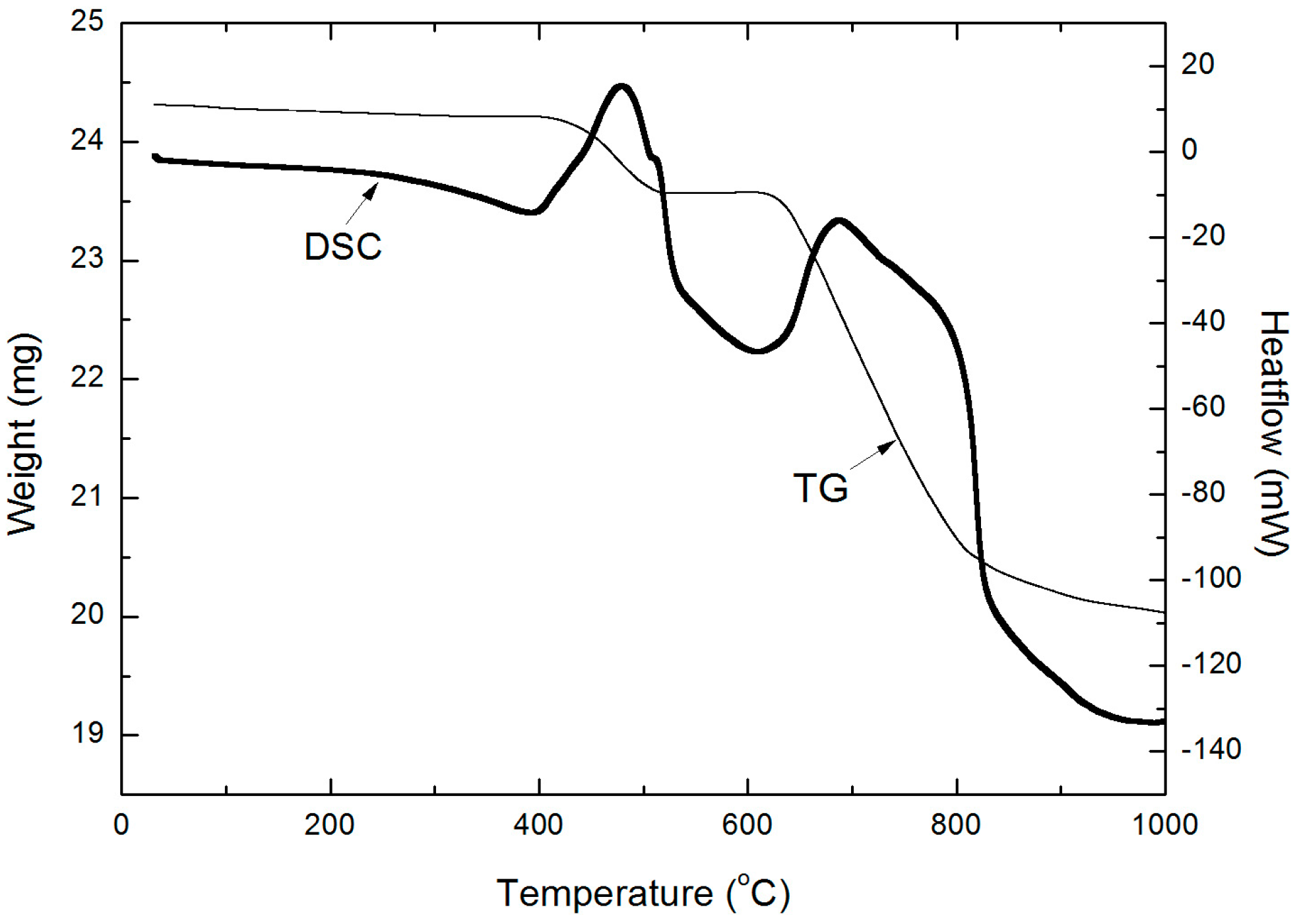
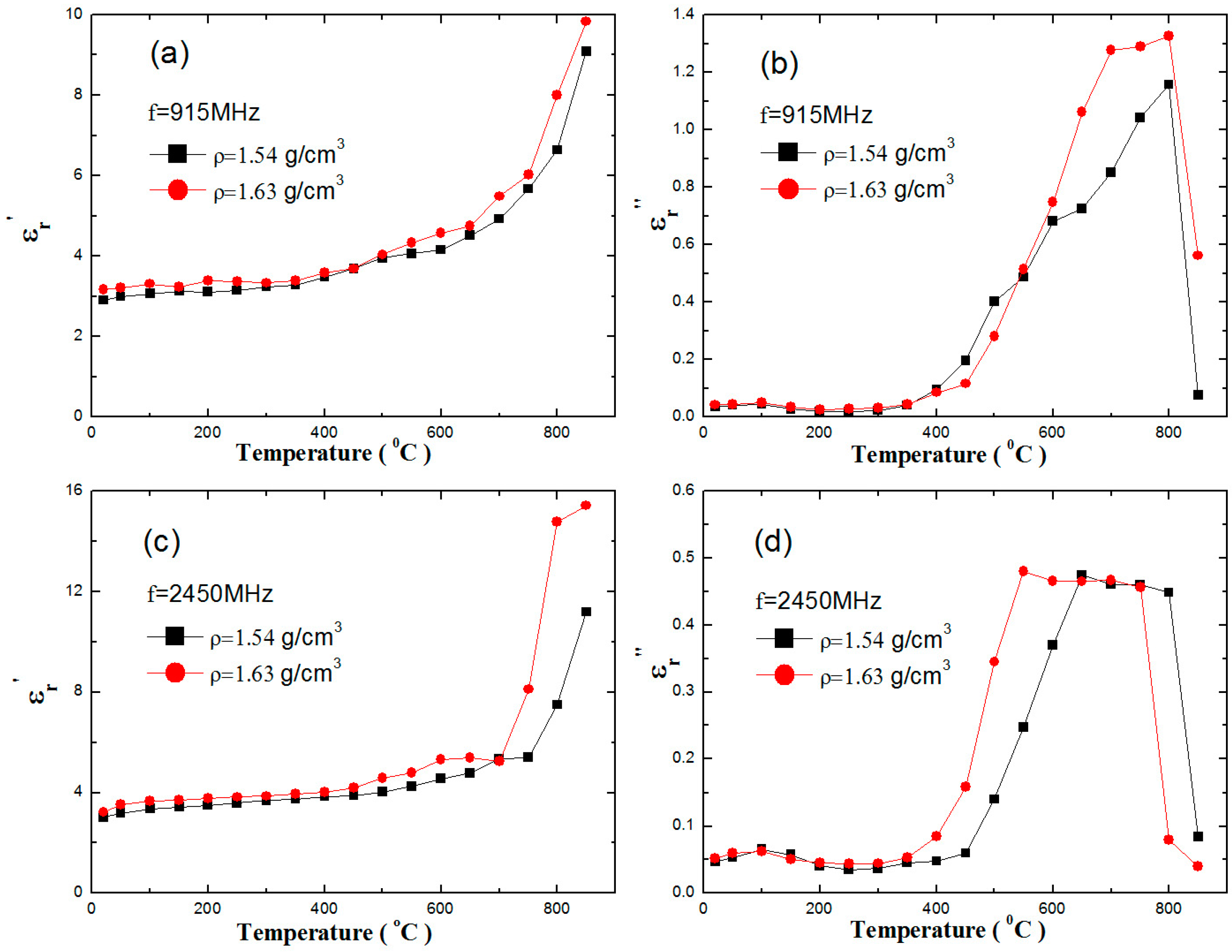
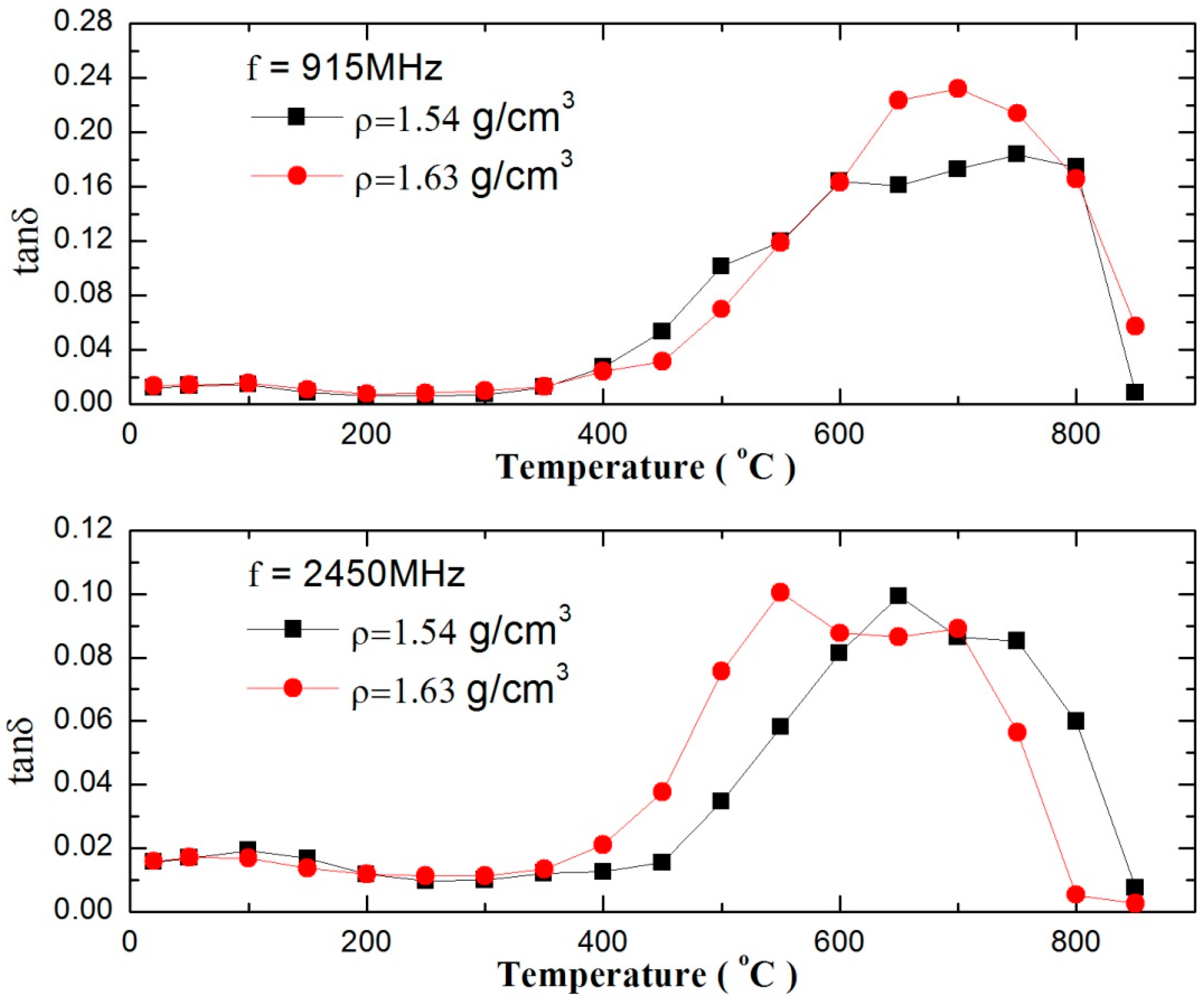
| ZnT | S | Fe | Pb |
|---|---|---|---|
| 51.16 | 32.98 | 8.26 | 1.95 |
| Zinc Phase | Zinc Sulfide | Zinc Carbonate | Zinc Silicate | Franklinite et al. | ZnT |
|---|---|---|---|---|---|
| Mass fraction/% | 49.55 | 0.80 | 0.64 | 0.17 | 51.16 |
| Distribution/% | 96.85 | 1.56 | 1.25 | 0.33 | 100 |
| Parameter | Model | Frequency | Regression Coefficients | R2 | |||
|---|---|---|---|---|---|---|---|
| Boltzmann model | A1 | A2 | T0 | dT | |||
| 915 MHz | 3.27 | 46,800.40 | 2108.67 | 141.71 | 0.99 | ||
| 2450 MHz | 4.16 | 15.79 | 760.94 | 19.47 | 0.97 | ||
| Gauss model | y0 | T0 | w | A | |||
| 915 MHz | 0.04 | 724.12 | 228.83 | 374.52 | 0.97 | ||
| 2450 MHz | 0.04 | 626.37 | 225.13 | 139.98 | 0.90 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Li, S.; Yang, K.; Liu, J.; Liu, P.; Zhang, L.; Peng, J. Dielectric Properties of Zinc Sulfide Concentrate during the Roasting at Microwave Frequencies. Minerals 2017, 7, 31. https://doi.org/10.3390/min7020031
He G, Li S, Yang K, Liu J, Liu P, Zhang L, Peng J. Dielectric Properties of Zinc Sulfide Concentrate during the Roasting at Microwave Frequencies. Minerals. 2017; 7(2):31. https://doi.org/10.3390/min7020031
Chicago/Turabian StyleHe, Guangjun, Shiwei Li, Kun Yang, Jian Liu, Peng Liu, Libo Zhang, and Jinhui Peng. 2017. "Dielectric Properties of Zinc Sulfide Concentrate during the Roasting at Microwave Frequencies" Minerals 7, no. 2: 31. https://doi.org/10.3390/min7020031






