Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis
Abstract
:1. Introduction
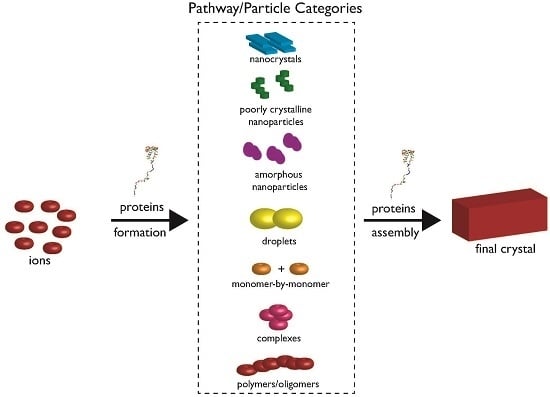
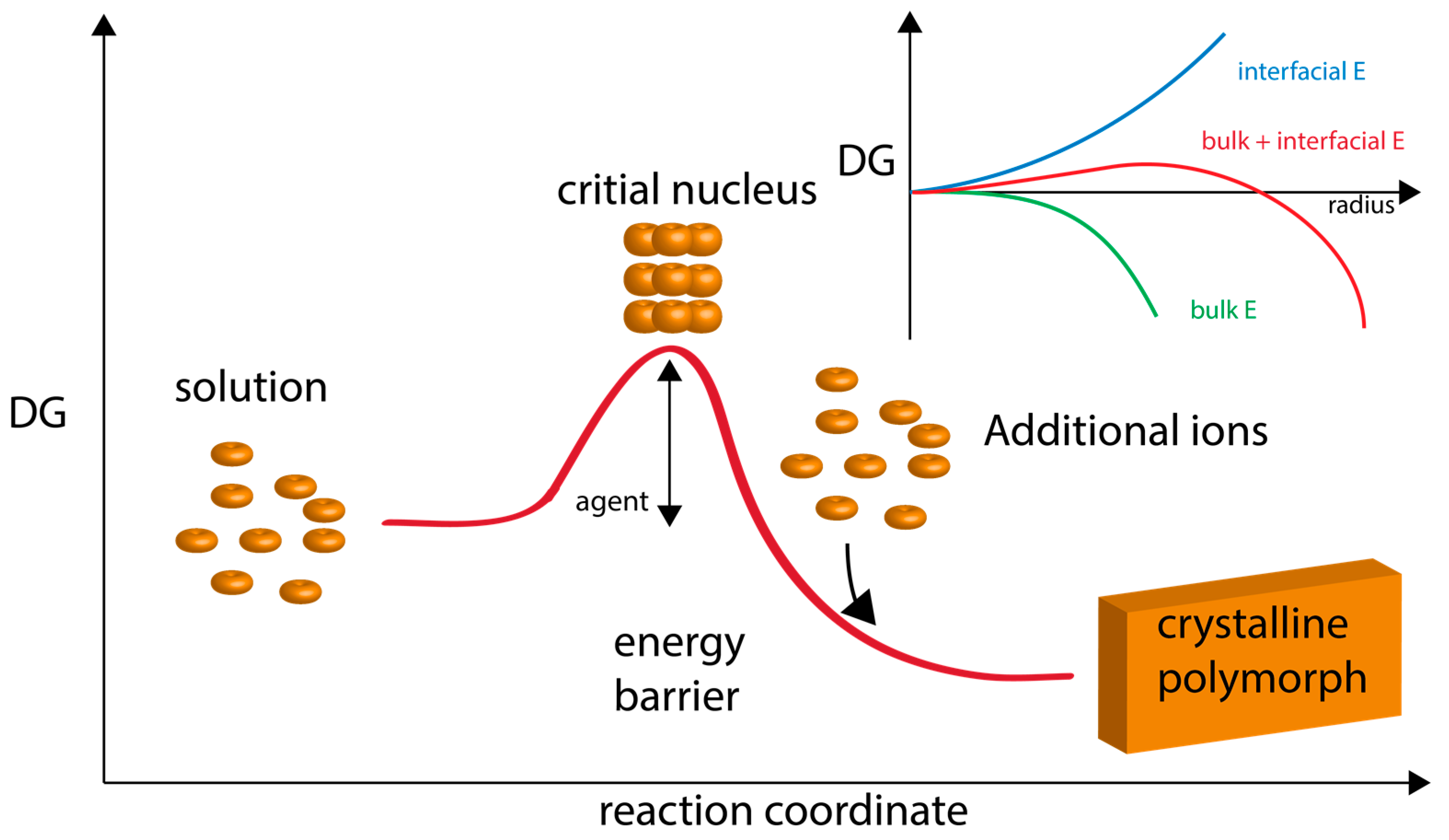
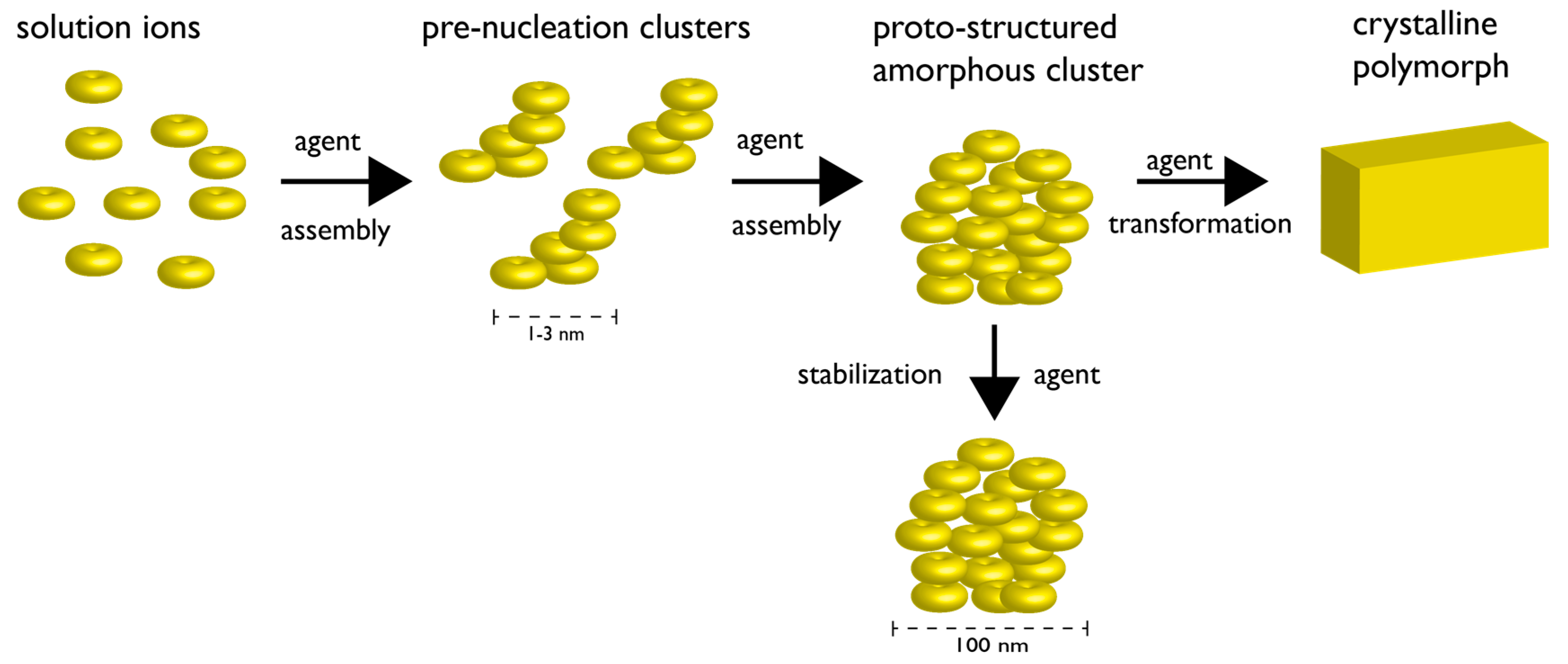
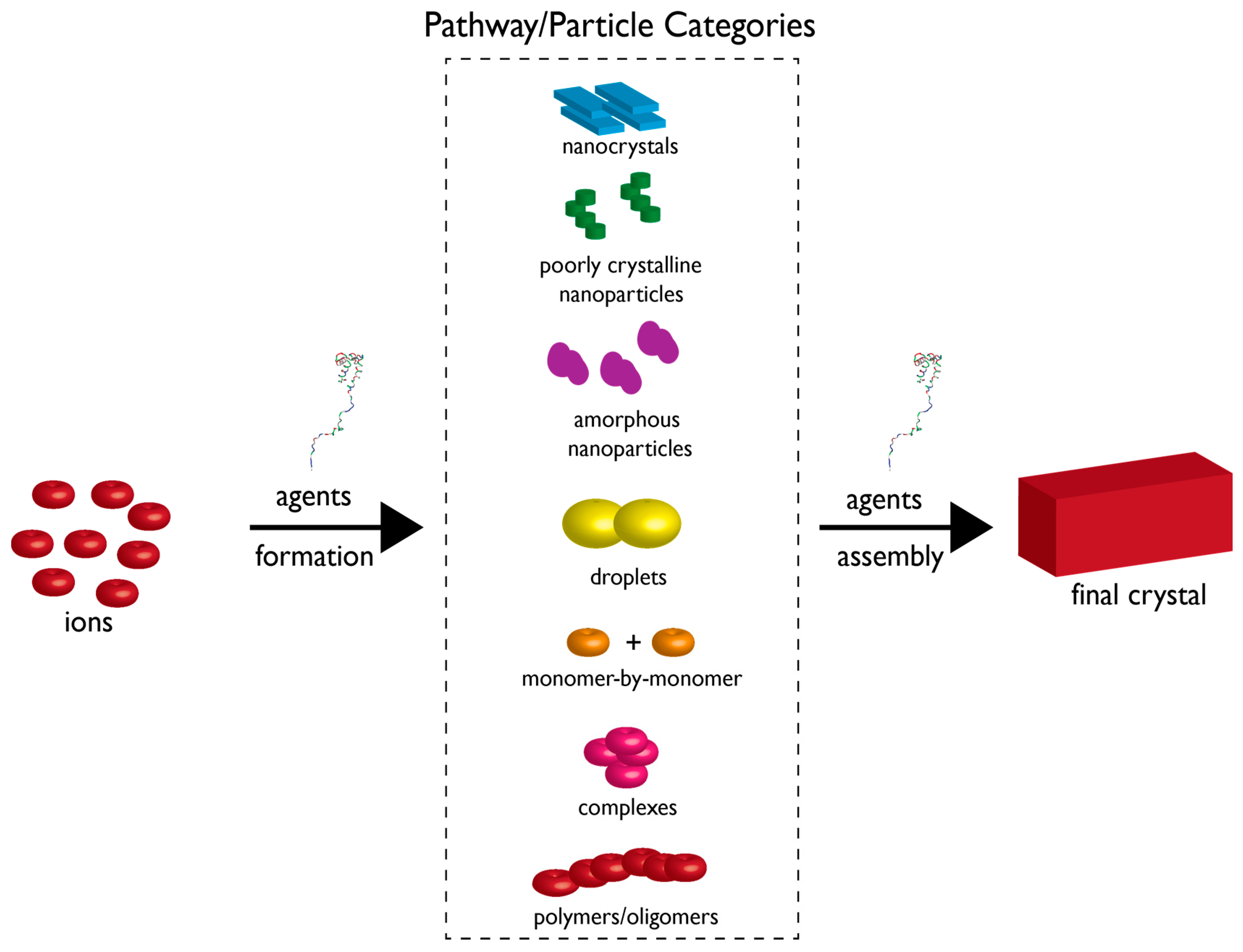
2. The Current State of Knowledge Regarding Nucleation and Polymorph Formation
3. How Biomineralization Proteomes Might Fit into Recently Proposed Nucleation Schemes
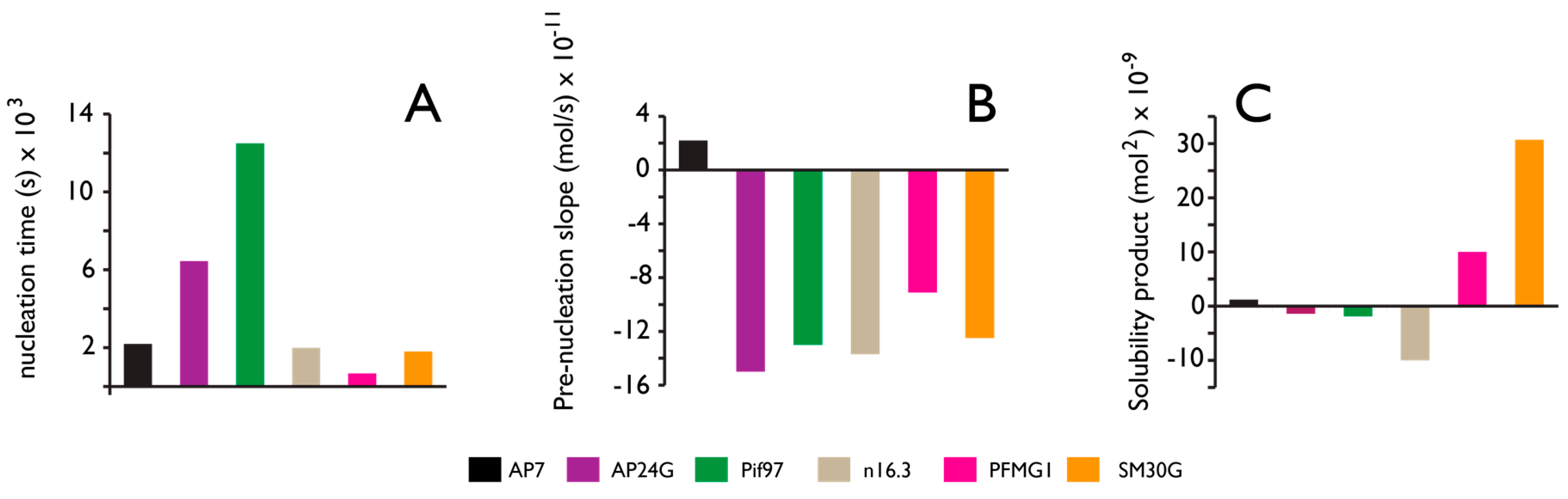
4. Protein-Polymorph Formation and Stabilization—What Do We Currently Know?
5. Moving towards More Informative Studies
6. The Next Steps
Acknowledgments
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, X. Uncovering aragonite nanoparticle self-assembly in nacre—A natural armor. Cryst. Growth Des. 2012, 12, 4306–4310. [Google Scholar] [CrossRef]
- Li, X.; Chang, W.C.; Chao, Y.J.; Wang, R.; Chang, M. Nanoscale structural and mechanical characterization of a natural nanocomposite material: The shell of red abalone. NanoLetters 2004, 4, 613–617. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Berman, A.; Addadi, L.; Kvick, A.; Leiserowitz, L.; Nelson, M.; Weiner, S. Intercalation of sea urchin proteins in calcite: Study of a crystalline composite material. Science 1990, 250, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar]
- Mann, K.; Poustka, A.J.; Mann, M. The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes. Proteome Sci. 2008, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Wilt, F.H.; Poustka, A.J. Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix. Proteome Sci. 2010, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tester, C.C.; Wu, C.H.; Krejci, M.R.; Mueller, L.; Park, A.; Lai, B.; Chen, S.; Sun, C.; Joester, D. Time-resolved evolution of short- and long-range order during the transformation of ACC to calcite in the sea urchin embryo. Adv. Funct. Mater. 2013, 23, 4185–4194. [Google Scholar] [CrossRef]
- Politi, Y.; Metzler, R.A.; Abrecht, M.; Gilbert, B.; Wilt, F.H.; Sagi, I.; Addadi, L.; Weiner, S.; Gilbert, P. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc. Natl. Acad. Sci. USA 2008, 105, 17362–17366. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.U.T.; Killian, C.E.; Olson, I.C.; Appathurai, N.P.; Amasino, A.L.; Martin, M.C.; Holt, L.J.; Wilt, F.H.; Gilbert, P. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2012, 109, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.L.; Mass, T.; Haramaty, L.; Zelzion, E.; Bhattacharya, D.; Falkowski, P.G. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proc. Natl. Acad. Sci. USA 2013, 110, 3788–3793. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Luisman, L.; Ball, E.E.; Hayward, D.C.; Grasso, L.C.; Chua, C.M.; Woo, H.N.; Gattuso, J.P.; Forêt, S.; Miller, D.J. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol. Ecol. 2012, 21, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, S.; Vergni, P.; Reggi, M.; Caroselli, E.; Sparla, F.; Levy, O.; Dubinsky, Z.; Falini, G. The skeletal organic matrix from Mediterranean coral Balanophyllia europaea influences calcium carbonate precipitation. PLoS ONE 2011, 6, e22338. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Liquid-liquid separation in supersaturated CaCO3 solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wagermaier, W.; Masic, A.; Kommareddy, K.P.; Bennet, M.; Manjubala, I.; Lee, S.W.; Park, S.B.; Cölfen, H.; Fratzl, P. Self-assembly of amorphous calcium carbonate microlens arrays. Nat. Commun. 2012, 3, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 2002, 293, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J. Polymorphism—A perspective. Cryst. Growth Des. 2011, 11, 632–650. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, M.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in water-alcohol mixtures: Spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth. Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Olderoy, M.O.; Xie, M.; Strand, B.L.; Draget, K.I.; Sikorski, P.; Andreassen, J.P. Polymorph switching in the calcium carbonate system by well-defined alginate oligomers. Cryst. Growth Des. 2011, 11, 520–529. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Liu, Y.; Liu, H.; Xu, F.; Wang, L. Vaterite selection by chitosan gel: An example of polymorph selection by morphology of biomacromolecules. Cryst. Growth Des. 2008, 8, 2887–2891. [Google Scholar] [CrossRef]
- Sun, W.; Jayaraman, S.; Chen, W.; Persson, K.A.; Ceder, G. Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl. Acad. Sci. USA 2015, 112, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Kababya, S.; Gal., A.; Kahil, K.; Weiner, S.; Addadi, L.; Schmidt, A. Phosphate-water interplay tunes amorphous calcium carbonate metastability: Spontaneous phase separation and crystallization versus stabilization viewed by solid-state NMR. J. Am. Chem. Soc. 2015, 137, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Immel, F.; Gaspard, D.; Marie, A.; Guichard, N.; Cusack, M.; Marin, F. Shell proteome of rhynchonelliform brachiopods. J. Struct. Biol. 2015, 190, 360–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, X.D.; Guo, X.M.; Li, L.; Luo, R.B.; Xu, F.; Yang, P.C.; Zhang, L.L.; Wang, X.T.; Qi, H.G.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; McDougall, C.; Woodcroft, B.; Moase, P.; Rose, R.A.; Kube, M.; Reinhart, R.; Rokhsar, D.S.; Montagnani, C.; Joube, C.; et al. Parallel evolution of nacre building gene sets in mollusks. Mol. Biol. Evol. 2010, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergstrom, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Volkel, A.; Cölfen, H. Stable prenucleation of calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bascik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergstrom, L.; Tai, C.W.; Sham, T.K.; et al. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef] [PubMed]
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusk shell proteins. Nature 1996, 381, 56–58. [Google Scholar] [CrossRef]
- Fritz, M.; Belcher, A.M.; Radmacher, M.; Walters, D.A.; Hansma, P.K.; Stucky, G.D.; Morse, D.E.; Mann, S. Flat pearls from biofabrication of organized composites on inorganic substrates. Nature 1994, 371, 49–51. [Google Scholar] [CrossRef]
- Su, J.; Zhu, F.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Transformation of amorphous calcium carbonate nanoparticles into aragonite controlled by ACCBP. CrystEngComm 2016, 18, 2125–2134. [Google Scholar] [CrossRef]
- Thompson, J.B.; Paloczi, G.T.; Kindt, J.H.; Michenfelder, M.; Smith, B.L.; Stucky, G.; Morse, D.E.; Hansma, P.K. Direct observation of the transition from calcite to aragonite growth as induced by abalone shell proteins. Biophys. J. 2000, 79, 3307–3312. [Google Scholar] [CrossRef]
- Seto, J.; Picker, A.; Evans, J.S.; Cölfen, H. A nacre protein sequence organizes the mineralization space for polymorph formation. Cryst. Growth Des. 2014, 14, 1501–1505. [Google Scholar] [CrossRef]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Su, J.; Zheng, J.G.; Liang, J.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Patterns of expression in the matrix proteins responsible for nucleation and growth of aragonite crystals in flat pearls of Pinctada fucata. PLoS ONE 2013, 8, e66564. [Google Scholar] [CrossRef]
- Yan, Z.; Jing, G.; Gong, N.; Li, C.; Zhou, Y.; Xie, L.; Zhang, R. N40, a novel nonacidic matrix protein from pearl oyster nacre, facilitates nucleation of aragonite in vitro. Biomacromolecules 2007, 8, 3597–3601. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, H.; Du, X.; Zhao, X.; Wang, Q.; Huang, R.; Deng, R. Dermatopontin, a shell matrix protein gene from pearl oyster Pinctada martensii, participates in nacre formation. Biochem. Biophys. Res. Commun. 2012, 425, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Huang, J.; Sun, J.; Wang, G.; Li, C.; Xie, L.; Zhang, R. A novel extrapallial fluid protein controls the morphology of nacre lamellae in the pearl oyster, Pinctada fucata. J. Biol. Chem. 2007, 282, 23253–23263. [Google Scholar] [CrossRef] [PubMed]
- Samata, T.; Hayashi, N.; Kono, M.; Hagesawa, K.; Horita, C.; Akera, S. A new matrix protein family related to the nacreous layer formation of Pinctada fucata. FEBS Lett. 1999, 462, 225–229. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Amos, F.F.; Evans, J.S. AP7, a partially disordered pseudo C-RING protein, is capable of forming stabilized aragonite in vitro. Biochemistry 2009, 48, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Ponce, C.B; Evans, J.S. Polymorph crystal selection by n16, an intrinsically disordered nacre framework protein. Cryst. Growth Des. 2011, 11, 4690–4696. [Google Scholar] [CrossRef]
- Amos, F.F.; Ponce, C.B.; Evans, J.S. Formation of framework nacre polypeptide supramolecular assemblies that nucleate polymorphs. Biomacromolecules 2011, 12, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Metzler, R.A.; Evans, J.S.; Kilian, C.E.; Zhou, D.; Churchill, T.H.; Appathurai, P.N.; Coppersmith, S.N.; Gilbert, P.U.P.A. Lamellar self-assembly and aragonite polymorph selection by a single intrinsically disordered protein fragment. J. Am. Chem. Soc. 2010, 132, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Keene, E.C.; Evans, J.S.; Estroff, L.A. Matrix interactions in biomineralization: Aragonite nucleation by an intrinsically disordered nacre polypeptide, n16N, associated with a β-chitin substrate. Cryst. Growth Des. 2010, 10, 1383–1389. [Google Scholar] [CrossRef]
- Amos, F.F.; Destine, E.; Ponce, C.B.; Evans, J.S. The N- and C-terminal regions of the pearl-associated EF Hand protein, PFMG1, promote the formation of the aragonite polymorph in vitro. Cryst. Growth Des. 2010, 10, 4211–4216. [Google Scholar] [CrossRef]
- Natoli, A.; Wiens, M.; Schroder, H.C.; Stifanic, M.; Batel, R.; Soldati, A.L.; Jacob, D.E.; Muller, W.E.G. Bio-vaterite formation by glycoproteins from freshwater pearls. Micron 2010, 41, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yao, Q.Z.; Zhou, G.T.; Sheng, Y.M. Formation of vaterite mesocrystals in biomineral-like structures and implication for biomineralization. Cryst. Growth Des. 2015, 15, 1714–1725. [Google Scholar] [CrossRef]
- Kalikmanov, V.I. Nucleation Theory in Lecture Notes in Physics; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; Volume 860, pp. 17–41. [Google Scholar]
- Bano, A.M.; Rodger, P.M.; Quigley, D. New insight into the stability of CaCO3 surfaces and nanoparticles via molecular simulation. Langmuir 2014, 30, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.W.; Yazaydin, A.O.; Kirkpatrick, R.J.; Bowers, G.M. Structure and transformation of amorphous calcium carbonate: A solid-state 43Ca NMR and computational molecular dynamics investigation. Chem. Mater. 2012, 24, 1828–1836. [Google Scholar] [CrossRef]
- Ren, D.; Albert, O.; Sun, M.; Muller, W.E.G.; Feng, Q. Primary cell culture of fresh water Hyriopsis comingii mantle/pearl sac tissues and its effect on calcium carbonate mineralization. Cryst. Growth Des. 2014, 14, 1149–1157. [Google Scholar] [CrossRef]
- Chang, E.P.; Roncal-Herrero, T.; Morgan, T.; Dunn, K.E.; Rao, A.; Kunitake, J.A.M.R.; Lui, S.; Bilton, M.; Estroff, L.A.; Kröger, R.; et al. Synergistic biomineralization phenomena created by a nacre protein model system. Biochemistry 2016, 55, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Perovic, I.; Rao, A.; Cölfen, H.; Evans, J.S. Insect cell glycosylation and its impact on the functionality of a recombinant intracrystalline nacre protein, AP24. Biochemistry 2016, 55, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Evans, J.S. Pif97, a von Willebrand and Peritrophin biomineralization protein, organizes mineral nanoparticles and creates intracrystalline nanochambers. Biochemistry 2015, 54, 5348–5355. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Williamson, G.; Evans, J.S. Focused ion beam tomography reveals the presence of micro-, meso-, and macroporous intracrystalline regions introduced into calcite crystals by the gastropod nacre protein AP7. Cryst. Growth Des. 2015, 15, 1577–1582. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Verch, A.; Rao, A.; Cölfen, H.; Kröger, R.; Evans, J.S. An oligomeric C-RING nacre protein influences pre-nucleation events and organizes mineral nanoparticles. Biochemistry 2014, 53, 7259–7268. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kröger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by an intracrystalline biomineralization protein. Biochemistry 2014, 53, 4317–4319. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kröger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by framework biomineralization protein phases. CrystEngComm 2014, 16, 7406–7409. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Lui, M.; Rao, A.; Cölfen, H.; Evans, J.S. A framework nacre protein, n16.3, self-assembles to form protein oligomers that participate in the post-nucleation spatial organization of mineral deposits. Biochemistry 2014, 53, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Perovic, I.; Mandal, T.; Evans, J.S. A pseudo EF-hand pearl protein self-assembles to form protein complexes that amplify mineralization. Biochemistry 2013, 52, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Rao, A.; Cölfen, H.; Evans, J.S. A model sea urchin spicule matrix protein self-associates to form mineral-modifying hydrogels. Biochemistry 2016, 55, 4410–4421. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Seto, J.; Berg, J.K.; Kreft, S.G.; Scheffner, M.; Cölfen, H. Roles of larval sea urchin spicule SM50 domains in organic matrix self-assembly and calcium carbonate mineralization. J. Struct. Biol. 2013, 183, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S. Identification of intrinsically disordered and aggregation—Promoting sequences within the aragonite-associated nacre proteome. Bioinformatics 2012, 28, 3182–3185. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S. “Liquid-like” biomineralization protein assemblies: A key to the regulation of non-classical nucleation. CrystEngComm 2013, 15, 8388–8394. [Google Scholar] [CrossRef]
- Yan, F.; Jiao, Y.; Deng, Y.; Du, X.; Huang, R.; Wang, Q.; Chen, W. Tissue inhibitor of metalloprotease gene from pearl oyster Pinctada martensii participates in nacre formation. Biochem. Biophys. Res. Commun. 2014, 450, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Liu, S.F.; Ge, Y.J.; Liu, J.; Wang, X.Y.; Xie, L.P.; Zhang, R.; Wang, Z. Indentification and characterization of a biomineralization related gene PFMG1 highly expresses in the mantle of Pinctada fucata. Biochemistry 2007, 46, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fang, Z.; Ma, Z.; Deng, J.; Li, S.; Xie, L.; Zhang, R. Biomineralization: Functions of calmodulin-like protein in the shell formation of pearl oyster. Biochim. Biophys. Acta 2007, 1770, 1338–1344. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.S. Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals 2017, 7, 62. https://doi.org/10.3390/min7040062
Evans JS. Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals. 2017; 7(4):62. https://doi.org/10.3390/min7040062
Chicago/Turabian StyleEvans, John Spencer. 2017. "Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis" Minerals 7, no. 4: 62. https://doi.org/10.3390/min7040062
APA StyleEvans, J. S. (2017). Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals, 7(4), 62. https://doi.org/10.3390/min7040062