Characterization of Germanium Speciation in Sphalerite (ZnS) from Central and Eastern Tennessee, USA, by X-ray Absorption Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Sphalerite Samples
2.3. Method
2.4. Beamline Configuration
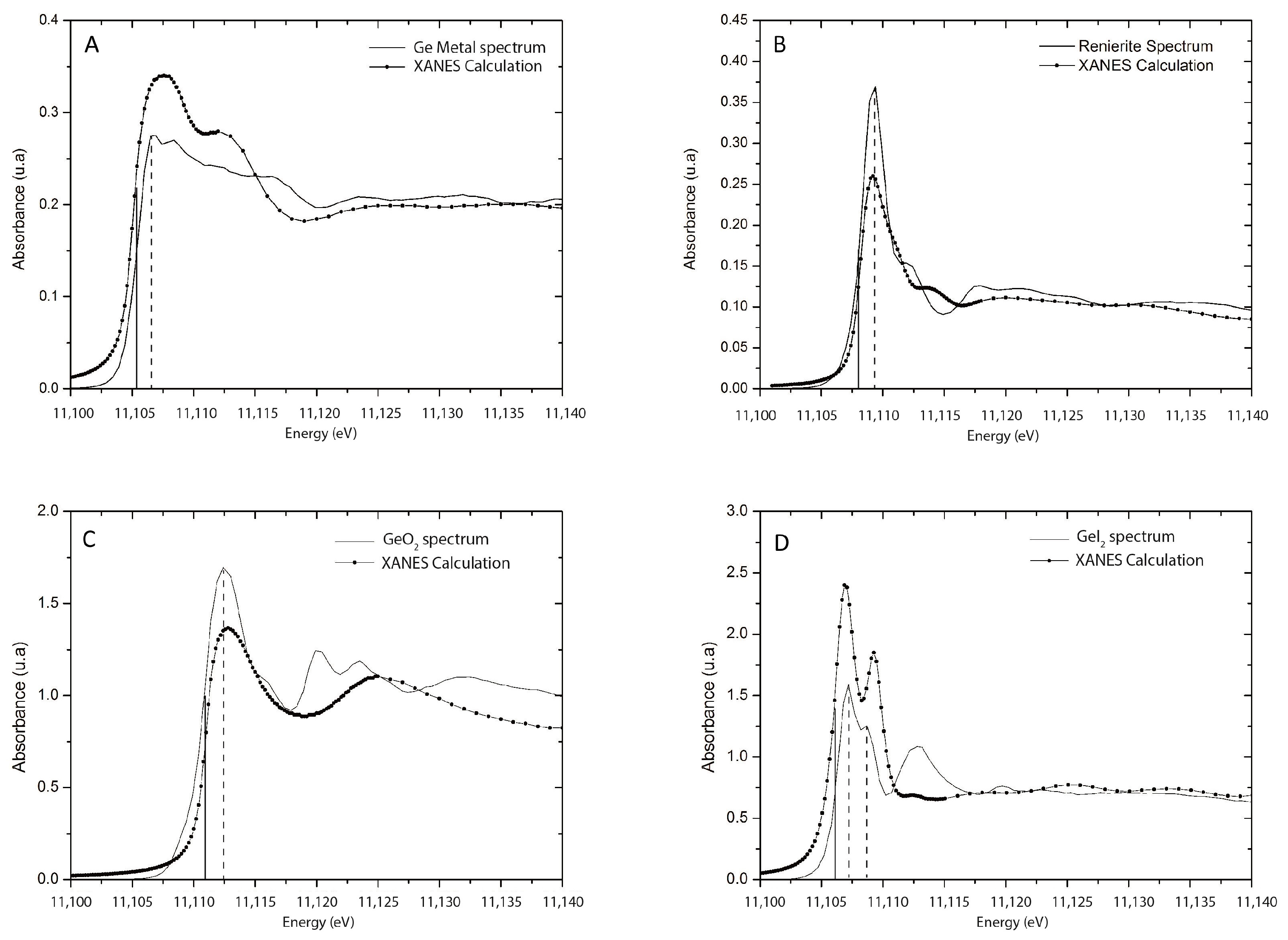
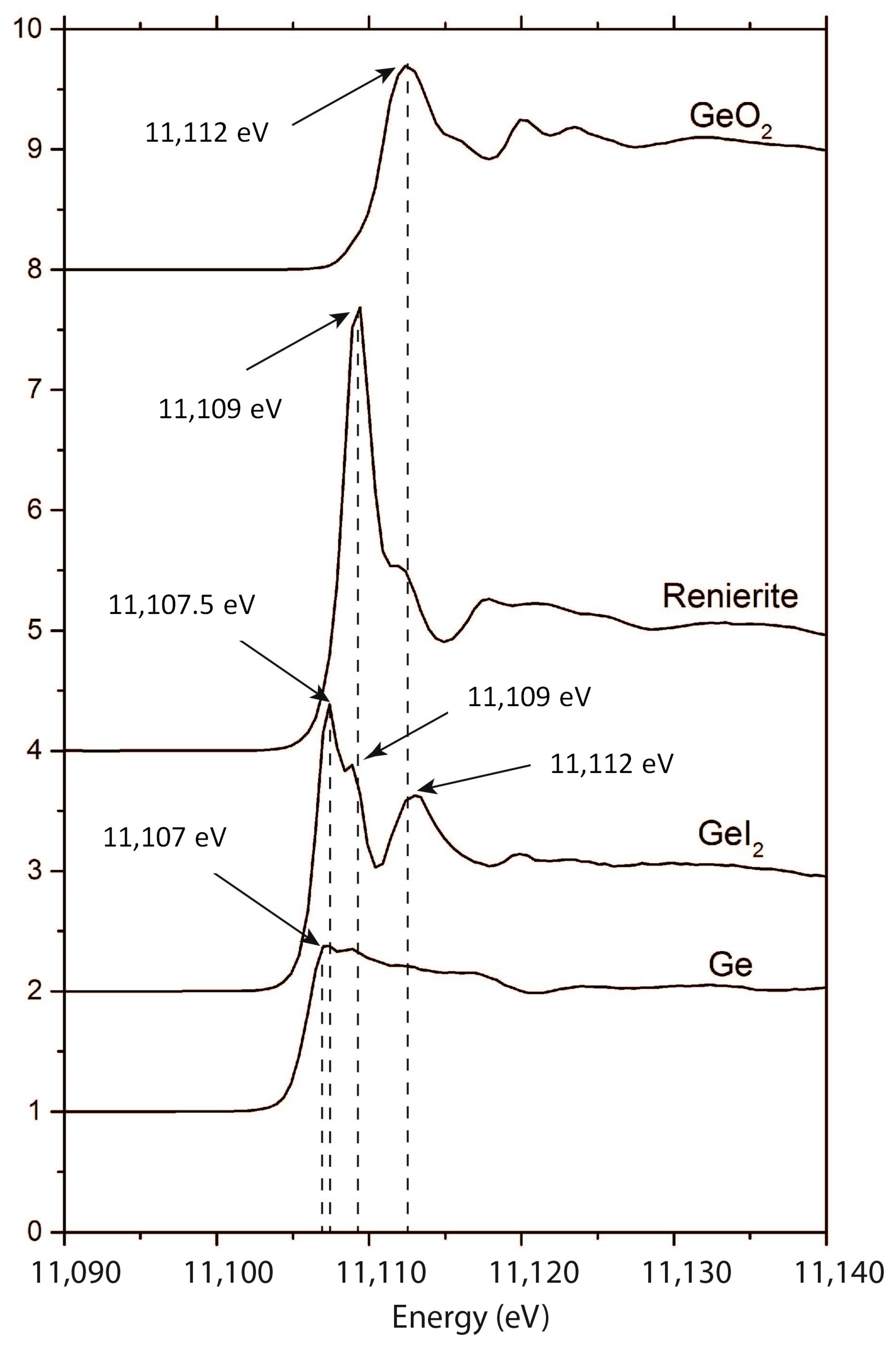
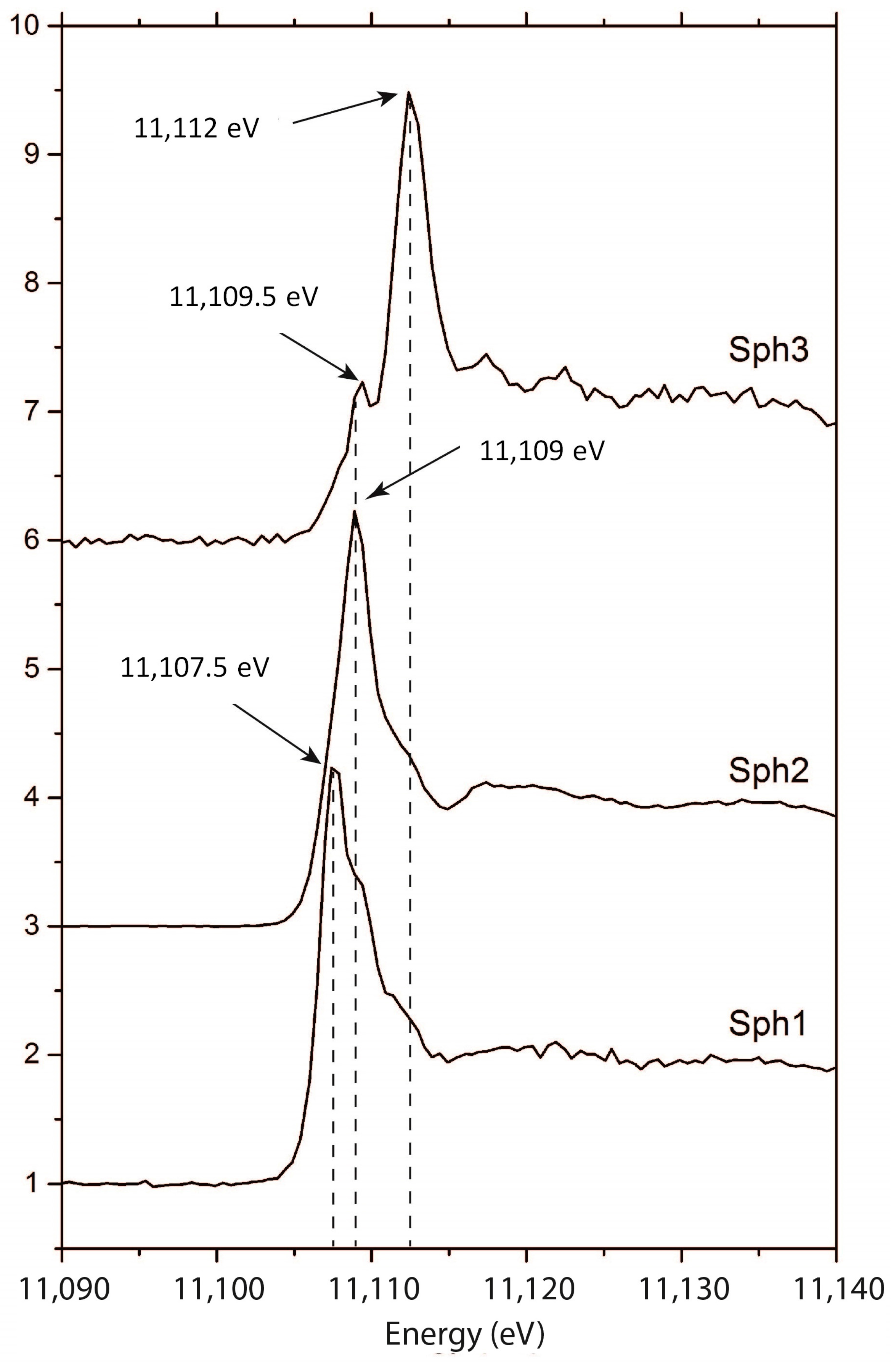
3. Results
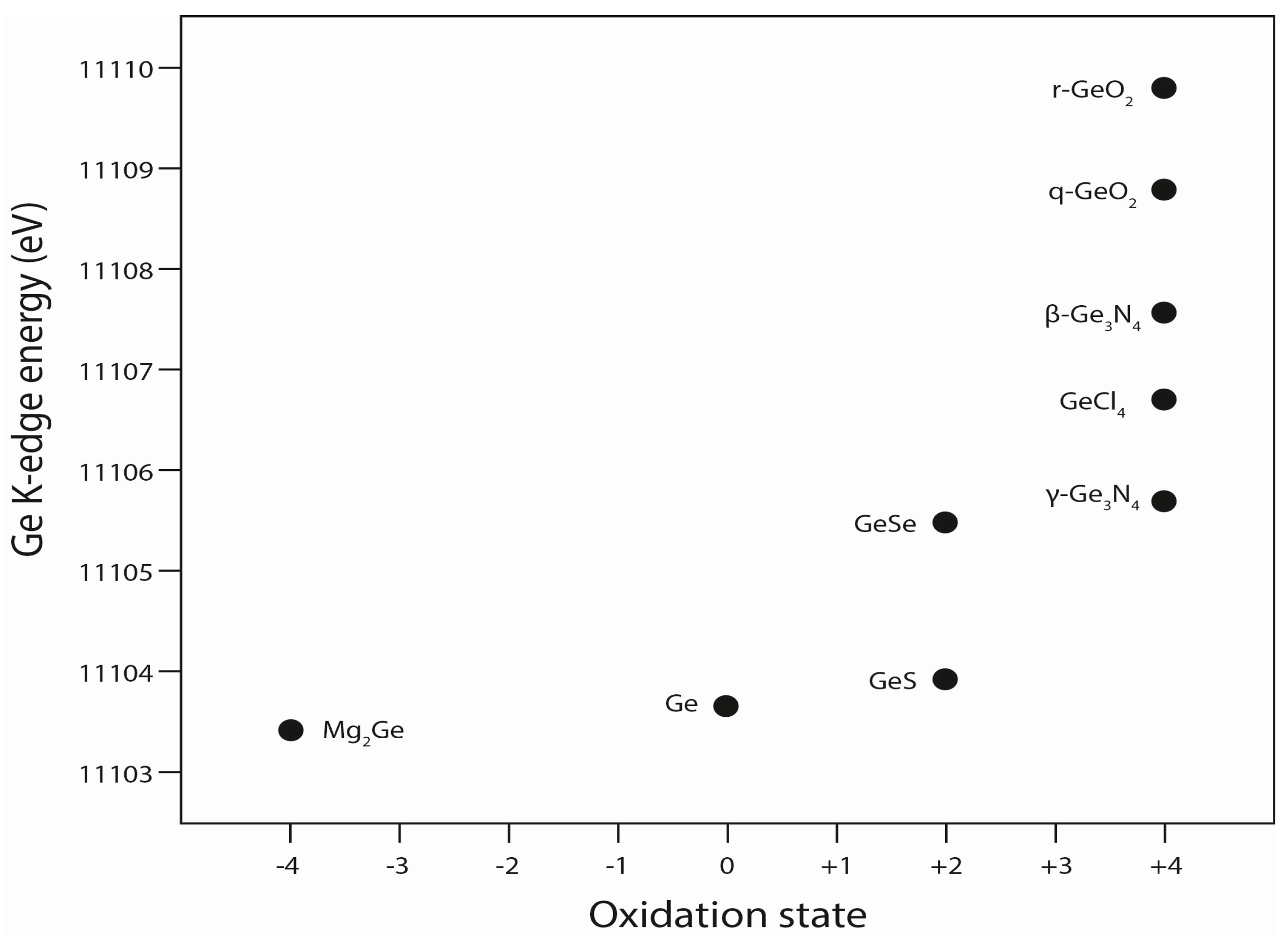
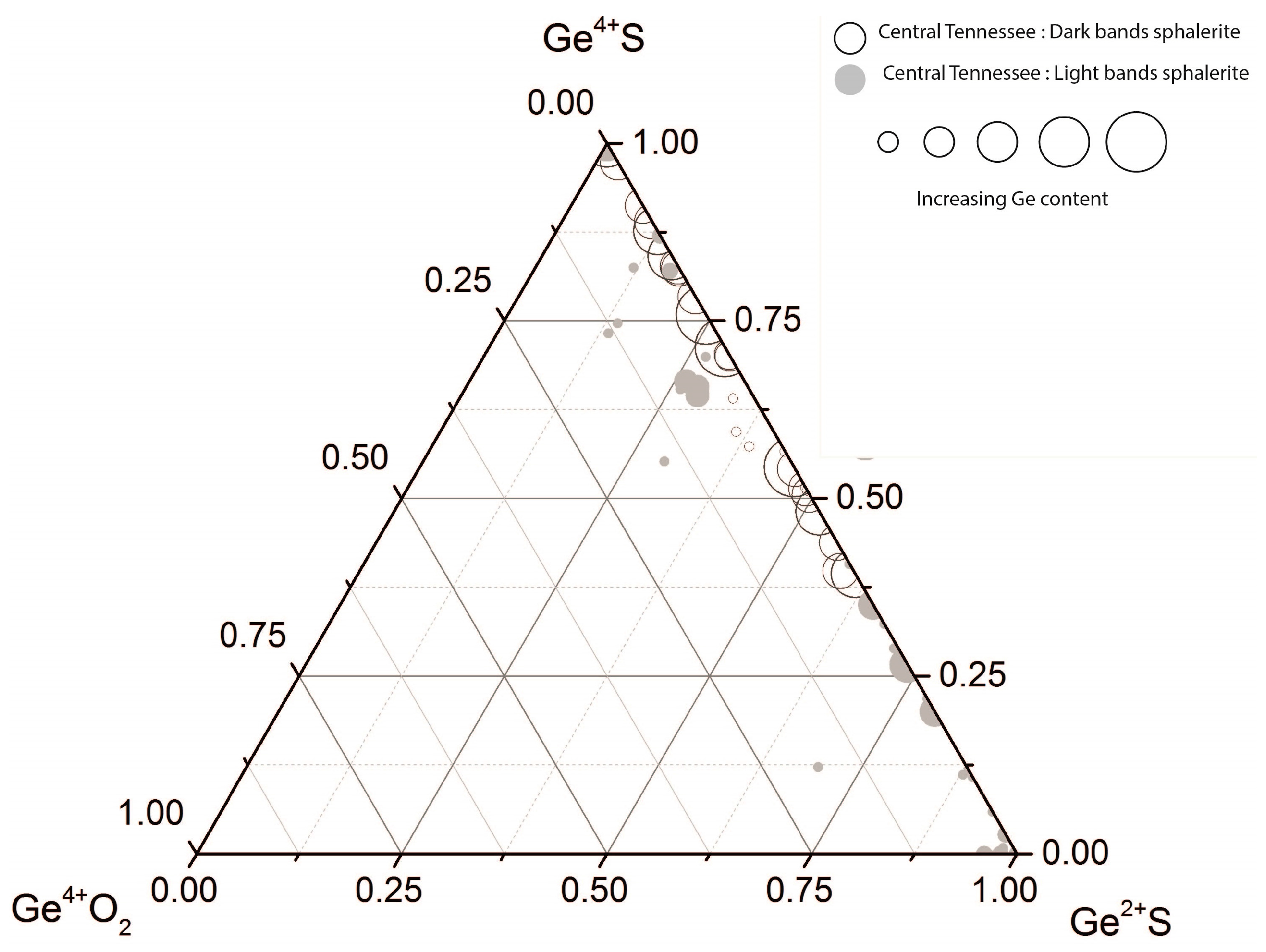
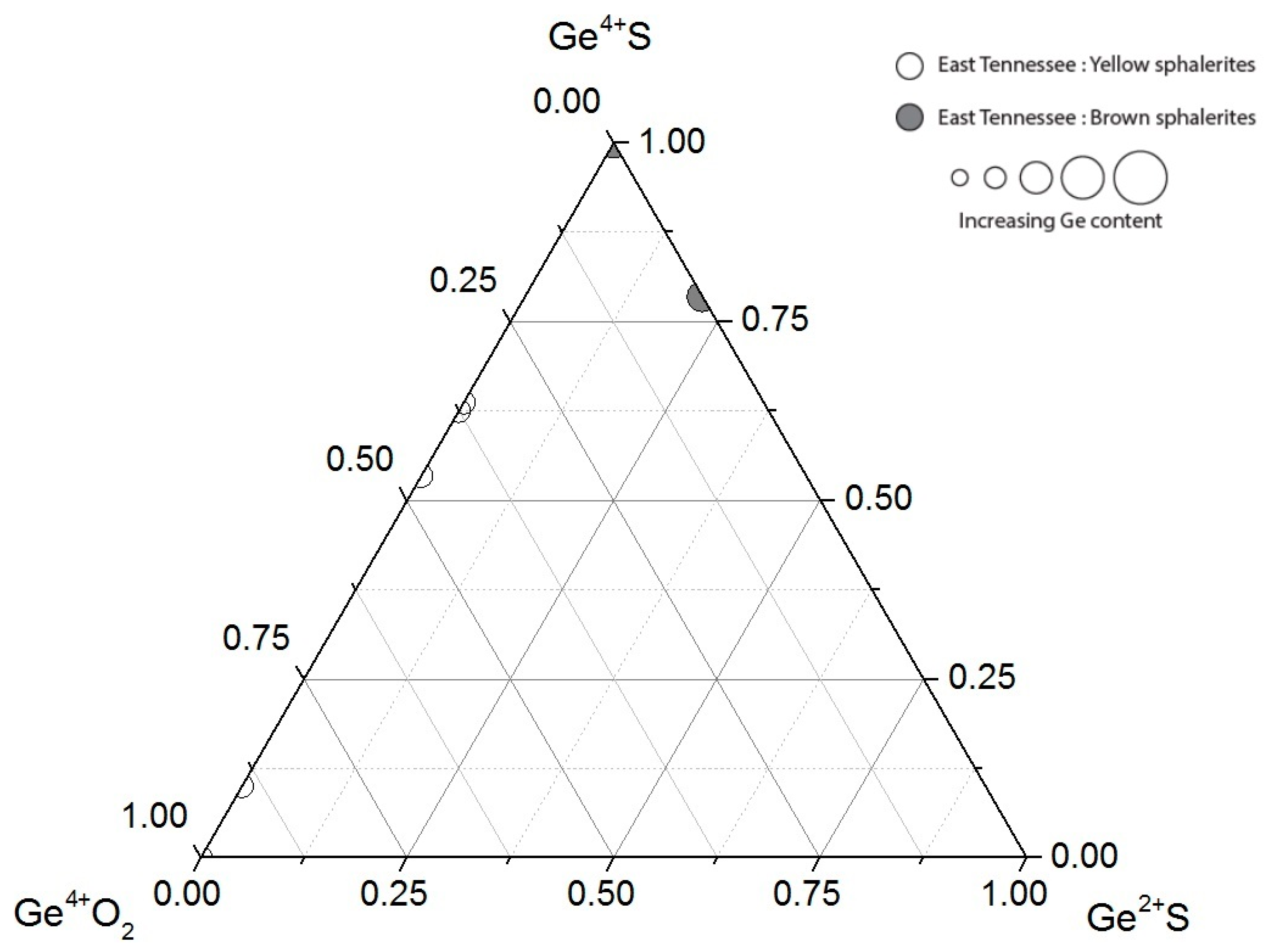
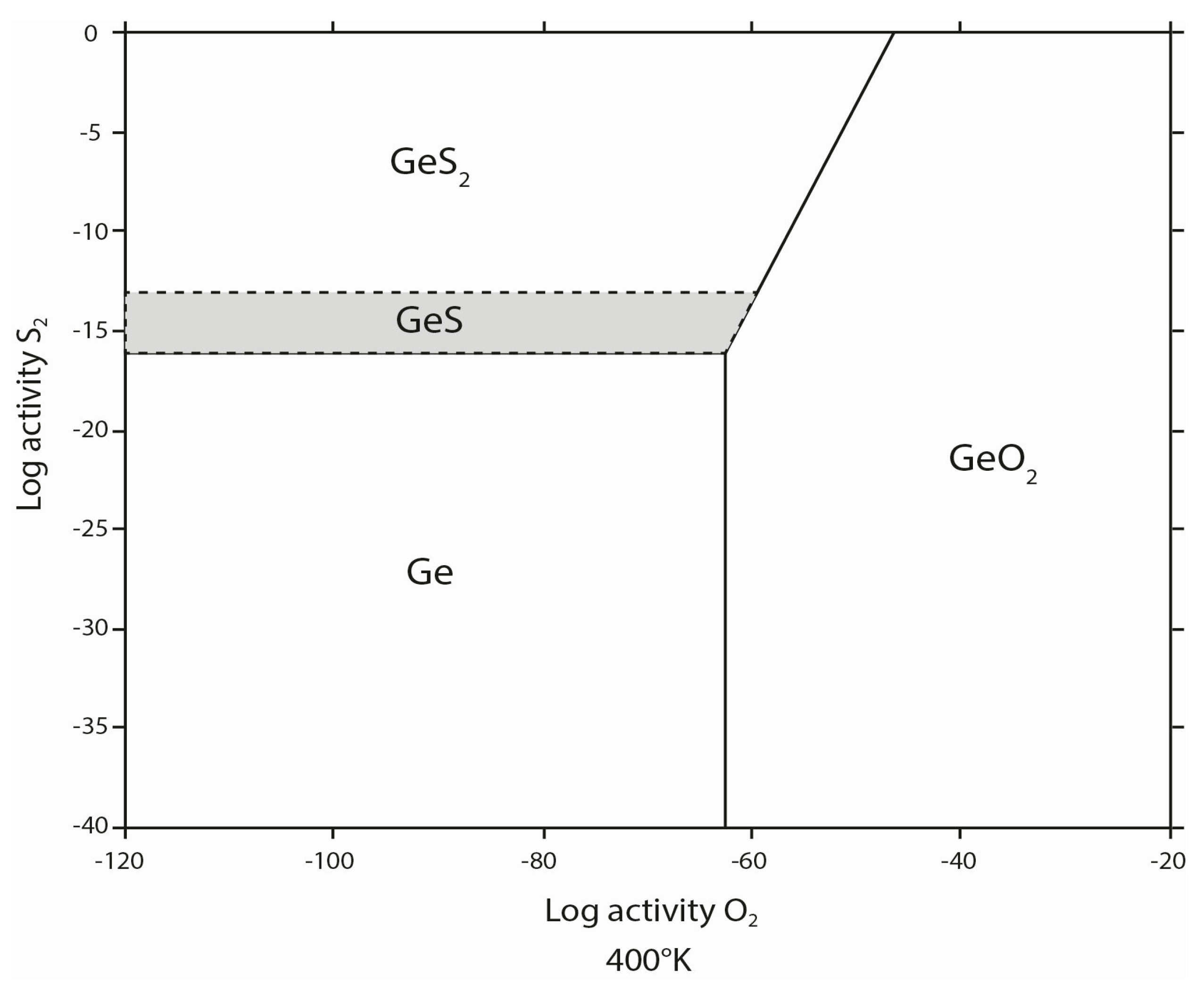
4. Discussion
4.1. Implications of Experimental Conditions
4.2. Implications for the Conditions of Sphalerite Formation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butterman, W.C.; Jorgenson, J.D. Mineral Commodity Profiles: Germanium; USGS Open-File Report 2004-1218; USGS: Washington, DC, USA, 2015. Available online: https://pubs.er.usgs.gov/publication/ofr20041218 (accessed on 9 May 2017).
- Guberman, D.E. Germanium in 2014 Minerals Yearbook; USGS Open-File Report; USGS: Washington, DC, USA, 2014. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/germanium/myb1-2014-germa.pdf (accessed on 9 May 2017).
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Yuping, L.; Qian, Z.; Tiegeng, L.; Wei, G.; Yulong, Y.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite–galena–chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; De Jonge, M.D.; Ryan, C.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via μ-XANES spectroscopy. Am. Miner. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Beaudoin, G. Acicular sphalerite enriched in Ag, Sb, and Cu embedded within color-banded sphalerite from the Kokanee Range, British Columbia, Canada. Can. Miner. 2000, 38, 1387–1398. [Google Scholar] [CrossRef]
- Benedetto, F.D.; Bernardini, G.P.; Costagliola, P.; Plant, D.; Vaughan, D.J. Compositional zoning in sphalerite crystals. Am. Miner. 2005, 90, 1384–1392. [Google Scholar] [CrossRef]
- Pfaff, K.; Koenig, A.; Wenzel, T.; Ridley, I.; Hildebrandt, L.H.; Leach, D.L.; Markl, G. Trace and minor element variations and sulfur isotopes in crystalline and colloform ZnS: Incorporation mechanisms and implications for their genesis. Chem. Geol. 2011, 286, 118–134. [Google Scholar] [CrossRef]
- Johan, Z. Indium and germanium in the structure of sphalerite: An example of coupled substitution with Copper. Miner. Pet. 1988, 39, 211–229. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Martin, F.; Hazemann, J.-L.; Schott, J. An X-ray absorption fine structure spectroscopy study of germanium-organic ligand complexes in aqueous solution. Chem. Geol. 2000, 163, 151–165. [Google Scholar] [CrossRef]
- Valeev, R.G.; Surnin, D.V.; Beltyukov, A.N.; Vetoshkin, V.M.; Kriventsov, V.V.; Zubavichus, Y.V.; Mezentsev, N.A.; Eliseev, A.A. Synthesis and structural study of the ordered germanium nanorod arrays. J. Struct. Chem. 2010, 51, 132–136. [Google Scholar] [CrossRef]
- Bowron, D.T.; Newport, R.J.; Rigden, J.S.; Tarbox, E.J.; Oversluizen, M. An X-ray absorption study of doped silicate glass, fibre optic preforms. J. Mater. Sci. 1996, 31, 485–490. [Google Scholar] [CrossRef]
- Gurman, S.J. EXAFS studies in materials science. J. Mater. Sci. 1982, 17, 1541–1570. [Google Scholar] [CrossRef]
- Matsubara, E.; Harada, K.; Waseda, Y.; Inoue, A.; Bizen, Y.; Masumoto, T. X-ray diffraction study of an amorphous Al60Ge30Ni10 alloy. J. Mater. Sci. 1988, 23, 3485–3489. [Google Scholar] [CrossRef]
- Bernstein, L.R.; Waychunas, G.A. Germanium crystal chemistry in hematite and goethite from the Apex Mine, Utah, and some new data on germanium in aqueous solution and in stottite. Geochim. Cosmochim. Acta 1987, 51, 623–630. [Google Scholar] [CrossRef]
- Etschmann, B.; Liu, W.; Li, K.; Dai, S.; Reith, F.; Falconer, D.; Kerr, G.; Paterson, D.; Howard, D.; Kappen, P.; et al. Enrichment of germanium and associated arsenic and tungsten in coal and roll-front U deposits. Chem. Geol. 2017, in press. [Google Scholar] [CrossRef]
- Cook, N.J.; Etschmann, B.; Ciobanu, C.L.; Geraki, K.; Howard, D.L.; Williams, T.; Rae, N.; Pring, A.; Chen, G.; Johannessen, B. Distribution and substitution mechanism of Ge in a Ge-(Fe)-bearing sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef]
- Belissont, R.; Muñoz, M.; Boiron, M.-C.; Luais, B.; Mathon, O. Distribution and oxidation state of Ge, Cu and Fe in sphalerite by μ-XRF and K-edge μ-XANES: Insights into Ge incorporation, partitioning and isotopic fractionation. Geochim. Cosmochim. Acta 2016, 177, 298–314. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Pugsley, A.J.; Bull, C.L.; Sella, A.; Sankar, G.; McMillan, P.F. XAS/EXAFS studies of Ge nanoparticles produced by reaction between Mg2Ge and GeCl4. J. Solid State Chem. 2011, 184, 2345–2352. [Google Scholar] [CrossRef]
- Bernstein, L.R.; Reichel, D.G.; Merlino, S. Renierite crystal structure refined from Rietveld analysis of powder neutron-diffraction data. Am. Miner. 1989, 74, 1177–1181. [Google Scholar]
- Bonnet, J.; Mosser-Ruck, R.; Caumon, M.-C.; Rouer, O.; Andre-Mayer, A.-S.; Cauzid, J.; Peiffert, C. Trace element distribution (Cu, Ga, Ge, Cd and Fe) in sphalerite from the Tennessee MVT deposits, USA, by combined EMPA, LA-ICP-MS, Raman spectroscopy and crystallography. Can. Miner. (accepted).
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Newville, M.; Līviņš, P.; Yacoby, Y.; Rehr, J.J.; Stern, E.A. Near-edge X-ray-absorption fine structure of Pb: A comparison of theory and experiment. Phys. Rev. 1993, 47, 14126–14131. [Google Scholar] [CrossRef]
- Proux, O.; Biquard, X.; Lahera, E.; Menthonnex, J.-J.; Prat, A.; Ulrich, O.; Soldo, Y.; Trévisson, P.; Kapoujyan, G.; Perroux, G.; et al. FAME: A new beamline for X-ray absorption investigations of very-diluted systems of environmental, material and biological interests. Phys. Scr. 2005, 2005, 970. [Google Scholar] [CrossRef]
- Proux, O.; Nassif, V.; Prat, A.; Ulrich, O.; Lahera, E.; Biquard, X.; Menthonnex, J.J.; Hazemann, J.L. Feedback system of a liquid-nitrogen-cooled double-crystal monochromator: Design and performances. J. Synchrotron Radiat. 2006, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hazemann, J.L.; Proux, O.; Nassif, V.; Palancher, H.; Lahera, E.; Da Silva, C.; Braillard, A.; Testemale, D.; Diot, M.A.; Alliot, I.; et al. High-resolution spectroscopy on an X-ray absorption beamline. J. Synchrotron Radiat. 2009, 16, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Llorens, I.; Lahera, E.; Delnet, W.; Proux, O.; Braillard, A.; Hazemann, J.-L.; Prat, A.; Testemale, D.; Dermigny, Q.; Gelebart, F.; et al. High energy resolution five-crystal spectrometer for high quality fluorescence and absorption measurements on an X-ray absorption spectroscopy beamline. Rev. Sci. Instrum. 2012, 83, 063104. [Google Scholar] [CrossRef] [PubMed]
- Johan, Z.; Oudin, E.; Picot, P. Analogues germanifères et gallifères des silicates et oxydes dans les gisements de zinc des Pyrénées centrales, France; argutite et carboirite, deux nouvelles espèces minérales. Tschermaks Mineral. Petrogr. Mitt. 1983, 31, 97–119. [Google Scholar] [CrossRef]
- Gratz, J.F.; Misra, K.C. Fluid inclusion study of the Gordonsville zinc deposit, central Tennessee. Econ. Geol. 1987, 82, 1790–1804. [Google Scholar] [CrossRef]
- Murray, W.T.; O’hare, P.A.G. Thermochemistry of inorganic sulfur compounds. II: Standard enthalpy of formation of germanium disulfide. J. Chem. Thermodyn. 1984, 16, 335–341. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (10^5 Pascals) Pressure and at Higher Temperatures; U.S. Geological Survey Bulletin; U.S. GPO: Washington, DC, USA, 1995.



| District | Sphalerite | Statistics | Zn (%) | S (%) | Fe (ppm) | Cu (ppm) | Ga (ppm) | Ge (ppm) | Cd (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| East TN | YET n = 91 | Average | 49.0 | 50.7 | 734 | 79 | 94 | 11 | 1565 |
| Max | 49.2 | 51.1 | 4043 | 953 | 801 | 48 | 3531 | ||
| Min | 48.8 | 50.2 | 163 | 0 | 0 | 0 | 267 | ||
| BET n = 27 | Average | 49.5 | 49.2 | 9613 | 188 | 9 | 131 | 2820 | |
| Max | 50.1 | 50.3 | 30400 | 520 | 88 | 357 | 7367 | ||
| Min | 49.1 | 46.7 | 4162 | 37 | <4 | <7 | 882 | ||
| Central TN | LCT n = 35 | Average | 49.1 | 50.3 | 1899 | 470 | 807 | 182 | 1867 |
| Max | 49.5 | 50.9 | 3607 | 979 | 2787 | 612 | 4996 | ||
| Min | 48.9 | 49.7 | 773 | 61 | 117 | 9 | 447 | ||
| BCT n = 55 | Average | 49.1 | 50.5 | 1252 | 586 | 765 | 381 | 1306 | |
| Max | 49.3 | 50.9 | 2404 | 1441 | 4536 | 790 | 3153 | ||
| Min | 48.9 | 50.0 | 701 | 82 | 4 | <7 | 493 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnet, J.; Cauzid, J.; Testemale, D.; Kieffer, I.; Proux, O.; Lecomte, A.; Bailly, L. Characterization of Germanium Speciation in Sphalerite (ZnS) from Central and Eastern Tennessee, USA, by X-ray Absorption Spectroscopy. Minerals 2017, 7, 79. https://doi.org/10.3390/min7050079
Bonnet J, Cauzid J, Testemale D, Kieffer I, Proux O, Lecomte A, Bailly L. Characterization of Germanium Speciation in Sphalerite (ZnS) from Central and Eastern Tennessee, USA, by X-ray Absorption Spectroscopy. Minerals. 2017; 7(5):79. https://doi.org/10.3390/min7050079
Chicago/Turabian StyleBonnet, Julien, Jean Cauzid, Denis Testemale, Isabelle Kieffer, Olivier Proux, Andreï Lecomte, and Laurent Bailly. 2017. "Characterization of Germanium Speciation in Sphalerite (ZnS) from Central and Eastern Tennessee, USA, by X-ray Absorption Spectroscopy" Minerals 7, no. 5: 79. https://doi.org/10.3390/min7050079







