The Hydrothermal Fluid Evolution of Vein Sets at the Pipeline Gold Mine, Nevada
Abstract
:1. Introduction
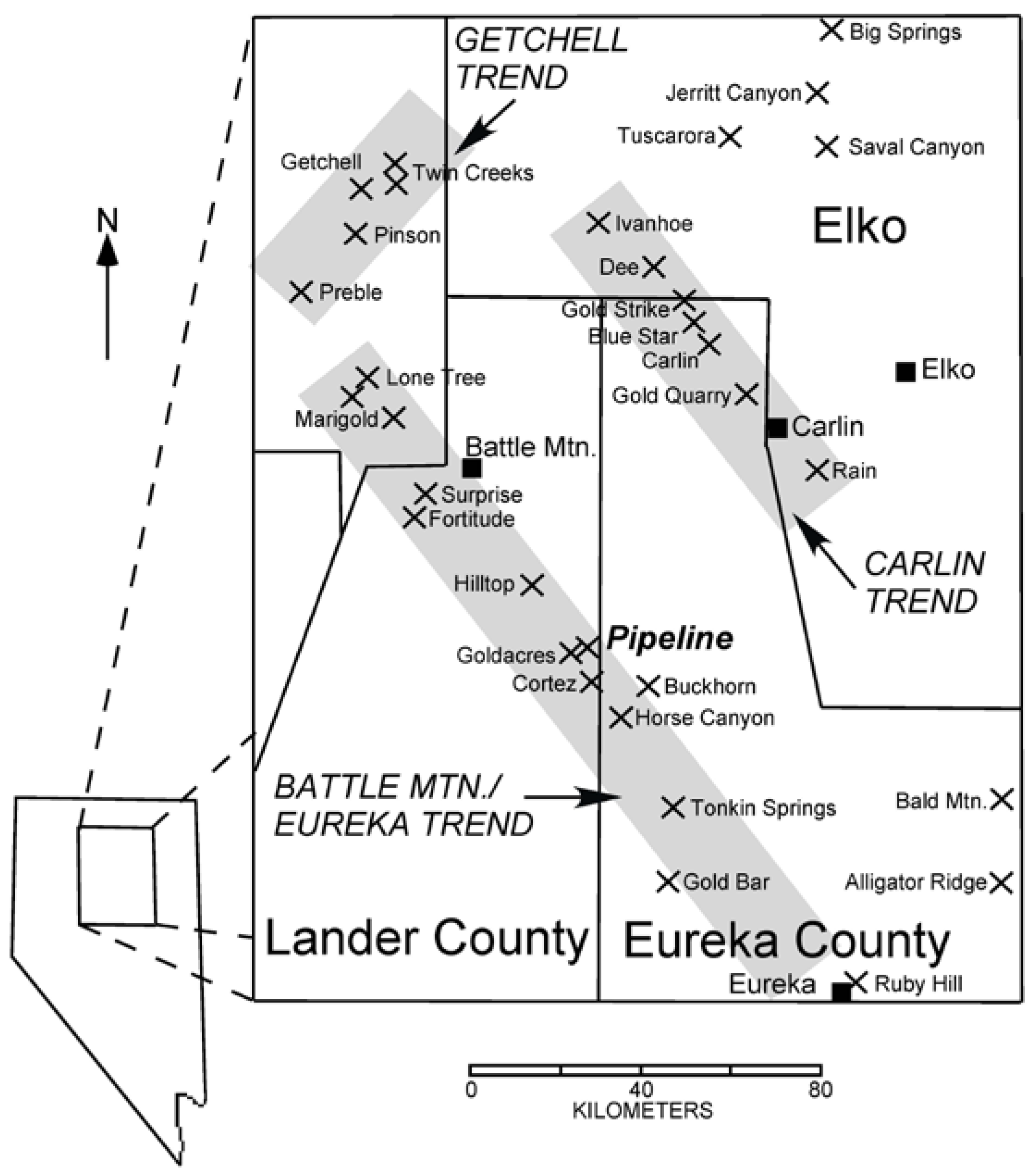
1.1. Regional Geologic Setting
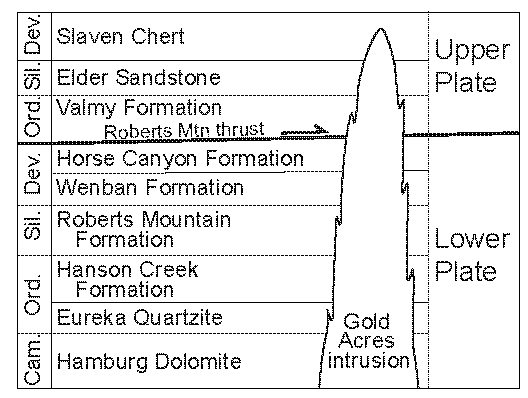
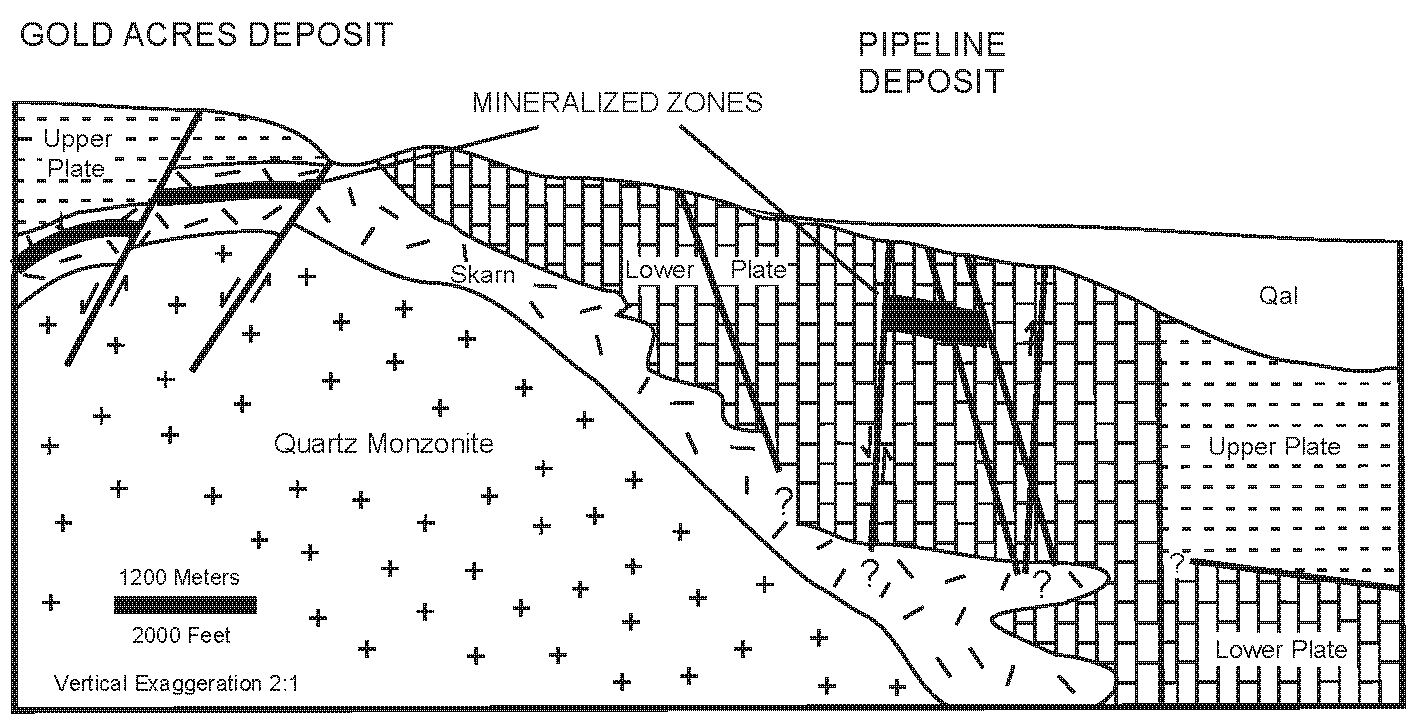
1.2. Local Geologic Setting
1.2.1. Alteration
1.2.2. Gold Mineralization
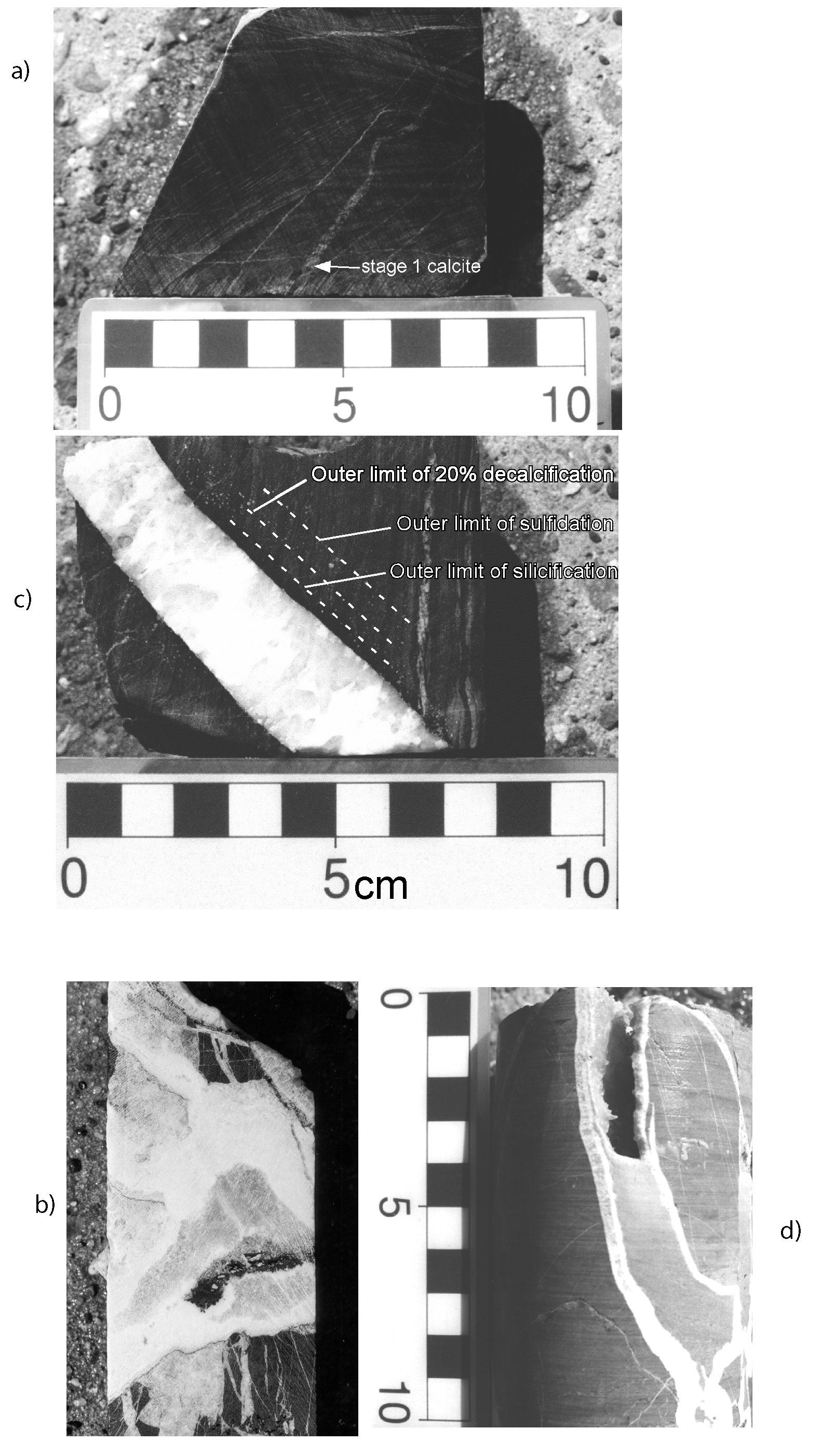
1.2.3. Vein Paragenesis
2. Materials and Methods
3. Results
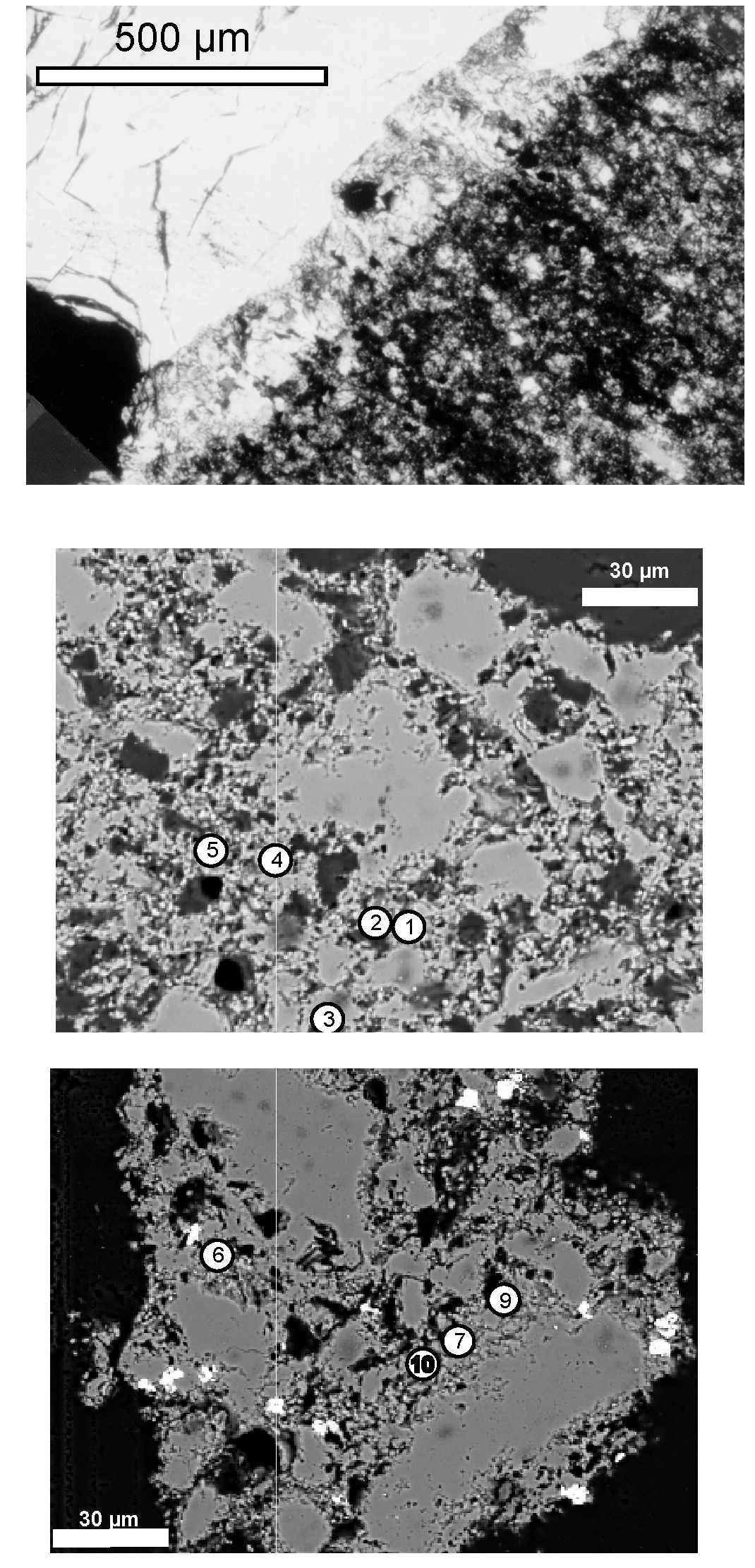
3.1. Fluid Inclusion Classification and Analysis
3.2. Microthermometry
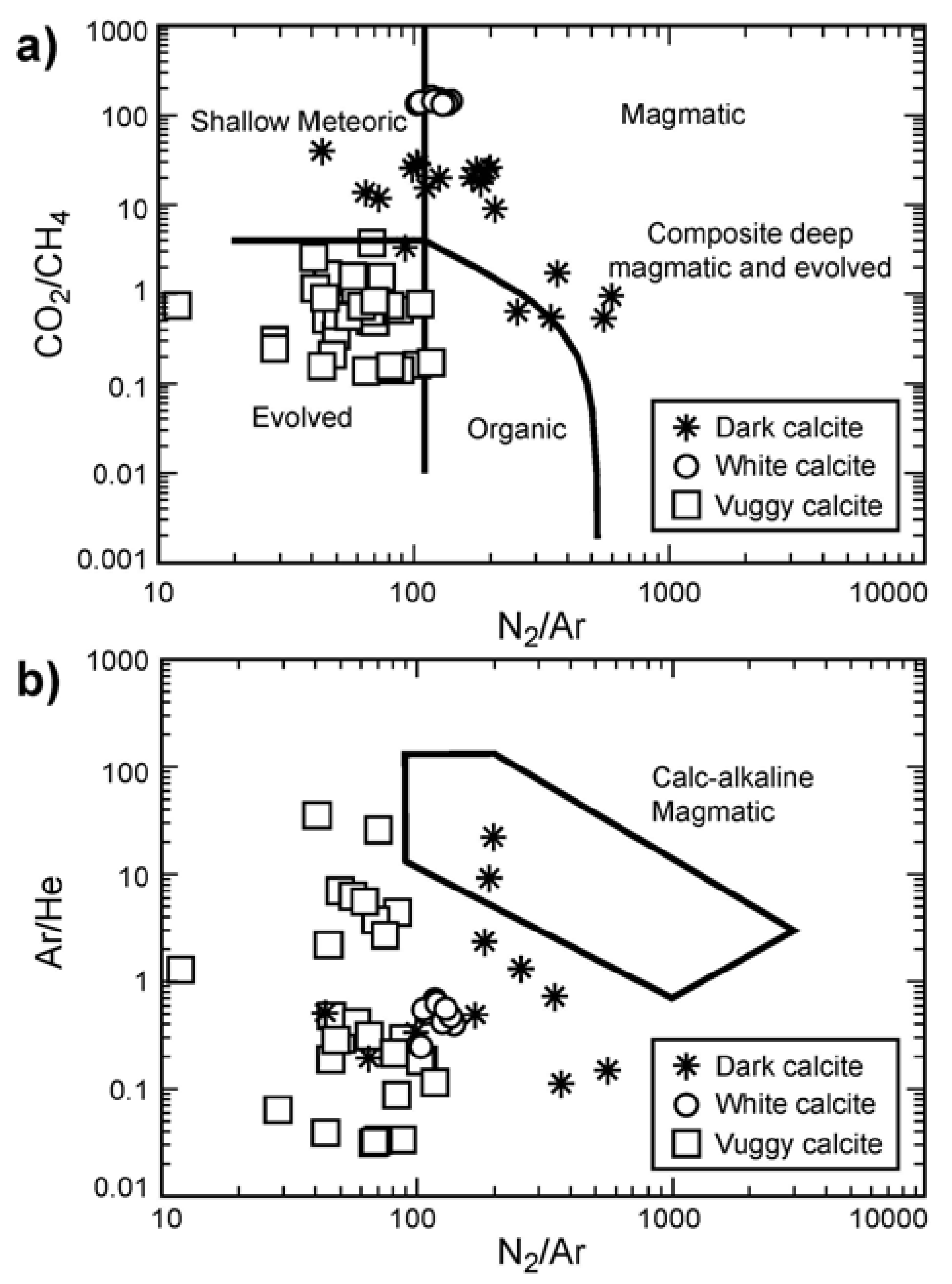
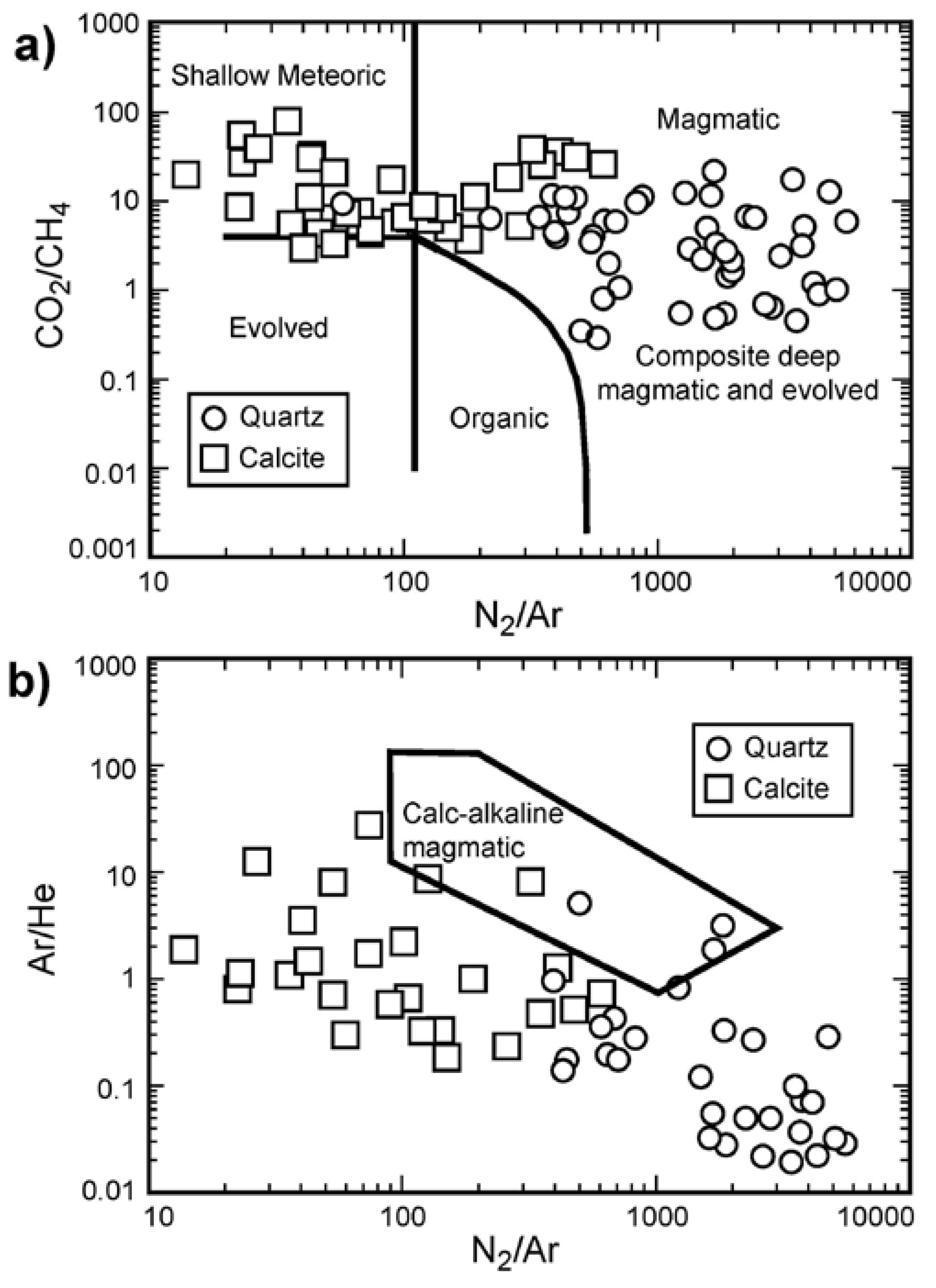
3.3. Fluid Inclusion Gas Chemistry
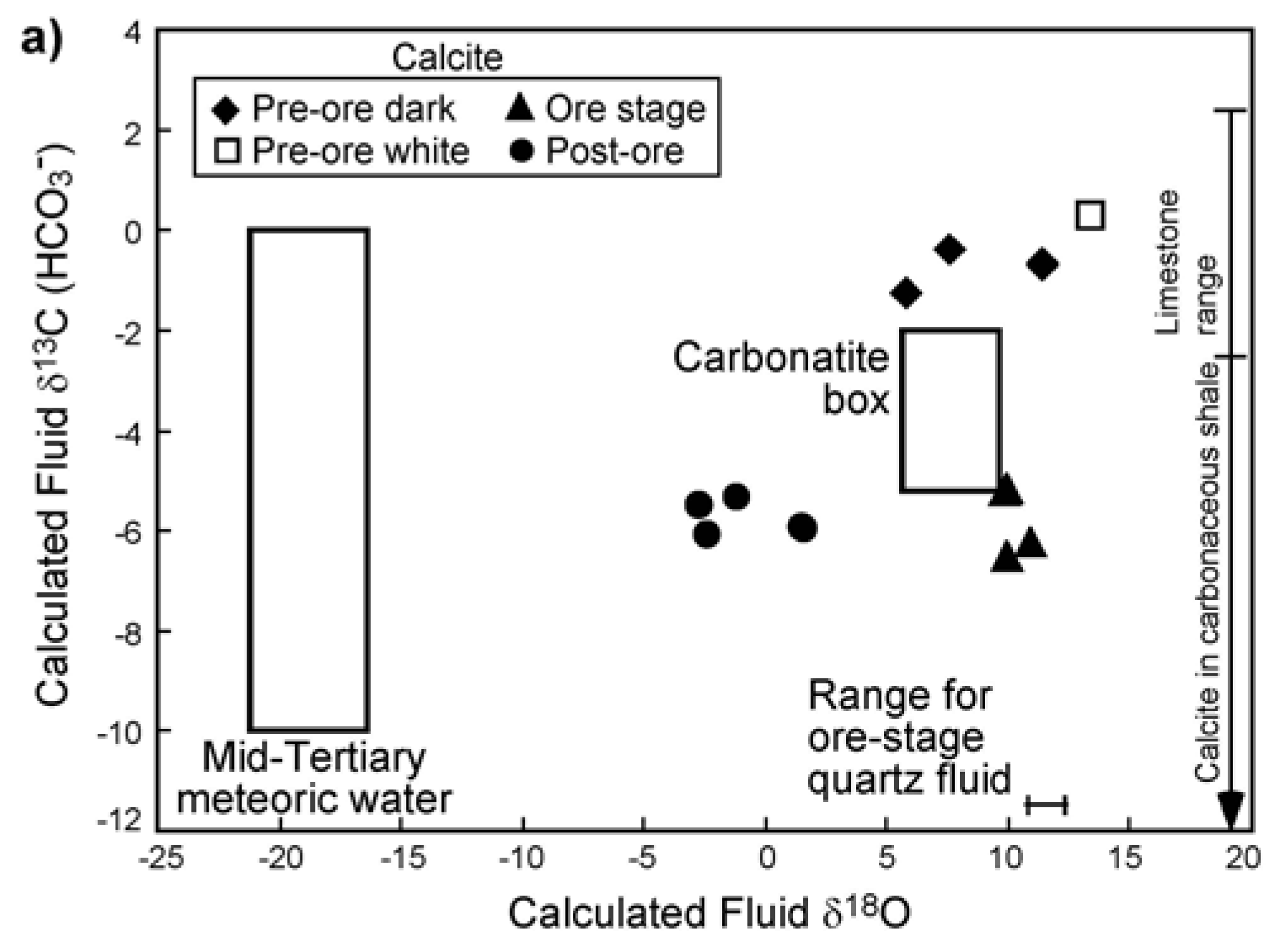
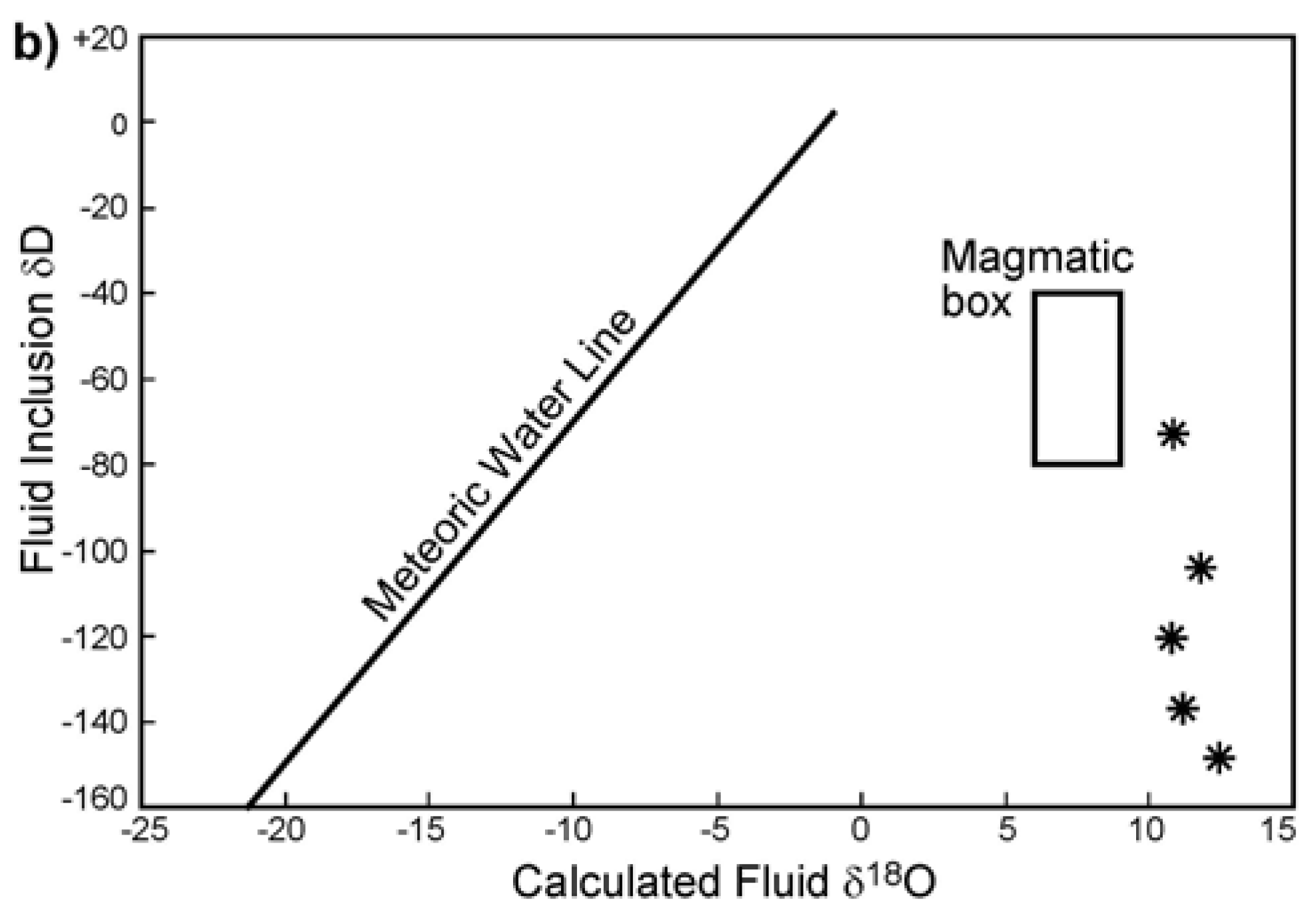
3.4. Stable Isotopes
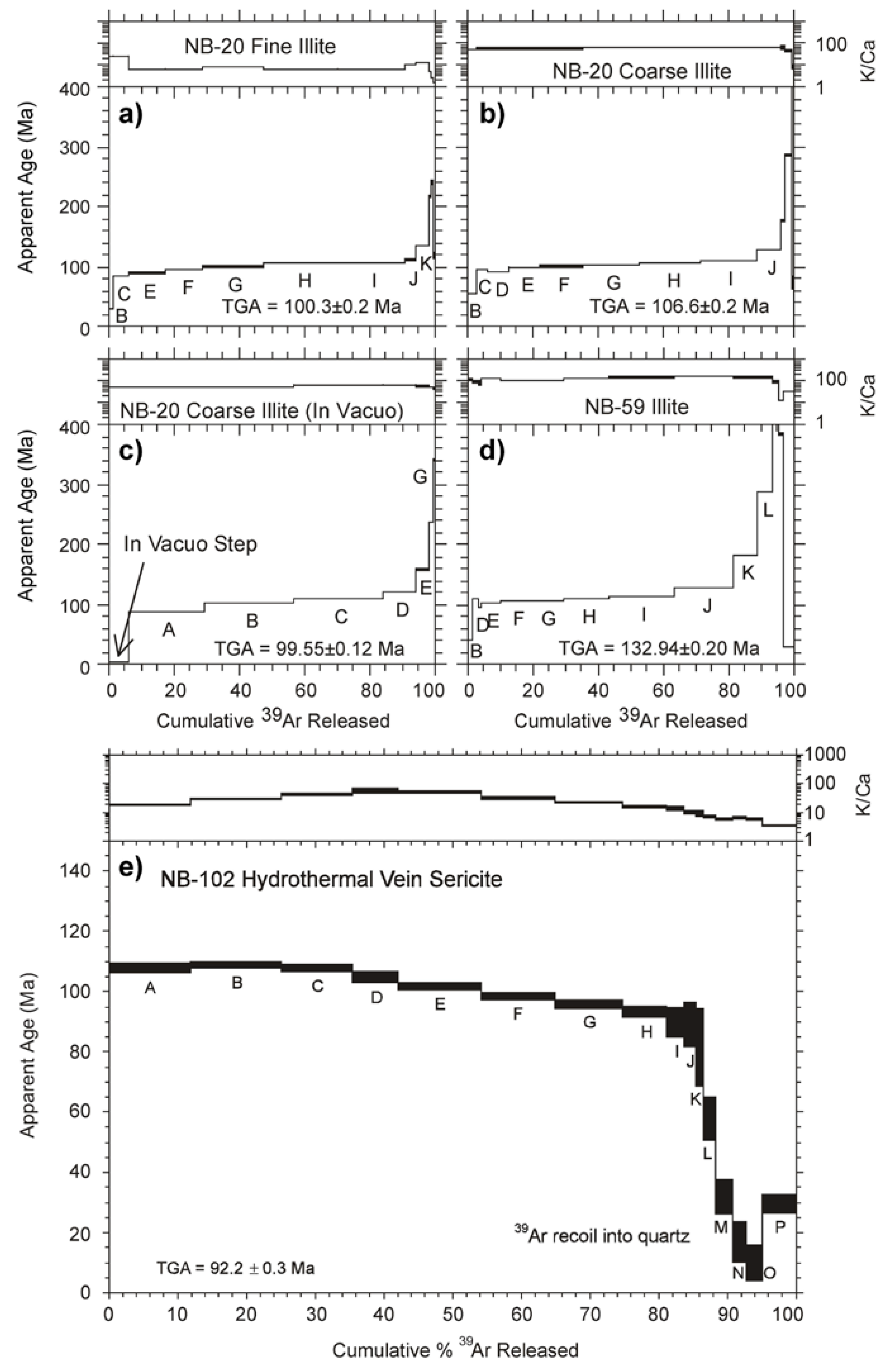
3.5. Geochronology
4. Discussion
4.1. Microthermometry
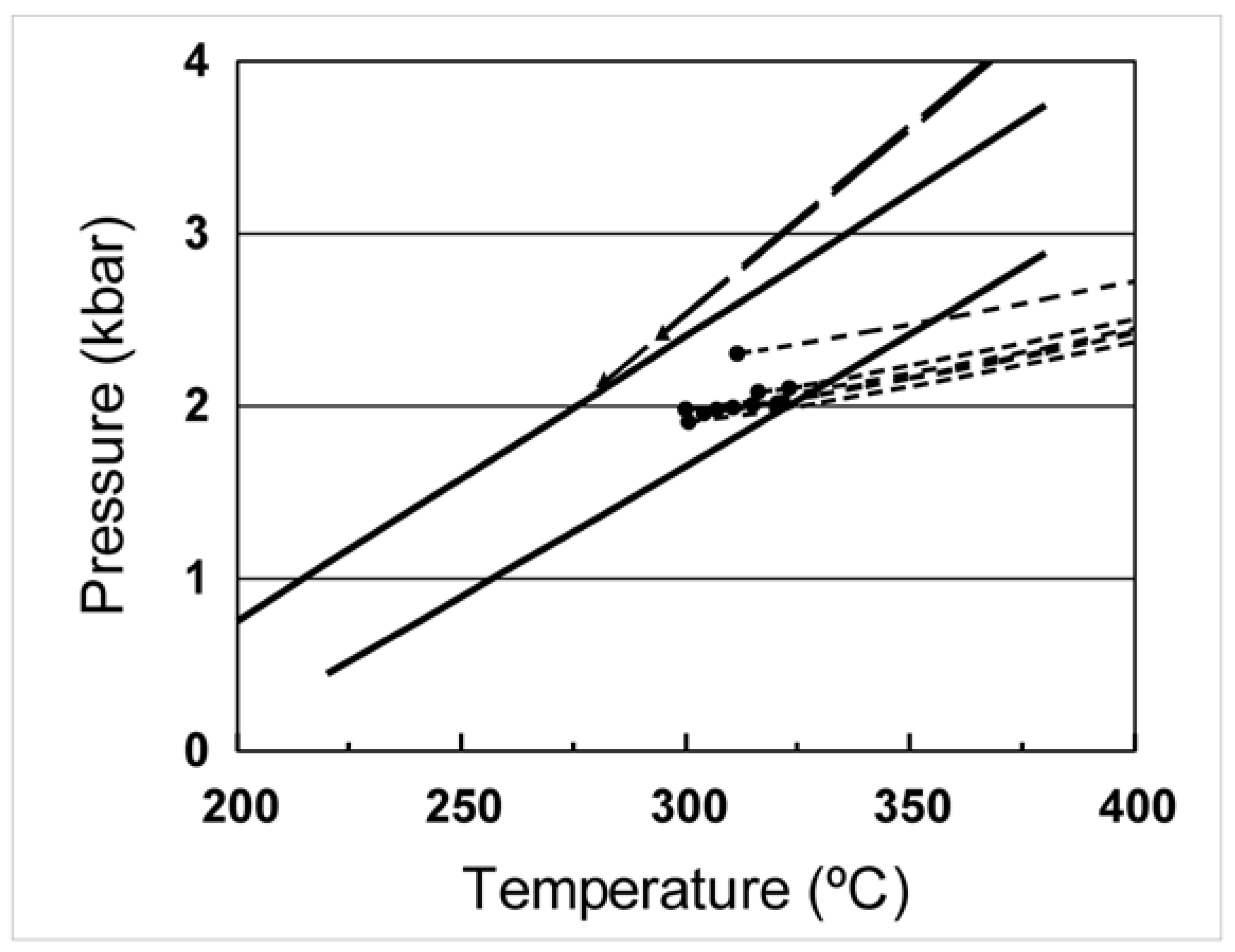
4.2. Temperature, Pressure and Depth
4.3. Fluid Sources Indicated by Fluid Inclusion Gas Analysis
4.4. Fluid Sources Indicated by Stable Isotopes
4.5. Geochronology
4.6. Genetic Model for Vein Formation
4.7. Gold Solubility and Depositional Mechanism
4.8. Genetic Model
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arehart, G.B.; Chryssoulis, S.L.; Kesler, S.E. 40Ar/39Ar, K/Ar, and fission track geochronology of sediment-hosted disseminated gold deposits at the Post-Betze, Carlin-trend, northeastern Nevada. Econ. Geol. 1993, 88, 622–646. [Google Scholar] [CrossRef]
- Kuehn, C.A.; Rose, A.W. Carlin gold deposits, Nevada: Origin in a deep zone of mixing between normally pressured and over-pressured fluids. Econ. Geol. 1995, 90, 17–36. [Google Scholar] [CrossRef]
- Cline, J.S.; Hofstra, A.H.; Rye, R.O.; Landis, G.P. Stable isotope and fluid inclusion evidence for a deep sourced ore fluid at the Getchell, Carlin-type, gold deposit, Nevada. PACROFI VI 1996, 33–35. [Google Scholar]
- Stenger, D.P.; Kesler, S.E.; Peltonen, D.R.; Tapper, C.J. Deposition of gold in Carlin-type deposits: The role of sulfidation and decarbonation at Twin Creeks, Nevada. Econ. Geol. 1998, 93, 201–215. [Google Scholar] [CrossRef]
- Hofstra, A.H.; Cline, J.S. Characteristics and models for Carlin-type gold deposits. SEG Rev. 2000, 13, 163–220. [Google Scholar]
- Hofstra, A.H.; John, D.A.; Theodore, T.G. A special issue devoted to gold deposits in Northern Nevada: Part 2. Carlin-type deposits. Econ. Geol. 2003, 98, 1063–1067. [Google Scholar] [CrossRef]
- Muntean, J.L.; Cline, J.S.; Johnston, M.K.; Ressel, M.W.; Seedorf, E.; Barton, M.D. Controversies of the origin of world-class gold deposits: Part 1. Carlin-type gold deposits in Nevada: SEG Newsletter. Soc. Econ. Geol. 2004, 59, 1. [Google Scholar]
- Cline, J.S.; Hofstra, A.H.; Muntean, J.L.; Tosdal, R.M.; Hickey, K.A. Carlin-type gold deposits in Nevada: Critical geologic characteristics and viable models. In Econ Geol 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfard, R.J., Richards, J.P., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005; Volume 100, pp. 451–484. [Google Scholar]
- Teal, L.; Jackson, M. Geologic overview of the Carlin Trend Gold Deposits and descriptions of recent deep discoveries. SEG Newsl. 1997, 31, 12–25. [Google Scholar]
- Groff, J.A.; Heizler, M.T.; McIntosh, W.C.; Norman, D.I. 40Ar/39Ar dating and mineral paragenesis for Carlin-type gold deposits along the Getchell trend, Nevada: Evidence for Cretaceous and Tertiary gold mineralization. Econ. Geol. 1997, 92, 601–622. [Google Scholar] [CrossRef]
- Teal, L.; Branham, A. Geology of the Mike gold-copper deposit, Eureka County, Nevada. In Carlin-Type Gold Deposits Field Conference; Vikre, P., Thompson, T.B., Bettles, K., Christensen, O., Parratt, R., Eds.; Society of Economic Geology: Littleton, CO, USA, 1997; Volume 28, pp. 257–276. [Google Scholar]
- Hall, C.M.; Kesler, S.E.; Simon, G.; Fortuna, J. Overlapping Cretaceous and Eocene alteration, Twin Creeks Carlin-type deposit, Nevada. Econ. Geol. 2000, 95, 1739–1752. [Google Scholar] [CrossRef]
- Gilluly, J.; Gates, O. Tectonic and Igneous Geology of the Northern Shoshone Range Nevada; U.S. Government Printing Office: Washington, DC, USA, 1965; p. 153.
- Gilluly, J.; Masursky, H. Geology of the Cortez Quadrangle Nevada: Geological Survey Bulletin 1175; U.S. Government Printing Office: Washington, DC, USA, 1965; p. 117.
- Roberts, R.J.; Hotz, P.E.; Gilluly, J.; Ferguson, H.G. Paleozoic rocks of north-central Nevada. Am. Assoc. Petro. Geol. Bull. 1958, 42, 2813–2857. [Google Scholar]
- Leonardson, R.W. Barrick Cortez gold acres structure. In Great Basin Evolution and Metallogeny Geological Society of Nevada 2010 Symposium; Steininger, R.C., Pennell, W.M., Eds.; DEstech Publications, Inc.: Lancaster, PA, USA, 2011; pp. 17–30. [Google Scholar]
- McCormack, J.K.; Hays, R.C., Jr. Crescent Valley: A model for reconstruction of district mineralization in the Basin and Range. In Geology and Ore Deposits of the American Cordillera; Coyner, A.R., Fahey, P.L., Eds.; Geological Society of Nevada: Reno/Sparks, NV, USA, 1996; pp. 635–646. [Google Scholar]
- Colgan, J.P.; John, D.A.; Henry, C.D.; Fleck, R.J. Large-magnitude Miocene extension of the Eocene Caetano caldera, Shoshone and Toiyabe Ranges, Nevada. Geosphere 2008, 4, 107–131. [Google Scholar] [CrossRef]
- Colgan, J.P.; Henry, C.D.; John, D.A. Evidence for large-magnitude, post-Eocene extension in the northern Shoshone Range, Nevada, and its implications for Carlin-type gold deposits in the lower plate of the Roberts Mountains allochthon. Econ. Geol. 2014, 109, 1843–1862. [Google Scholar] [CrossRef]
- Rhys, D.; Valli, F.; Burgess, R.; Heitt, D.; Greisel, G.; Hart, K. Controls of fault and fold geometry on the distribution of gold mineralization on the Carlin trend. In New Concepts and Discoveries (Geological Society of Nevada 2015 Symposium); Pennell, W.M., Garside, L.J., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2015; pp. 333–389. [Google Scholar]
- Foo, S.T.; Hays, R.C., Jr.; McCormack, J.K. Geology and mineralization of the Pipeline Gold Deposit, Lander County, Nevada. In Geology and Ore Deposits of the American Cordillera: Symposium Proceedings; Coyner, A.R., Fahey, P.L., Eds.; Geological Society of Nevada: Reno/Sparks, NV, USA, 1996; pp. 95–109. [Google Scholar]
- Tretbar, D.R.; Arehart, G.B.; Christensen, J.N. Dating gold deposition in a Carlin-type gold deposit using Rb/Sr methods on the mineral galkhaite. Geology 2000, 28, 947–950. [Google Scholar] [CrossRef]
- Cline, J.S. Timing of gold and arsenic sulfide mineral deposition at the Getchell Carlin-type gold deposit, North-Central Nevada. Econ. Geol. 2001, 96, 75–89. [Google Scholar] [CrossRef]
- Bradley, M.A.; Eck, N. The Goldrush discovery, Cortez District, Nevada—The stratigraphic story. In New Concepts and Discoveries; Pennell, W.M., Garside, L.J., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2015; pp. 435–452. [Google Scholar]
- Blamey, N.J.F. Composition and evolution of crustal, geothermal and hydrothermal fluids interpreted using quantitative fluid inclusion gas analysis. J. Geochem. Explor. 2012, 116–117, 17–27. [Google Scholar] [CrossRef]
- Blamey, N.J.F.; Parnell, J.; McMahon, S.; Mark, D.; Tomkinson, T.; Lee, M.; Shivak, J.; Izawa, M.; Banerjee, N.; Flemming, R. Evidence for methane in Martian meteorites. Nat. Commun. 2015, 6, 7399. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.I.; Ting, W.; Putnam, B.R.; Smith, R.W. Mineralization of the Hansonburg Mississippi-Valley type deposit, New Mexico: Insight from composition of gases in fluid inclusions. Can. Miner. 1985, 23, 353–368. [Google Scholar]
- Norman, D.I. Geology and Geochemistry of the Tribag Mine, Batchawana Bay, Ontario. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 1977; p. 257. [Google Scholar]
- Brown, P.E.; Hageman, S.G.; Vanko, D.A. Macflincor and its application to fluids in Archean lodegold deposits. Geochim. Cosmochim. Acta 1995, 59, 3943–3952. [Google Scholar] [CrossRef]
- Katz, D.L.; Cornell, D.; Kobayashi, R.; Poetmann, F.H.; Vary, J.A.; Elenbaas, J.R.; Weinaug, C.F. Handbook of Natural Gas Engineering; McGraw-Hill Book Company: New York, NY, USA, 1959; p. 802. [Google Scholar]
- Blamey, N.J.F. The Evolution of Hydrothermal Fluids at the Pipeline Gold Mine, Lander County, Nevada. Unpublished Ph.D. Dissertation, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2000; p. 200. [Google Scholar]
- Shepherd, T.J.; Rankin, A.H.; Alderron, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie & Son Ltd.: Glasgow/London, UK, 1985; p. 239. [Google Scholar]
- Norman, D.I.; Blamey, N.J.F.; Kurilovich, L. New applications of geothermal gas analysis to exploration. Trans. Geotherm. Res. Council 2002, 26, 345–353. [Google Scholar]
- Sharp, Z.D.; Krischner, D.L. Quartz-calcite oxygen isotope thermometry: A calibration based on natural isotopic variations. Geochim. Cosmochim. Acta 1994, 58, 4491–4501. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Clayton, R.N.; Mayeda, T.K. Oxygen isotope fractionation in divalent metal carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Deines, P.; Langmuir, D.; Harmon, R.S. Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate groundwaters. Geochim. Cosmochim. Acta 1974, 38, 1147–1164. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Deposits; Barnes, H.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1979; pp. 509–567. [Google Scholar]
- Dong, H.; Hall, C.M.; Peacor, C.R.; Halliday, C.N. Mechanisms of argon retention in clays revealed by laser 40Ar/39Ar dating. Science 1995, 267, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Cromie, P.W.; Zaw, K. Geological setting, nature of ore fluids and sulfur isotope geochemistry of the Fu Ning Carlin-type gold deposits, Yunnan Province, China. Geofluids 2003, 3, 133–143. [Google Scholar] [CrossRef]
- Bowers, T.S.; Helgeson, H.C. Calculation of the thermodynamic and geochemical consequences of non-ideal mixing in the system H2O-CO2-NaCl on phase relations in geologic systems. Equation of state for H2O-CO2-NaCl fluids at high pressures and temperatures. Geochim. Cosmochim. Acta 1983, 47, 1247–1275. [Google Scholar] [CrossRef]
- Drummond, S.E. Boiling and Mixing of Hydrothermal Fluids: Chemical Effects on Mineral Precipitation. Unpublished Ph.D. Dissertation, Pennsylvania State University, State College, PA, USA, 1981; p. 380. [Google Scholar]
- Lamb, J.B.; Cline, J.S. Depths of formation of the Meikle and Betze/Post deposits. In Society Economic Geologists Guidebook Series; Society of Economic Geology: Littleton, CO, USA, 1997; Volume 28, pp. 101–108. [Google Scholar]
- Emsbo, P.; Hofstra, A.H.; Lauha, E.A. Jurassic auriferous polymetallic mineralization at the Goldstrike Mine, Carlin Trend, Nevada. In Geology and Ore Deposits 2000: The Great Basin and Beyond; Cluer, J.K., Price, J.G., Struhsacker, E.M., Hardyman, R.F., Morris, C.L., Eds.; Geological Society of Nevada: Reno, NV, USA, 2000; Appendix B2. [Google Scholar]
- Groff, J.A. Geochronology and Origin of Auriferous Fluids for the Getchell and Twin Creeks mines, Humbolt County, Nevada. Unpublished Ph.D. Dissertation, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 1996; p. 291. [Google Scholar]
- Cline, J.S.; Hofstra, A.H. Ore-fluid evolution at the Getchell Carlin-type gold deposit, Nevada, USA. Eur. J. Miner. 2000, 12, 195–212. [Google Scholar] [CrossRef]
- Bagby, W.C.; Cline, J.S. Constraints on the pressure of formation of the Getchell gold deposit, Humbolt County, Nevada, as interpreted from secondary fluid inclusion data. In Geology and Ore Deposits of the Great Basin; Raines, G.L., Lisle, R.E., Schaeffer, R., Wilkinson, W., Eds.; Geological Society of Nevada: Reno, NV, USA, 1991; pp. 793–804. [Google Scholar]
- Norman, D.I.; Moore, J.N. Methane and excess N2 and Ar in geothermal fluid inclusions. In Proceedings of the Twenty-Fourth Workshop of Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 22–24 January 1999; pp. 233–240. [Google Scholar]
- Heinrich, C.A.; Henley, R.W.; Seward, T.M. Hydrothermal Systems; Australian Mineral Foundation: Adelaide, SA, Australia, 1989; p. 74. [Google Scholar]
- Simmons, S.F.; Arehart, G.B.; Simpson, M.P.; Mauk, J.L. Origin of massive calcite veins in the Golden Cross low-sulphidation, epithermal Au-Ag deposit, New Zealand. Econ. Geol. 1999, 95, 99–112. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Redox control of gas compositions in Phillippine volcanic-hydrothermal systems. Geothermics 1993, 22, 575–587. [Google Scholar] [CrossRef]
- Blamey, N.J.F.; Norman, D.I. New interpretations of geothermal volatiles: Ar/He and N2/Ar ratios—Better indicator of magmatic volatiles, and equilibrium gas geothermometry. In Proceedings of the Twenty-Seventh Workshop of Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 28–30 January 2002. [Google Scholar]
- Pasteris, J.D.; Kuehn, C.A.; Bodnar, R.J. Applications of the laser Raman microprobe RAMANOR U-1000 to hydrothermal ore deposits: Carlin as an example. Econ. Geol. 1986, 81, 915–930. [Google Scholar] [CrossRef]
- Kesler, S.E.; Riciputi, L.C.; Ye, Z. Evidence for a magmatic origin for Carlin-type gold deposits: Isotopic composition of sulfur in the Betse-Post-Screamer Deposit, Nevada, USA. Miner. Depos. 2005, 40, 127–136. [Google Scholar] [CrossRef]
- Thompson, T.B. Origin of fluids associated with Carlin-type ore systems. In Great Basin Evolution and Metallogeny Geological Society of Nevada 2010 Symposium; Steininger, R.C., Pennell, W.M., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2010; pp. 201–219. [Google Scholar]
- Muntean, J.L.; Cline, J.S.; Simon, A.C.; Longo, A.A. Magmatic–hydrothermal origin of Nevada’s Carlin-type gold deposits. Nat. Geosci. 2011, 4, 122–127. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Heinrich, C.A. Vapor transport of metals and the formation of magmatic-hydrothermal ore deposits. Econ. Geol. 2005, 100, 1287–1312. [Google Scholar] [CrossRef]
- Hickey, K.A.; Barker, S.L.L.; Dipple, G.M.; Arehart, G.B.; Donelick, R.A. The Brevity of Hydrothermal Fluid Flow Revealed by Thermal Halos around Giant Gold Deposits: Implications for Carlin-Type Gold Systems. Econ. Geol. 2014, 109, 1461–1487. [Google Scholar] [CrossRef]
- Young, C.M.; Campbell, A.R.; Chavez, W.X., Jr. Whole-rock isotope traverses across gold-bearing and barren structures, Lone Tree Complex, Nevada. In Geology and Ore Deposits 2000: The Great Basin and Beyond; Cluer, J.K., Price, J.G., Struhsacker, E.M., Hardyman, R.F., Morris, C.L., Eds.; Geological Society of Nevada: Reno, NV, USA, 2000; pp. 969–987. [Google Scholar]
- Hickey, K.A.; Ahmed, A.D.; Barker, S.L.L.; Leonardson, R. Fault-controlled lateral fluid flow underneath and into a Carlin-type gold deposit: Isotopic and geochemical footprints. Econ. Geol. 2014, 109, 1431–1460. [Google Scholar] [CrossRef]
- Kesler, S.E.; Fortuna, J.; Ye, Z.; Alt, J.C.; Core, D.P.; Zohar, P.; Borhauer, J.; Chryssoulis, S.L. Evaluation of the role of sulfidation in deposition of gold, Screamer Section of the Betze-Post Carlin-type deposit, Nevada. Econ. Geol. 2003, 98, 1137–1157. [Google Scholar] [CrossRef]
- Silberman, M.L.; McKee, E.H. K-Ar ages of granitic plutons in north-central Nevada. Isochron/West 1971, 1, 15–32. [Google Scholar]
- Arehart, G.B.; Donelick, R.A. Thermal and isotopic profiling of the Pipeline hydrothermal system: Application to exploration for Carlin-type gold deposits. J. Geochem. Explor. 2006, 91, 27–40. [Google Scholar] [CrossRef]
- Folger, H.W.; Snee, L.W.; Mehnert, H.H.; Hofstra, A.H. Significance of K-Ar and 40Ar/39Ar dates of mica from Carlin-type deposits: Evidence from the Jerritt Canyon District in Nevada. In Geology and Ore Deposits of the American Cordillera; Coyner, A.R., Fahey, P.L., Eds.; Geological Society of Nevada: Reno/Sparks, NV, USA, 1996; pp. 41–60. [Google Scholar]
- Arehart, G.B.; Chakurian, A.M.; Tretbar, D.R.; Christensen, J.N.; McInnes, B.A.; Donelick, R.A. Evaluation of radioisotope dating of Carlin-type deposits in the Great Basin, western North America, and implications for deposit genesis. Econ. Geol. 2003, 98, 235–248. [Google Scholar]
- Harrison, T.M.; Celerier, J.; Aikman, A.B.; Hermann, J.; Heizler, M.T. Diffusion of 40-Ar in muscovite. Geochim. Cosmochim. Acta 2009, 73, 1039–1051. [Google Scholar] [CrossRef]
- Mortensen, J.K.; Thompson, J.F.H.; Tosdal, R.M. U-Pb age constraints on magmatism and mineralization in the northern Great Basin, Nevada. In Geology and Ore Deposits 2000: The Great Basin and Beyond; Cluer, J.K., Price, J.G., Struhsacker, E.M., Hardyman, R.F., Morris, C.L., Eds.; Geological Society of Nevada: Reno, NV, USA, 2000; pp. 419–438. [Google Scholar]
- Clark Maroun, L.R.; Cline, J.S.; Simon, A.; Anderson, P.; Muntean, J. High-grade gold deposition and collapse breccia formation, Cortez Hills Carlin-type gold deposit, Nevada, USA. Econ. Geol. 2017, 112, 707–740. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations; USGS Water-Resources Investigations Report; U.S. Geological Survey: Reston, VA, USA, 1999; pp. 99–4259.
- Benning, L.G.; Seward, T.M. Hydrosulphide complexing of Au(I) in hydrothermal solutions from 150–400 °C and 500–1500 bar. Geochim. Cosmochim. Acta 1996, 60, 1849–1871. [Google Scholar] [CrossRef]
- Cail, T.L.; Cline, J.S. Alteration associated with gold deposition at the Getchell Carlin-type gold deposit, North-Central Nevada. Econ. Geol. 2001, 96, 1343–1359. [Google Scholar] [CrossRef]



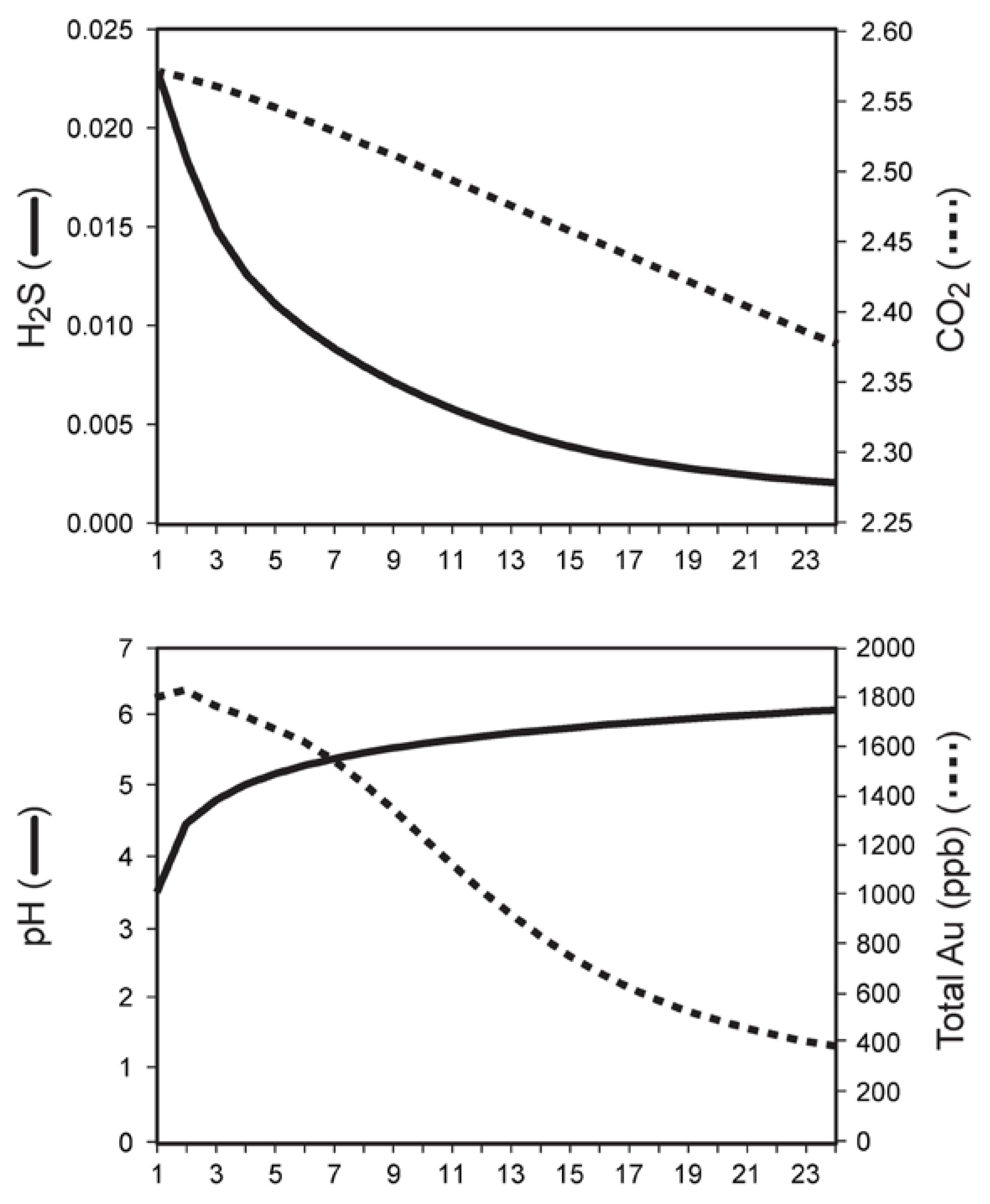
| Stage Mineral Analysis# | Early Dark Calcite 5390L | White Calcite Stockwork 6492L | Qsp-Stage Quartz 5830D | Qsp-Stage Quartz 6208D | Qsp-Stage Calcite 5847L | Late Vuggy Calcite 6058B |
| H2 (mol %) | 0.58 | 0.091 | 0.04 | <0.0001 | <0.0001 | <0.0001 |
| He (mol %) | 0.0003 | 0.007 | 0.0009 | 0.0006 | 0.0001 | 0.0003 |
| CH4 (mol %) | 1.74 | 0.48 | 0.53 | 0.40 | 0.10 | 0.067 |
| H2O (mol %) | 96.4 | 35.7 | 93.5 | 98.8 | 99.2 | 99.9 |
| N2 (mol %) | 0.11 | 0.39 | 0.24 | 0.072 | 0.025 | 0.033 |
| H2S (mol %) | 0.0001 | 0.0028 | 0.011 | 0.0002 | 0.0002 | <0.0001 |
| Ar (mol %) | 0.0005 | 0.0036 | 0.00027 | 0.0001 | 0.0002 | 0.0007 |
| CO2 (mol %) | 1.11 | 63.1 | 5.6 | 0.76 | 0.66 | 0.060 |
| SO2 (mol %) | 0.0001 | 0.0002 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Analysis# | 5861H | 6492R | 5830G | 6208F | 5847J | 6058F |
| C2H4 (ppm) | 348 | 101 | 7 | - | 7 | 5 |
| C2H6 (ppm) | 826 | 705 | 96 | 237 | 23 | 14 |
| C3H6 (ppm) | 511 | 702 | 38 | - | - | - |
| C3H8 (ppm) | - | 25 | 83 | 54 | 21 | 16 |
| C4H8 (ppm) | - | - | 7 | 36 | 7 | - |
| C4H10 (ppm) | 152 | - | - | 9 | 12 | 14 |
| C6H6 (ppm) | - | - | - | - | 1 | 10 |
| C7H8 (ppm) | - | - | - | - | 0.3 | 1 |
| Sample# | Material | δ13C in ‰ | δ18O in ‰ | ||||
| NB-33 | SRM | −2.90 | +10.8 | ||||
| NB-106 | SRM | −3.00 | +15.9 | ||||
| NB-114 | SRM | −2.69 | +14.4 | ||||
| NB-115 | SRM | −3.07 | +14.8 | ||||
| NB-116 | SRM | +2.90 | +16.3 | ||||
| Sample# | Mineral | Stage | T (°C) | Mineral δ34S | δ34S H2S(a) | ||
| NB-111 | Pyrite | Qsp | 300 | +6.4 | +5.2 | ||
| NB-113 | Pyrite | Qsp | 300 | +4.8 | +3.6 | ||
| NB-114 | Pyrite | Qsp | 300 | +4.4 | +3.2 | ||
| Sample# | Mineral | Stage | T (°C) | Mineral δ18O | δ18O Fluid | F.I. δD | |
| NB-99 | Quartz | Qsp | 300 | +19.0 | +10.8 | −117 | |
| NB-100 | Quartz | Qsp | 300 | +20.6 | +12.4 | −148 | |
| NB-102 | Quartz | Qsp | 300 | +19.4 | +11.2 | −137 | |
| NB-103 | Quartz | Qsp | 300 | +19.0 | +10.8 | −73 | |
| NB-106 | Quartz | Qsp | 300 | −61 | |||
| NB-107 | Quartz | Qsp | 300 | +20.0 | +11.8 | −104 | |
| Sample# | Mineral | Stage | T (°C) | Mineral δ13C | δ13C Fluid | Mineral δ18O | δ18O Fluid |
| NB-77 | Calcite | Early dark | 150 | +0.8 | −0.7 | +24.0 | +11.4 |
| NB-77dup | Calcite | Early dark | 150 | +0.7 | −0.7 | +24.0 | +11.4 |
| NB-43 | Calcite | Early dark | 150 | +1.1 | −0.4 | +20.2 | +7.6 |
| NB-32 | Calcite | Early dark | 150 | +0.2 | −1.3 | +18.4 | +5.8 |
| NB-84 | Calcite | White stockwork | 300 | +1.5 | +0.3 | +19.1 | +13.5 |
| NB-84dup | Calcite | White stockwork | 300 | +1.5 | +0.3 | +19.1 | +13.5 |
| NB-84dup2 | Calcite | White stockwork | 300 | +1.5 | +0.3 | +18.9 | +13.3 |
| NB-84 | Calcite | White stockwork | 300 | +1.5 | +0.3 | +18.9 | +13.3 |
| NB-33 | Calcite | Qsp-calcite | 240 | −3.9 | −5.1 | +17.6 | +9.9 |
| NB-33dup | Calcite | Qsp-calcite | 240 | −4.0 | −5.2 | +17.6 | +9.9 |
| NB-34 | Calcite | Qsp-calcite | 240 | −4.9 | −5.2 | +18.5 | +10.9 |
| NB-106 | Calcite | Qsp-calcite | 240 | −5.2 | −6.5 | +17.6 | +9.9 |
| NB-78 | Calcite | Late vuggy | 150 | −4.5 | −6.0 | +14.2 | +1.6 |
| NB-86 | Calcite | Late vuggy | 150 | −4.1 | −5.5 | +10.0 | −2.6 |
| NB-85 | Calcite | Late vuggy | 150 | −4.7 | −6.1 | +10.3 | −2.3 |
| NB-7 | Calcite | Late vuggy | 150 | −3.9 | −5.3 | +11.5 | −1.1 |
| # | Al2O3 | P2O5 | TiO2 | MgO | CaO | MnO | Na2O | K2O | Al2O3 | SiO2 | Total | K2O/Al2O3 | SiO2/(Al2O3 + K2O) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.00 | 0.02 | 0.00 | 0.09 | 0.05 | 0.01 | 0.01 | 1.12 | 4.00 | 70.69 | 76.00 | 0.28 | 13.8 |
| 2 | 3.08 | 0.02 | 0.03 | 0.08 | 0.04 | 0.02 | 0.02 | 0.76 | 3.08 | 83.81 | 87.85 | 0.25 | 21.8 |
| 3 | 1.62 | 0.01 | 0.00 | 0.05 | 0.03 | 0.03 | 0.04 | 0.41 | 1.62 | 94.27 | 96.45 | 0.24 | 46.4 |
| 4 | 1.81 | 0.01 | 0.01 | 0.04 | 0.04 | 0.01 | 0.00 | 0.44 | 1.81 | 75.39 | 77.78 | 0.25 | 33.5 |
| 5 | 2.57 | 0.06 | 0.00 | 0.11 | 0.05 | 0.00 | 0.03 | 0.65 | 2.57 | 94.11 | 97.58 | 0.27 | 29.2 |
| 6 | 2.82 | 0.00 | 0.01 | 0.07 | 0.05 | 0.00 | 0.02 | 0.75 | 2.82 | 92.35 | 96.07 | 0.29 | 25.9 |
| 7 | 3.45 | 0.03 | 0.00 | 0.08 | 0.02 | 0.00 | 0.02 | 1.00 | 3.45 | 82.39 | 87.01 | 0.29 | 18.5 |
| 9 | 2.18 | 0.00 | 0.03 | 0.08 | 0.05 | 0.00 | 0.03 | 0.49 | 2.18 | 81.06 | 83.94 | 0.27 | 30.3 |
| 10 | 2.75 | 0.00 | 0.00 | 0.10 | 0.05 | 0.00 | 0.03 | 0.74 | 2.75 | 82.27 | 85.94 | 0.27 | 23.6 |
| Deposit | Salinity (wt % NaCl eq.) | Th (°C) | Reference |
|---|---|---|---|
| Carlin | 0 to 10 | 120 to 290 | Kuehn, 1989 |
| Getchell | 3 to 6 | 160 to 220 | Hofstra and Cline, 2000 |
| Getchell | 1.4 to 42 | 159 to 350 | Groff, 1996 |
| Twin Creeks | 4.8 to 6.0 | 148 to 225 | Groff, 1996 |
| Meikle | 10 to 20 | 200 to 225 | Lamb, 1996 |
| Betze-Post | - | 150 to 210 | Lamb, 1996 |
| Lone Tree | 1.2 to 41.4 | 210 to 411 | Kamali, 1996 |
| Bashishan | 4.3 to 9.3 | 210 to 330 | Cromie and Zaw, 2003 |
| Pipeline | 5 to 10 | 179 to 323 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blamey, N.J.F.; Campbell, A.R.; Heizler, M.T. The Hydrothermal Fluid Evolution of Vein Sets at the Pipeline Gold Mine, Nevada. Minerals 2017, 7, 100. https://doi.org/10.3390/min7060100
Blamey NJF, Campbell AR, Heizler MT. The Hydrothermal Fluid Evolution of Vein Sets at the Pipeline Gold Mine, Nevada. Minerals. 2017; 7(6):100. https://doi.org/10.3390/min7060100
Chicago/Turabian StyleBlamey, Nigel J.F., Andrew R. Campbell, and Matt T. Heizler. 2017. "The Hydrothermal Fluid Evolution of Vein Sets at the Pipeline Gold Mine, Nevada" Minerals 7, no. 6: 100. https://doi.org/10.3390/min7060100





