Mineralogy of Zn–Hg–S and Hg–Se–S Series Minerals in Carbonate-Hosted Mercury Deposits in Western Hunan, South China
Abstract
:1. Introduction
2. Geological Setting
2.1. Regional Geology of the Tongren–Fenghuang Hg Belt
2.2. Geology of the Studied Deposits
2.2.1. The Chashula Hg–Zn Deposit
2.2.2. The Dongping Hg–Ag–Se Deposit
3. Sampling and Analytical Methods
3.1. Sampling Site
3.2. Electron-Probe Microanalyses
3.3. X-ray Diffraction Analysis
4. Results
4.1. Ore Petrology
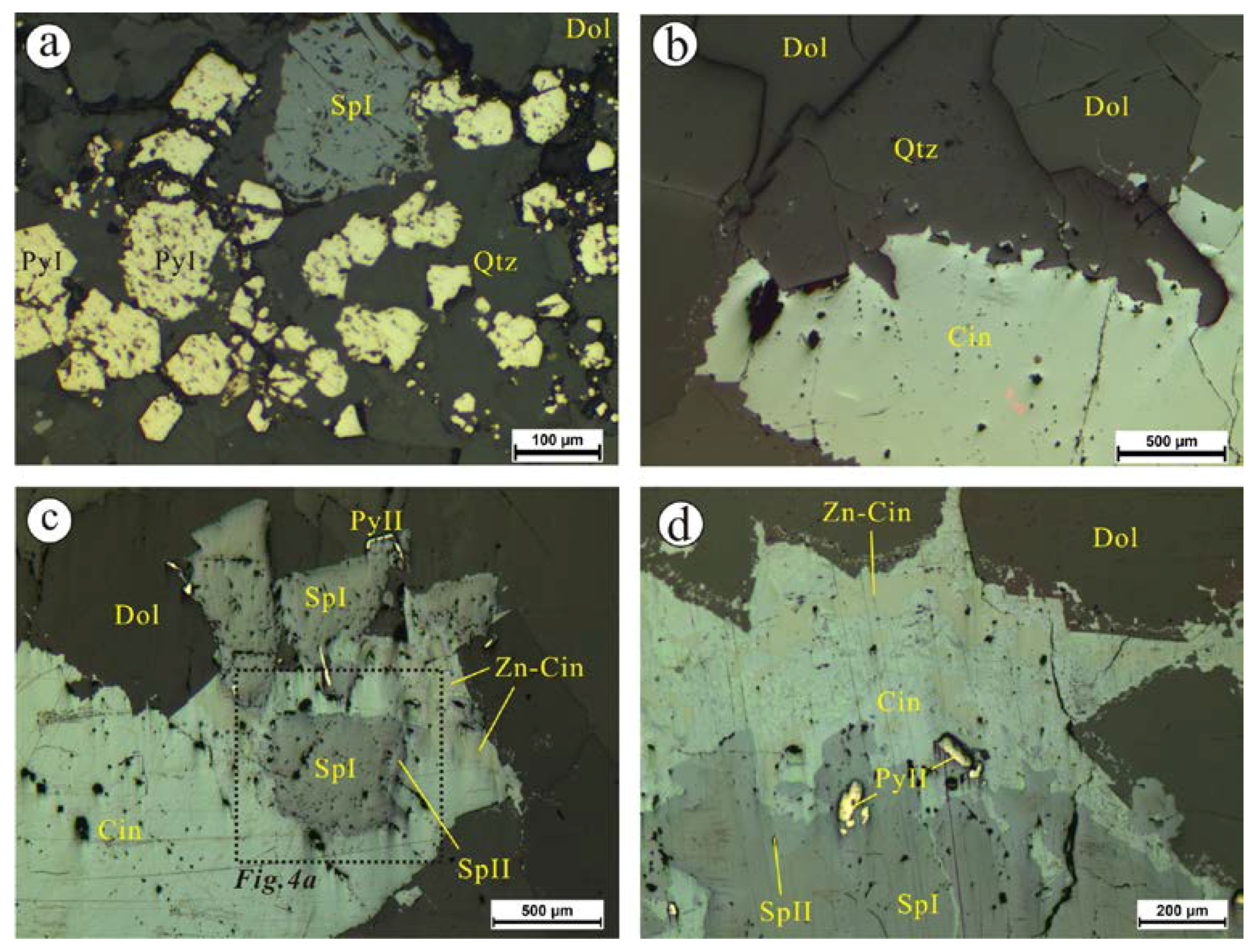
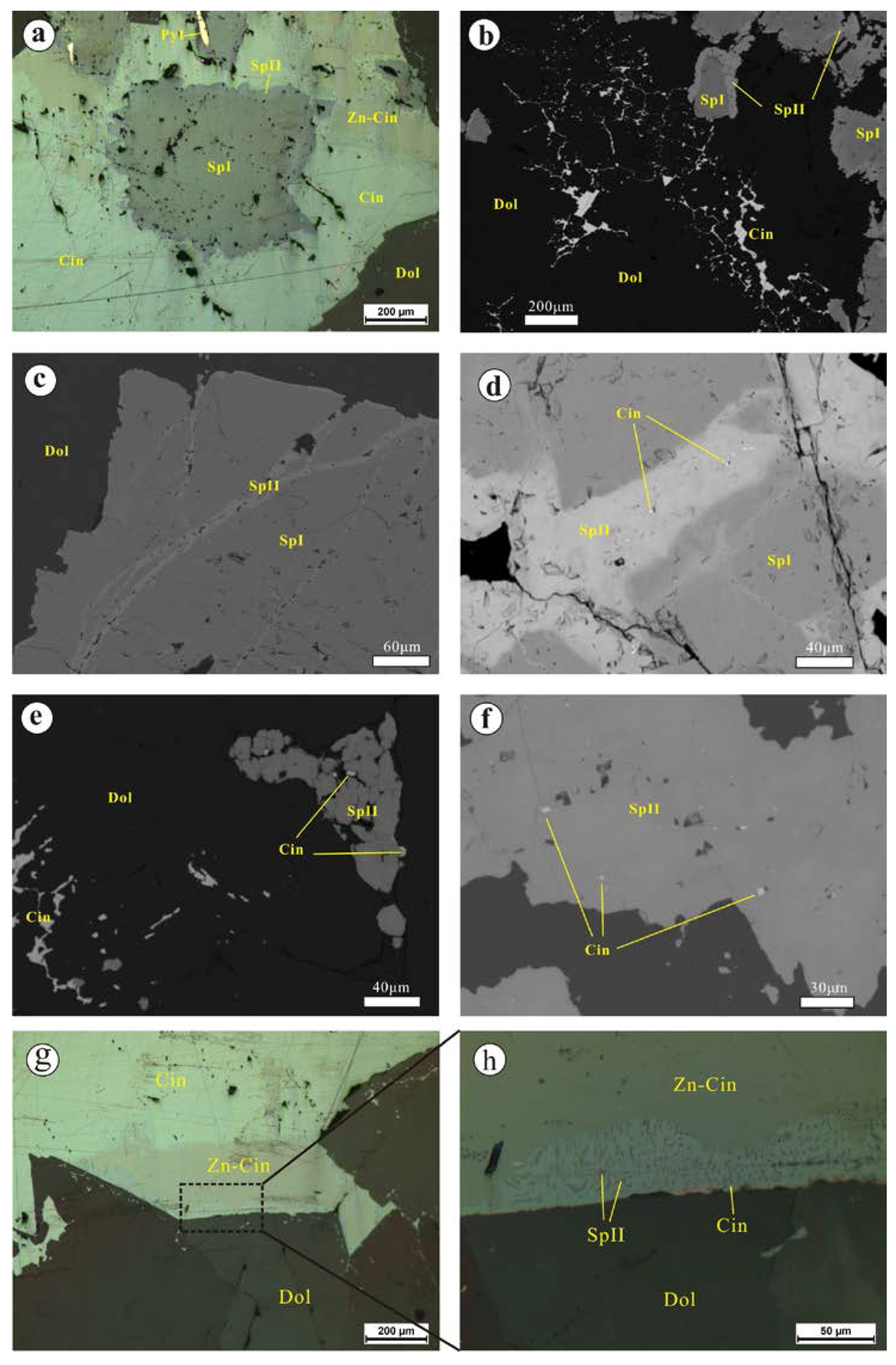
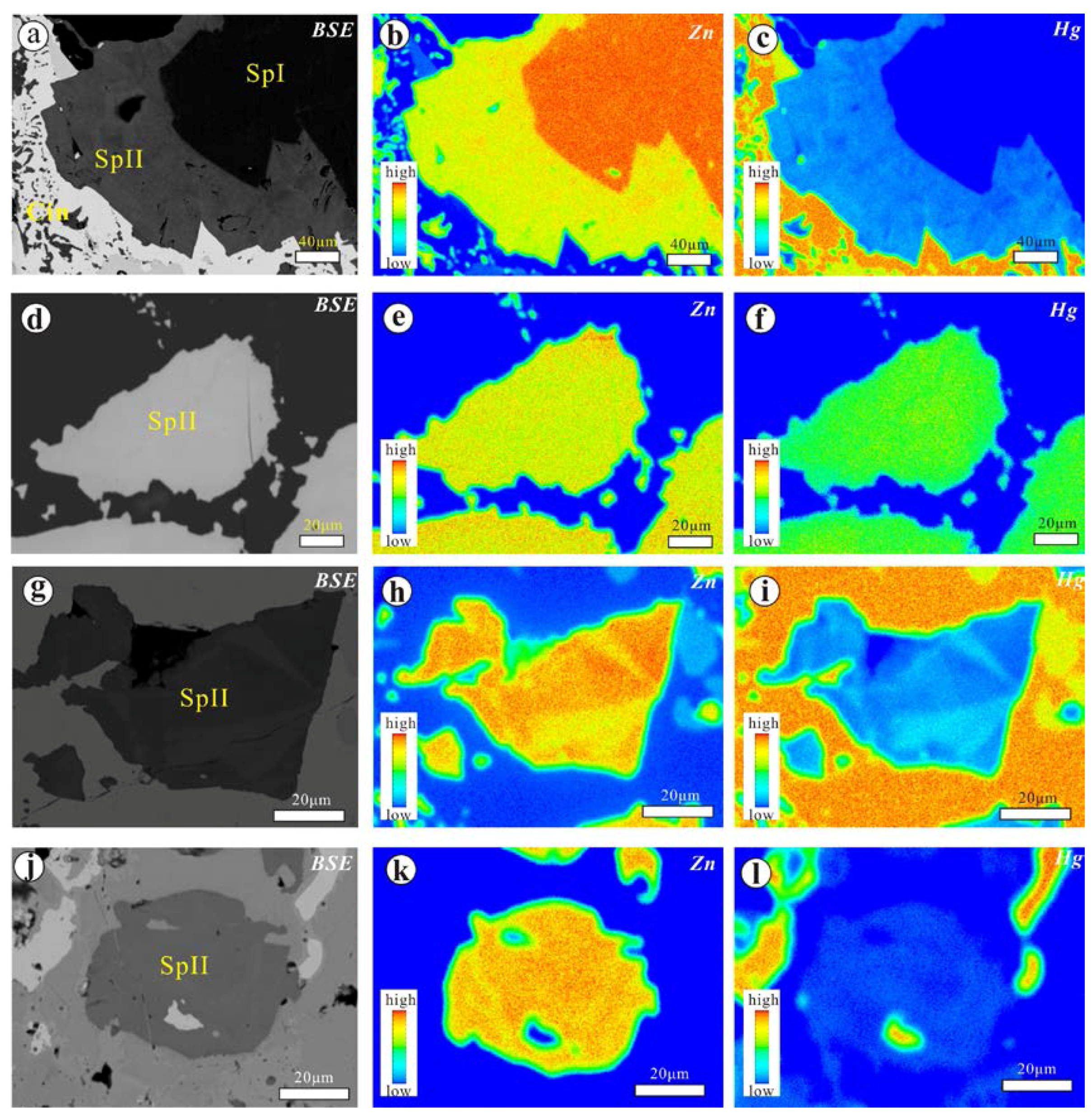
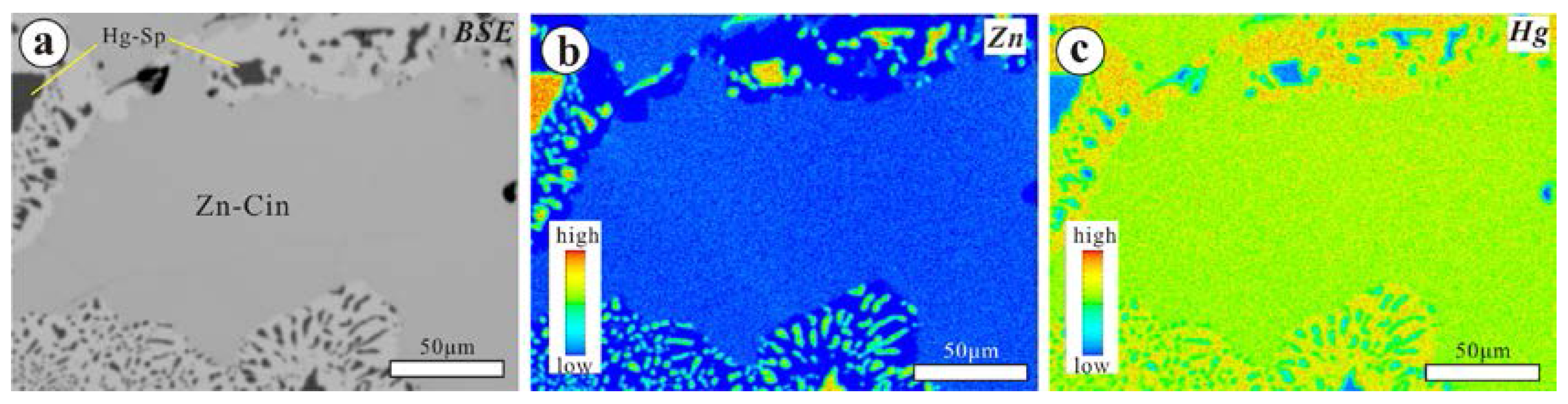
4.1.1. The Chashula Deposit
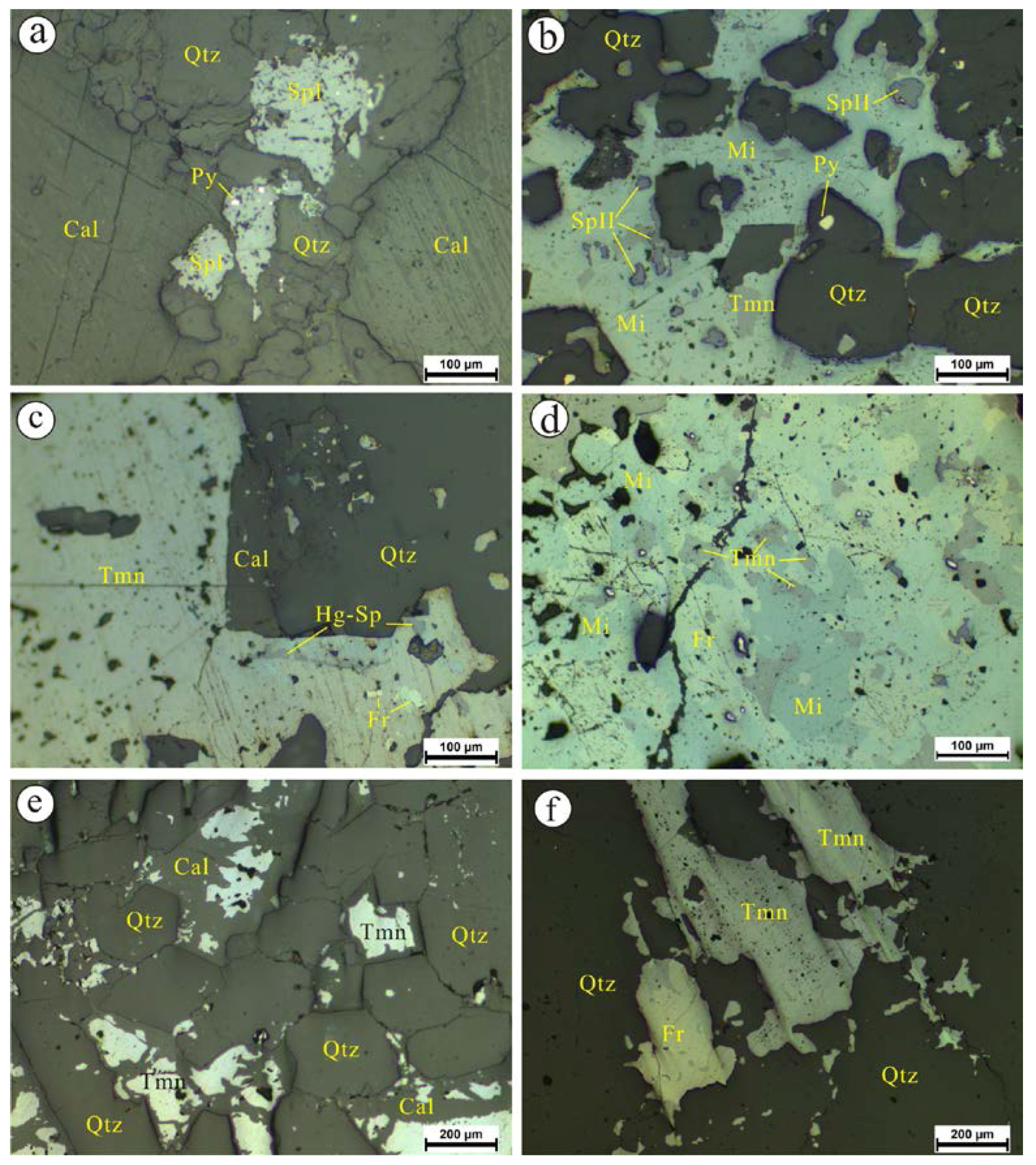
4.1.2. The Dongping Deposit
4.2. Chemical Composition of Hg-Bearing Sphalerite
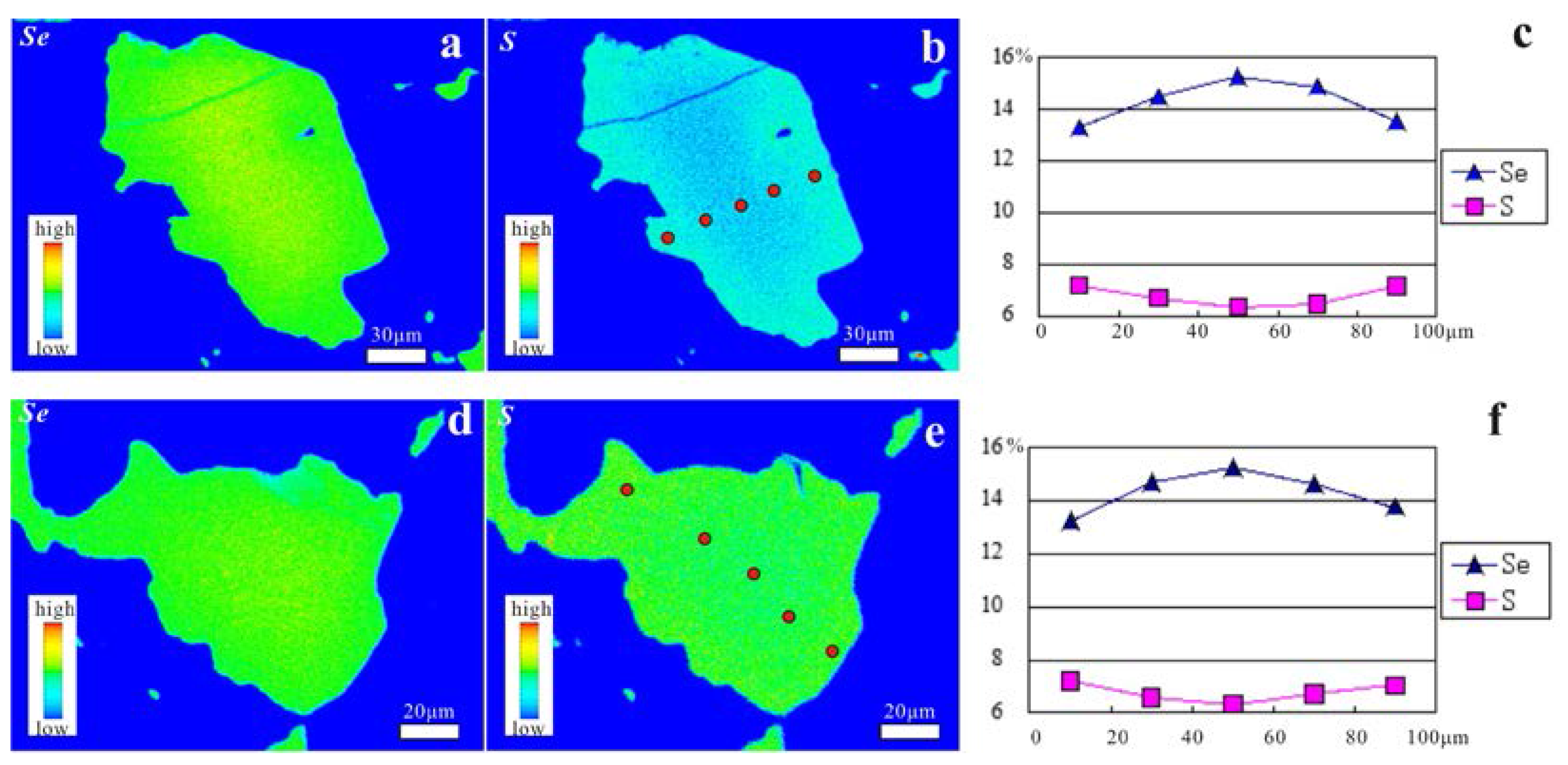
4.3. Chemical Compositions of Zn-Bearing Cinnabar and Selenium Metacinnabar
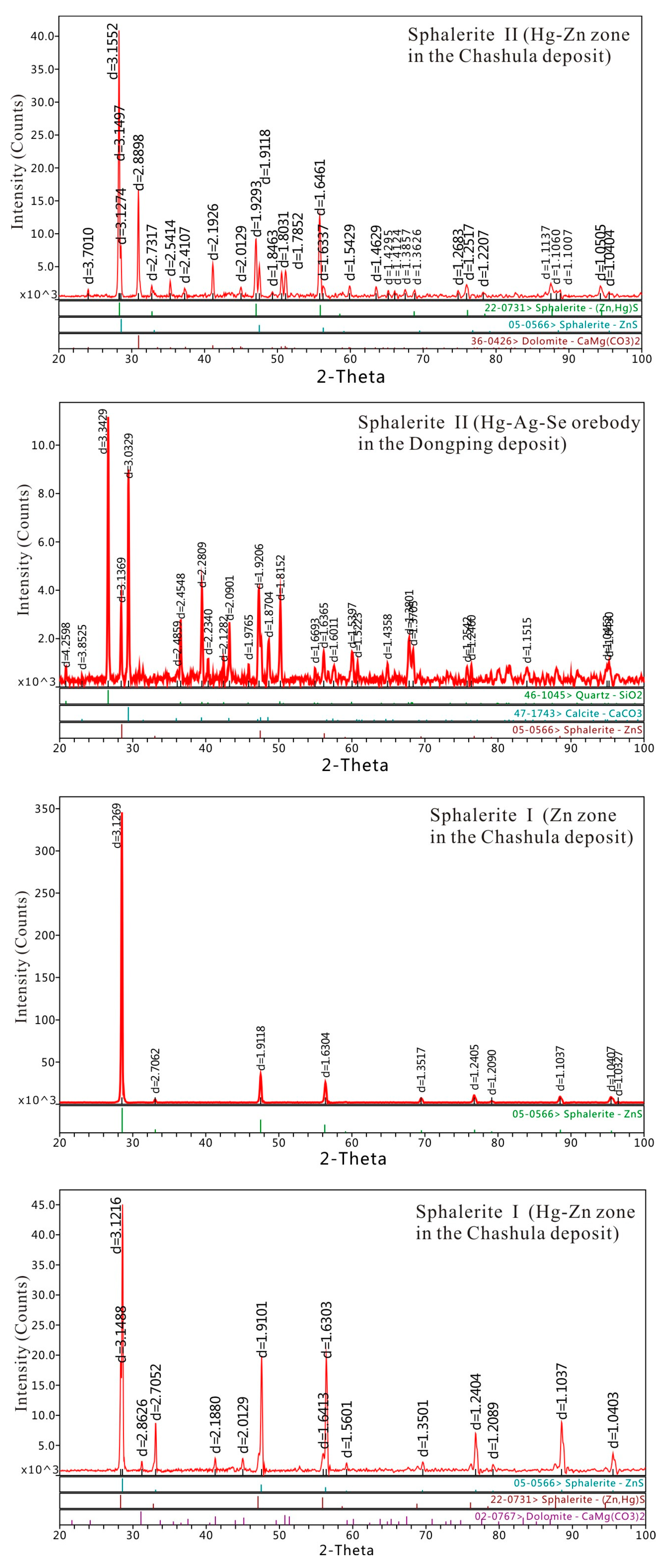
4.4. XRD Data of Hg-Bearing Sphalerite and Metacinnabar
5. Discussion
5.1. Hg Contents of Sphalerite Reflect Spatial and Temporal Variations in the Carbonate-Hosted Hg Deposits
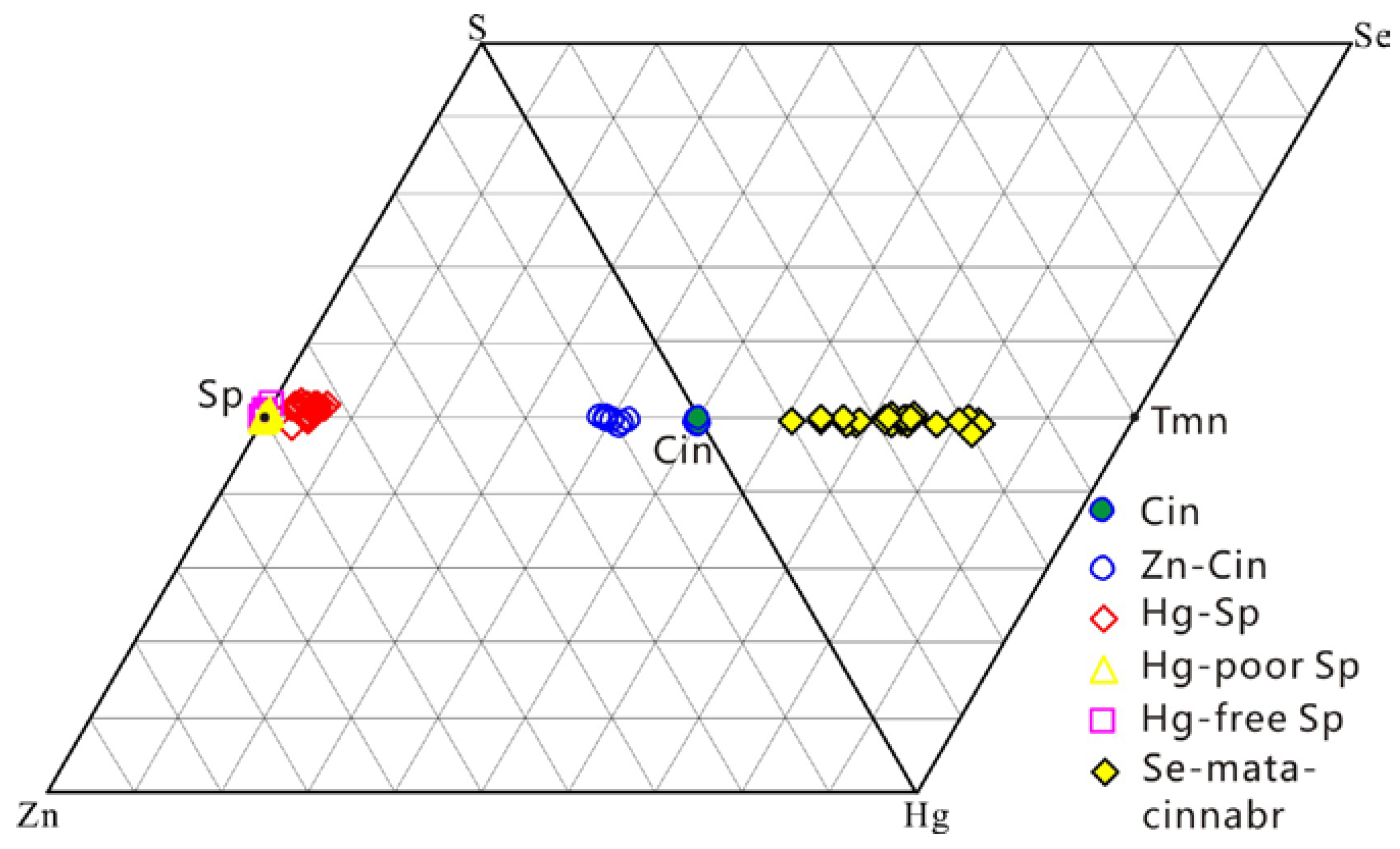
5.2. Zn–Hg–S and Hg–Se–S Series Minerals
5.3. Hg–Zn–Se Mineralization Style in the TFHB
6. Conclusions
- (1)
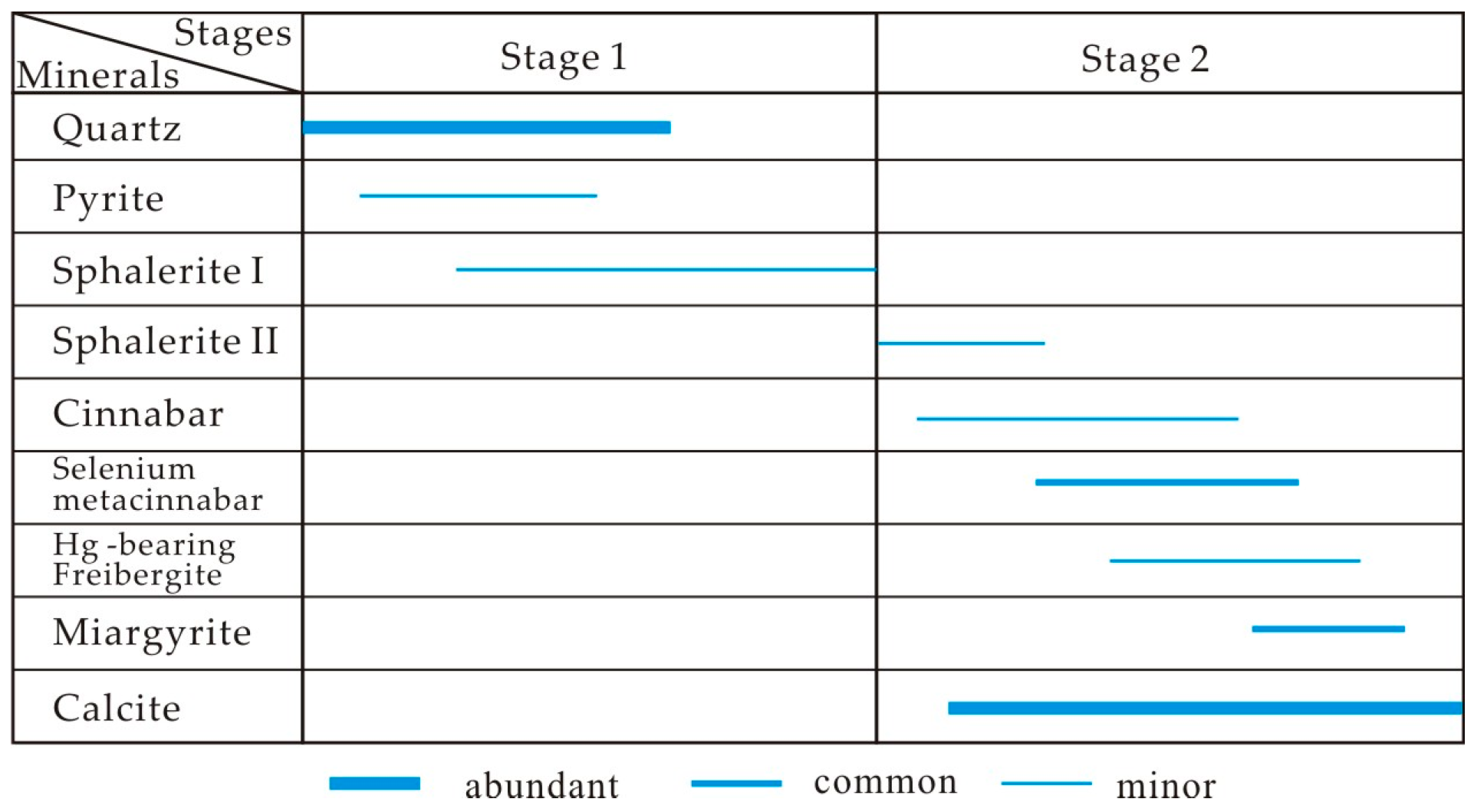
- Microscopic observations of the mineralization of the Chashula and Dongping deposits show that the carbonate-hosted Hg deposits in the TFHB experienced two stages. Pyrite, sphalerite I (Hg-poor sphalerite), and quartz were formed in Stage 1, while Zn-bearing cinnabar, sphalerite II (Hg-bearing sphalerite), cinnabar, selenium metacinnabar, and Ag minerals were formed in Stage 2.
- (2)
- The EPMA data show Hg contents of sphalerite I from the Chashula deposit contain low Hg (0.00–1.31 wt %), while sphalerite II is enriched in Hg (13.36–22.26 wt % Hg), which is the richest Hg in sphalerite within the TFHB. The sphalerite II of the Dongping deposit contains lower Hg (10.12–14.67 wt %) than that of Chashula deposit. The XRD data of sphalerite of the Chashula and Dongping deposits show that cubic unit cell parameter a gradually increases with increasing Hg content.
- (3)
- The texture of the Zn-bearing cinnabar of the Chashula deposit shows that it is not stable, and that it easily breaks down to Hg-bearing sphalerite and cinnabar through the chemical reaction: (Hg,Zn)S → (Zn,Hg)S + HgS. The isomorphic substitution between Se and S in selenium metacinnabar of the Dongping deposit is continuous, forming a wide range of selenium metacinnabar compositions.
- (4)
- The Zn, Hg–Zn, Hg, and Hg–Se mineralization styles in the deposits of the TFHB are the products of different stages in the mineralization process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry (Vol. 3 The Crust); Rudnick, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–64. [Google Scholar]
- George, M.W. Mercury. U.S. Geological Survey, 2015 Minerals Yearbook; USGS: Reston, VA, USA, 2016; pp. 102–103.
- George, M.W. Mercury. U.S. Geological Survey, Mineral Commodity Summaries; USGS: Reston, VA, USA, 2017; pp. 108–109.
- Yates, R.G.; Thompson, G.A. Geology and Quicksilver Deposits of the Terlingua District, Texas; U.S. Government Printing Office: Washington, DC, USA, 1959.
- Zen, R. A preliminary observation on the regional distribution regularity and the main characteristics of mercury mineralizations in China. Miner. Depos. 1983, 1, 30–37. (In Chinese) [Google Scholar]
- Song, S.H. Mineral Deposits of China (Volume 1); Geological Publishing House: Beijing, China, 1989; pp. 414–446. (In Chinese) [Google Scholar]
- Zou, Y.; Shi, D.; Zeng, L. China’s mercury resource development and management countermeasures under the new situation. China Min. Mag. 2017, 26, 6–8. (In Chinese) [Google Scholar]
- Hazen, R.M.; Golden, J.; Downs, R.T.; Hystad, G.; Grew, E.S.; Azzolini, D.; Sverjensky, D.A. Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestrial biosphere. Am. Mineral. 2012, 97, 1013–1042. [Google Scholar] [CrossRef]
- Vasil’ev, V.I. New data on the composition of metacinnabar and Hg-sphalerite with an isomorphous Cd admixture. Russ. Geol. Geophys. 2011, 52, 701–708. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.A.; Valeyev, O.; Roth, T. Compositional variation in Hg-bearing sphalerite from the polymetallic Eskay creek deposit, British Columbia, Canada. Chem. Erde Geochem. 2006, 66, 307–314. [Google Scholar] [CrossRef]
- Radosavljević, S.C.; Stojanović, J.N.; Pačevski, A.M. Hg-bearing sphalerite from the Rujevac polymetallic ore deposit, Podrinje Metallogenic District, Serbia: Compositional variations and zoning. Chem. Erde Geochem. 2012, 72, 237–244. [Google Scholar] [CrossRef]
- Jolly, J.L.; Heyl, A.V. Mercury and Other Trace Elements in Sphalerite and Wallrocks from Central Kentucky, Tennessee and Appalachian Zinc Districts; U.S. Geological Survey Bulletin; U.S. Government Printing Office: Washington, DC, USA, 1968.
- Slim-Shimi, N.; Tlig, S. Mixed type sulfide deposits in Northern Tunisia, regenerated in relation to paleogeography and tectonism. J. Afr. Earth Sci. Middle East 1993, 16, 287–307. [Google Scholar] [CrossRef]
- Jemmali, N.; Souissi, F.; Carranza, E.J.; Bouabdellah, M. Lead and sulfur isotope constraints on the genesis of the polymetallic mineralization at Oued Maden, Jebel Hallouf and Fedj Hassene carbonate-hosted Pb–Zn (As–Cu–Hg–Sb) deposits, Northern Tunisia. J. Geochem. Explor. 2013, 132, 6–14. [Google Scholar] [CrossRef]
- Davidson, D.F. Selenium in Some Epithermal Deposits of Antimony, Mercury and Silver and Gold; U.S. Geological Survey Bulletin 1112-A; U.S. Government Printing Office: Washington, DC, USA, 1960.
- Xinhuang Mercury Ming Company; No. 237 Metallurgical Geological Team of Hunan; No. 245 Metallurgical Geological Team of Hunan; Metallurgical Geology Institute of Hunan. Discovery of tiemannite and its prospecting significance. Geol. Prospect. 1975, 1, 35–37. (In Chinese) [Google Scholar]
- Chen, D.; Sun, S. Coccinite in Shangguanxi, Hunan Province. J. Changchun Univ. Earth Sci. 1990, 4, 68. (In Chinese) [Google Scholar]
- Chen, D.; Sun, S. Metacinnabar and tiemannite in Hunan-Guizhou (Xiangqian) mercury metallogenic belt. Acta Petrol. Miner. 1991, 10, 58–62. (In Chinese) [Google Scholar]
- Huang, Z. Onofrite in the Dongping mercury deposit, Baojing, Hunan Province. Acta Mineral. Sin. 1991, 1, 274–277. (In Chinese) [Google Scholar]
- Bao, J. Coccinite and its geological characteristics in the Jiudiantang mercury deposit. Geol. Geochem. 1982, 9, 56–57. (In Chinese) [Google Scholar]
- Bao, Z. Geological characteristics and metallogenic effects of Xiangxi Hg–Pb–Zn deposit. Geol. Prospect. 1983, 5, 15–21. (In Chinese) [Google Scholar]
- Liao, Z. Feature and genesis of zinc deposit in Dadongna mercury orefield. Guizhou Geol. 1999, 16, 315–320. (In Chinese) [Google Scholar]
- Bao, Z.; Wan, R.; Bao, J. Geological feature of Shangguanqi-Gaozai Se–Hg deposit in Xinhuang of Hunan and significance of seeking for the deposit. Guizhou Geol. 1999, 16, 321–324. (In Chinese) [Google Scholar]
- Xiang, P.; Niu, Y.; Feng, Q.; Ou, L.; Huang, X. Research on silver recovery of Doingping mercury-containing silver ore. Met. Mine 2005, 34, 407–410. (In Chinese) [Google Scholar]
- Liu, A.; Zhang, M.; Chen, J.; Yang, T. Distribution Law of zinc ores at Dadongla mercury orefield, Tongren and predict for exploration. Guizhou Geol. 2007, 24, 188–192. (In Chinese) [Google Scholar]
- Chen, J.; Liu, A.; Long, Q.; Xiong, W. The mineralization prospect analysis on the zinc ore in Yunchangping mercury mine field, Guizhou. Guizhou Sci. 2007, 25, 118–121. (In Chinese) [Google Scholar]
- Yang, T.; Duan, Q.; Tian, W. Geological characteristics and prospecting potential of Chatian Pb–Zn–Hg deposit, Western Hunan Province. Geol. Miner. Resour. South China 2014, 30, 109–117. (In Chinese) [Google Scholar]
- Wei, X.; Guo, Y.; Chen, Z. Hg-sphalerite and cell parameter formula. Chin. Sci. Bull. 1979, 24, 405–409. (In Chinese) [Google Scholar]
- Zheng, P.; Liu, Z. Mineralogical features of mercury sphalerite from Chatian deposit, Fenghuang County. Hunan Geol. 1992, 11, 221–224. (In Chinese) [Google Scholar]
- Bao, Z. Metallogenic mechanism of mercury-lead-zinc deposit in Western Hunan and Eastern Guizhou. J. Guilin Coll. Geol. 1987, 7, 159–170. (In Chinese) [Google Scholar]
- Hua, Y. Preliminary study on the origin of mercury deposits in Guizhou and its vicinity. Acta Geol. Sin. 1981, 2, 139–148. (In Chinese) [Google Scholar]
- Hua, Y.; Liu, Y. A genetic model for the Wanshan super-large mercury deposit, Guizhou. Guizhou Geol. 1996, 2, 161–165. (In Chinese) [Google Scholar]
- Wang, H.; Hu, K.; Wu, G.; Chen, H.; Liu, C. Genesis of strata-bound mercury deposits in the border area between Hunan and Guizhou. In Geology of Mercury Deposits of Guizhou Province, China; Yan, J.P., Ed.; Geological Publish Hourse: Beijing, China, 1989; pp. 99–182. (In Chinese) [Google Scholar]
- Wang, J.; Wen, H.; Shi, S. Characteristics and implications of REE, Carbon and oxygen isotopes of hydrothermal calcite from the mercury metallogenic belt in Hunan and Guizhou Provinces, China. Acta Miner. Sin. 2010, 30, 185–193. (In Chinese) [Google Scholar]
- Lei, Y.; Dai, P.; Duan, Q.; Wang, G.; Zhao, W. Rotational shear tectonics and metallogeny of the mercury ore belt in Hunan and Guizhou Provinces, China. Geotecton. Metallog. 2012, 36, 525–529. (In Chinese) [Google Scholar]
- Ye, L.; Cook, N.J.; Liu, T.; Ciobanu, C.L.; Gao, W.; Yang, Y. The Niujiaotang Cd-rich zinc deposit, Duyun, Guizhou Province, Southwest China: Ore genesis and mechanisms of cadmium concentration. Miner. Depos. 2012, 47, 683–700. [Google Scholar] [CrossRef]
- Bao, J.; Wan, R.; Bao, Z. Discussion of the mercury mineralization related to the metallogenic belt of Hunan-Guizhou Province. Beijing Geol. 1999, 2, 5–12. (In Chinese) [Google Scholar]
- Bao, Z. Mode of occurrence of two different types of orebodies in the Western Hunan-Eastern Guizhou mercury ore belt. Miner. Depos. 1985, 4, 81–88. (In Chinese) [Google Scholar]
- Long, G.; Xie, S.; Peng, G. Exploration Report of the Dongping Hg–Ag Deposit in Baojing County, Hunan Province; No. 405 Geological Team Bureau of Geology and Mineral Resources of Hunan Province: Jishou, China, 1995; pp. 1–60. (In Chinese) [Google Scholar]
- Yang, S.; Lao, K. Geological characteristics and ore indicators of lead-zinc deposits in northwestern Hunan, China. Geol. Bull. China 2007, 26, 899–908. (In Chinese) [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Danyushevsky, L.; Shimizu, M.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICP-MS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Liu, Y.; Zhang, Q.; Liu, T.; Gao, W.; Yang, Y.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- He, J.; Ma, D.; Liu, Y. The metallization geochemistry and hydrothermal concealed explosion model of the Chatian mercury deposit in Western Hunan. J. Guilin Inst. Technol. 1995, 15, 319–327. (In Chinese) [Google Scholar]
- Schneider, J.; Boni, M.; Lapponi, F.; Bechstadt, T. Carbonate-hosted zinc-lead deposits in the lower Cambrian of Hunan, South China: A radiogenic (Pb, Sr) isotope study. Econ. Geol. 2002, 97, 1815–1827. [Google Scholar] [CrossRef]
- Zheng, P. Mineralogical characteristics of tiemannite in Western Hunan. Hunan Geol. 1992, 11, 7–9. (In Chinese) [Google Scholar]
- Hua, Y.; Cui, M. Geology of the Wanshan Mercury Deposits in Guizhou; Geological Publishing House: Beijing, China, 1995; pp. 1–143. (In Chinese) [Google Scholar]
- Simon, G.; Kesler, S.E.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides: II. Applications to selenide bearing ore deposits. Econ. Geol. 1997, 92, 468–484. [Google Scholar] [CrossRef]




| I.D. | Zn | Hg | Fe | Cd | Se | S | Total | I.D. | Zn | Hg | Fe | Cd | Se | S | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphalerite II in the Hg–Zn zone of the Chashula deposit | Sphalerite I in the Hg–Zn zone of the Chashula deposit | ||||||||||||||
| 1 | 51.27 | 19.04 | 0.05 | 0.74 | 0 | 29.4 | 100.5 | 9 | 66.05 | 0 | 0.29 | 1.44 | 0.02 | 33.21 | 101 |
| 2 | 51.5 | 19.27 | 0.05 | 0.97 | 0 | 28.99 | 100.78 | 10 | 64.68 | 0.08 | 0.91 | 1.01 | 0.02 | 32.4 | 99.09 |
| 3 | 51.01 | 18.83 | 0.03 | 0.89 | 0.01 | 29.29 | 100.05 | 11 | 66.87 | 0.3 | 0.07 | 0.9 | 0 | 32.58 | 100.73 |
| 4 | 53.73 | 16.81 | 0 | 0.39 | 0.02 | 29.82 | 100.76 | 12 | 64.95 | 0.06 | 0.88 | 1.24 | 0 | 32.79 | 99.92 |
| 5 | 51.27 | 19.89 | 0.01 | 0.74 | 0.01 | 29.62 | 101.54 | 13 | 65.91 | 0 | 0.81 | 0.85 | 0.04 | 32.4 | 100.02 |
| 6 | 50.24 | 19.03 | 0.02 | 0.34 | 0 | 29.07 | 98.69 | Mean (n = 13) | 65.69 | 0.06 | 0.57 | 0.95 | 0.01 | 32.82 | 100.1 |
| 7 | 49.53 | 19.47 | 0.04 | 0.28 | 0 | 29.37 | 98.69 | Sphalerite I in the Zn zone of the Chashula deposit | |||||||
| 8 | 47.91 | 22.26 | 0.05 | 0.51 | 0 | 28.89 | 99.62 | 1 | 66.28 | 0.02 | 0.56 | 0.43 | 0.01 | 33.13 | 100.43 |
| 9 | 51.87 | 19.34 | 0.06 | 0.34 | 0 | 30.34 | 101.95 | 2 | 65.69 | 0.96 | 0.5 | 0.3 | 0 | 33.4 | 100.85 |
| 10 | 53.57 | 18.14 | 0.1 | 0.26 | 0.16 | 29.36 | 101.58 | 3 | 65.36 | 0.53 | 0.28 | 0.9 | 0 | 32.87 | 99.93 |
| 11 | 57.23 | 13.36 | 0 | 0.83 | 0.02 | 28.92 | 100.37 | 4 | 66.88 | 0.44 | 0.1 | 0.73 | 0.02 | 32.85 | 101.01 |
| 12 | 53.34 | 19.31 | 0.01 | 0.31 | 0 | 28.95 | 101.92 | 5 | 64.87 | 1.31 | 0.19 | 0.53 | 0 | 32.92 | 99.82 |
| 13 | 51.94 | 19.21 | 0 | 0.38 | 0 | 28.53 | 100.06 | 6 | 66.75 | 0.21 | 0.26 | 0.25 | 0 | 32.96 | 100.4 |
| 14 | 50.67 | 19.7 | 0.08 | 0.86 | 0.03 | 29.44 | 100.78 | 7 | 66.18 | 0.01 | 0.35 | 0.18 | 0 | 32.58 | 99.3 |
| 15 | 52.65 | 17.06 | 0.09 | 0.95 | 0 | 30.33 | 101.07 | 8 | 64.84 | 1.11 | 0.28 | 0.39 | 0 | 32.36 | 98.98 |
| 16 | 52.44 | 17.53 | 0.03 | 0.96 | 0 | 30.81 | 101.76 | 9 | 64.04 | 1.22 | 0.12 | 0.64 | 0.03 | 32.44 | 98.49 |
| 17 | 49.73 | 21.46 | 0.06 | 0.38 | 0 | 29.58 | 101.21 | 10 | 65.84 | 0.23 | 0.46 | 0.15 | 0.03 | 32.46 | 99.17 |
| 18 | 51.88 | 17.35 | 0.03 | 1.25 | 0.02 | 29.76 | 100.29 | 11 | 66.66 | 0.04 | 0.11 | 0.36 | 0 | 32.16 | 99.32 |
| 19 | 52.38 | 18.79 | 0.07 | 0.79 | 0 | 29.91 | 101.94 | Mean (n = 11) | 65.76 | 0.55 | 0.29 | 0.44 | 0.01 | 32.74 | 99.79 |
| 20 | 52.17 | 18.85 | 0 | 0.89 | 0 | 29.74 | 101.65 | Sphalerite II in the Dongping deposit | |||||||
| Mean (n = 20) | 51.82 | 18.73 | 0.04 | 0.65 | 0.01 | 29.51 | 100.76 | 1 | 56.77 | 10.69 | 0.2 | 0.05 | 0.05 | 31.66 | 99.41 |
| Sphalerite I in the Hg–Zn zone of the Chashula deposit | 2 | 56.72 | 10.12 | 0.18 | 0 | 0.04 | 31.08 | 98.14 | |||||||
| 1 | 66.26 | 0.1 | 0.63 | 0.62 | 0 | 32.83 | 100.43 | 3 | 54.79 | 12.02 | 0.23 | 0.04 | 0.03 | 31.27 | 98.37 |
| 2 | 66.96 | 0 | 0.3 | 0.42 | 0.02 | 33.43 | 101.13 | 4 | 54.39 | 13.43 | 0.27 | 0.08 | 0.04 | 31.05 | 99.26 |
| 3 | 65.2 | 0.18 | 0.28 | 0.97 | 0 | 32.59 | 99.22 | 5 | 56.29 | 10.23 | 0.25 | 0 | 0.03 | 31.43 | 98.23 |
| 4 | 65.96 | 0.01 | 0.26 | 0.69 | 0 | 33.03 | 99.95 | 6 | 54.1 | 14.67 | 0.17 | 0.03 | 0.07 | 31.23 | 100.27 |
| 5 | 66.05 | 0 | 0.13 | 0.85 | 0 | 32.82 | 99.86 | 7 | 56.26 | 12.18 | 0.17 | 0.01 | 0.06 | 31.67 | 100.35 |
| 6 | 66.88 | 0 | 0.34 | 0.58 | 0 | 32.59 | 100.38 | 8 | 56.74 | 11.52 | 0.32 | 0.09 | 0.01 | 31.52 | 100.19 |
| 7 | 62.2 | 0 | 2.28 | 1.31 | 0 | 32.83 | 98.61 | 9 | 56.76 | 11.04 | 0.18 | 0 | 0.03 | 31.36 | 99.37 |
| 8 | 66.05 | 0 | 0.29 | 1.44 | 0.02 | 33.21 | 101.00 | Mean (n = 9) | 55.87 | 11.77 | 0.22 | 0.03 | 0.04 | 31.36 | 99.29 |
| Samples | Zn | Hg | Fe | Cd | Se | S | Total |
|---|---|---|---|---|---|---|---|
| Zn-bearing cinnabar | |||||||
| 4b2.1b | 6.06 | 77.87 | 0.00 | 0.54 | 0.09 | 14.57 | 99.12 |
| 4b2.2 | 5.77 | 77.10 | 0.03 | 0.59 | 0.08 | 14.49 | 98.05 |
| 4b2.3 | 6.08 | 76.09 | 0.05 | 1.04 | 0.00 | 15.02 | 98.28 |
| 2kp3a4.1 | 6.72 | 75.18 | 0.00 | 3.09 | 0.15 | 15.00 | 100.14 |
| 2kp3a4.2 | 7.05 | 73.16 | 0.00 | 3.55 | 0.10 | 15.18 | 99.04 |
| 2kp3a4.3 | 6.64 | 74.65 | 0.00 | 3.50 | 0.07 | 15.12 | 99.97 |
| 4c1.7a | 5.67 | 79.21 | 0.05 | 0.69 | 0.04 | 14.99 | 100.64 |
| 4c1.8a | 5.13 | 78.98 | 0.00 | 0.61 | 0.07 | 14.88 | 99.66 |
| Mean (n = 8) | 6.14 | 76.53 | 0.02 | 1.70 | 0.07 | 14.91 | 99.36 |
| Cinnabar | |||||||
| 2kp3a5.1 | 1.01 | 86.80 | 0.03 | 0.00 | 0.00 | 13.39 | 101.24 |
| 4c1.6c | 0.03 | 86.45 | 0.00 | 0.02 | 0.12 | 13.33 | 99.94 |
| zp2b1.1 | 0.00 | 85.10 | 0.00 | 0.00 | 0.03 | 13.55 | 98.67 |
| zp2b1.2a | 0.03 | 84.93 | 0.00 | 0.02 | 0.00 | 13.58 | 98.55 |
| zp2a1.2b | 0.03 | 86.52 | 0.03 | 0.00 | 0.00 | 13.91 | 100.48 |
| zp2b1.3 | 0.00 | 85.23 | 0.00 | 0.00 | 0.02 | 13.71 | 98.96 |
| Mean (n = 6) | 0.18 | 85.84 | 0.01 | 0.01 | 0.03 | 13.58 | 99.64 |
| I.D. | Samples | Zn | Hg | Fe | Cd | Se | S | Total | I.D. | Samples | Zn | Hg | Fe | Cd | Se | S | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | d7a1.1 | 0.00 | 79.73 | 0.00 | 0.00 | 13.91 | 6.64 | 100.28 | 16 | d5a1.1 | 0.02 | 77.42 | 0.00 | 0.00 | 19.04 | 4.20 | 100.68 |
| 2 | d7a1.5 | 0.13 | 80.10 | 0.02 | 0.01 | 13.24 | 7.13 | 100.62 | 17 | d5a1.2 | 0.07 | 76.81 | 0.00 | 0.00 | 17.60 | 4.14 | 98.61 |
| 3 | d7a1.6b | 1.08 | 81.34 | 0.03 | 0.05 | 8.59 | 9.18 | 100.26 | 18 | d5a1.3 | 0.17 | 80.18 | 0.02 | 0.00 | 14.63 | 6.44 | 101.45 |
| 4 | d7a1.7a | 0.59 | 83.97 | 0.00 | 0.01 | 6.81 | 10.32 | 101.71 | 19 | d5a1.4 | 0.05 | 78.92 | 0.05 | 0.00 | 14.66 | 6.62 | 100.29 |
| 5 | d7a1.8 | 0.22 | 77.20 | 0.02 | 0.00 | 19.21 | 4.34 | 100.99 | 20 | d7b3.3 | 0.00 | 78.58 | 0.02 | 0.00 | 13.24 | 7.20 | 99.04 |
| 6 | d7a1.9 | 0.00 | 81.13 | 0.00 | 0.00 | 11.07 | 8.01 | 100.21 | 21 | d7b3.2 | 0.19 | 78.30 | 0.01 | 0.00 | 14.69 | 6.58 | 99.77 |
| 7 | d7a1.10 | 0.61 | 80.75 | 0.06 | 0.04 | 10.30 | 8.33 | 100.09 | 22 | d7b3.1 | 0.09 | 78.43 | 0.02 | 0.02 | 15.26 | 6.32 | 100.14 |
| 8 | d7a1.11 | 0.25 | 79.65 | 0.01 | 0.00 | 13.18 | 6.89 | 99.97 | 23 | d7b3.4 | 0.04 | 79.33 | 0.04 | 0.00 | 14.64 | 6.71 | 100.76 |
| 9 | d7a1.14 | 0.26 | 79.65 | 0.04 | 0.06 | 11.22 | 7.98 | 99.21 | 24 | d7b3.5 | 0.05 | 78.37 | 0.00 | 0.04 | 13.76 | 7.04 | 99.25 |
| 10 | d7a1.16 | 0.08 | 77.76 | 0.02 | 0.00 | 13.95 | 6.58 | 98.38 | 25 | d7b4.1 | 0.19 | 80.31 | 0.02 | 0.00 | 13.29 | 7.19 | 101.01 |
| 11 | d7a1.17 | 0.23 | 80.95 | 0.03 | 0.03 | 10.40 | 8.69 | 100.32 | 26 | d7b4.2 | 0.11 | 78.75 | 0.00 | 0.04 | 14.47 | 6.70 | 100.07 |
| 12 | d7a1.19 | 0.60 | 81.00 | 0.06 | 0.02 | 8.91 | 9.34 | 99.94 | 27 | d7b4.3 | 0.00 | 76.88 | 0.01 | 0.00 | 15.25 | 6.35 | 98.49 |
| 13 | d7b1.1 | 0.03 | 76.57 | 0.01 | 0.00 | 18.39 | 4.65 | 99.65 | 28 | d7b4.4 | 0.05 | 77.95 | 0.02 | 0.00 | 14.86 | 6.47 | 99.35 |
| 14 | d7b1.2a | 0.13 | 78.92 | 0.00 | 0.00 | 16.35 | 5.47 | 100.87 | 29 | d7b4.5 | 0.13 | 79.38 | 0.03 | 0.00 | 13.54 | 7.16 | 100.23 |
| 15 | d7b1.3 | 0.07 | 77.79 | 0.02 | 0.02 | 17.85 | 4.85 | 100.60 | Mean (n = 29) | 0.19 | 79.18 | 0.02 | 0.01 | 13.87 | 6.81 | 100.08 | |
| Samples | Sphalerite II (Hg–Zn Aone in the Chashula Aeposit) ~20 wt % Hg | Sphalerite II (Hg–Ag–Se Orebody in the Dongping Deposit) ~10 wt % Hg | Sphalerite I (Zn Zone in the Chashula Aeposit) ~0.5 wt % Hg | Sphalerite I (Hg–Zn Aone in the Chashula Aeposit) ~0 wt % Hg | ||||
|---|---|---|---|---|---|---|---|---|
| h k l | deas | Iest | deas | Iest | deas | Iest | deas | Iest |
| 111 | 3.1552 | 100.0 | 3.1369 | 83.5 | 3.1320 | 100 | 3.1264 | 100.0 |
| 200 | 2.7317 | 4.2 | 2.7172 | 7.5 | 2.7062 | 1.0 | 2.7087 | 18.1 |
| 220 | 1.9293 | 21.6 | 1.9219 | 100.0 | 1.9118 | 9.6 | 1.9118 | 43.5 |
| 311 | 1.6461 | 30.5 | 1.6373 | 31.4 | 1.6322 | 6.9 | 1.6315 | 43.4 |
| 222 | - | - | - | - | - | - | 1.5612 | 2.7 |
| 400 | - | - | - | - | 1.3517 | 1.5 | 1.3626 | 1.7 |
| 331 | - | - | - | - | 1.2405 | 2.4 | 1.2498 | 2.2 |
| a, Å | 5.459 (0.001184) | 5.432 (0.001149) | 5.407 (0.000151) | 5.409 (0.000684) | ||||
| Samples | Tiemannite (PDF#08-0469, City Chemical Corporation, New York, USA) | Metacinnabar (PDF#22-0729, Gorkhon Aeposit, Russia) | Metacinnabar (Hg–Ag–Se ores in the Dongping Aeposit) | |||
|---|---|---|---|---|---|---|
| h k l | d | I | d | I | deas | Iest |
| 111 | 3.510 | 100.0 | 3.4100 | 100.0 | 3.4304 | 95.9 |
| 200 | 3.040 | 16.0 | 2.950 | 40.0 | 2.9745 | 33.4 |
| 220 | 2.151 | 50.0 | 2.090 | 80.0 | 2.1009 | 100.0 |
| 311 | 1.835 | 30.0 | 1.780 | 70.0 | 1.7913 | 44.2 |
| 331 | 1.396 | 10.0 | 1.355 | 30.0 | 1.3633 | 14.7 |
| 422 | 1.242 | 8.0 | 1.204 | 30.0 | 1.2124 | 11.2 |
| 511 | 1.170 | 4.0 | 1.136 | 30.0 | 1.1426 | 7.7 |
| a, Å | 6.085 | 5.903 | 5.940 (0.001) | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Rong, Y.; Zhang, S. Mineralogy of Zn–Hg–S and Hg–Se–S Series Minerals in Carbonate-Hosted Mercury Deposits in Western Hunan, South China. Minerals 2017, 7, 101. https://doi.org/10.3390/min7060101
Liu J, Rong Y, Zhang S. Mineralogy of Zn–Hg–S and Hg–Se–S Series Minerals in Carbonate-Hosted Mercury Deposits in Western Hunan, South China. Minerals. 2017; 7(6):101. https://doi.org/10.3390/min7060101
Chicago/Turabian StyleLiu, Jianping, Yanan Rong, and Shugen Zhang. 2017. "Mineralogy of Zn–Hg–S and Hg–Se–S Series Minerals in Carbonate-Hosted Mercury Deposits in Western Hunan, South China" Minerals 7, no. 6: 101. https://doi.org/10.3390/min7060101





