Optimization Mechanism of Additive of Composite Sodium Salts on Vanadium Oxidation of Siliceous Shale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Procedure
3. Results and Discussion
3.1. Influence of Composite Additive of Two Salts on the Change of Vanadium Valence
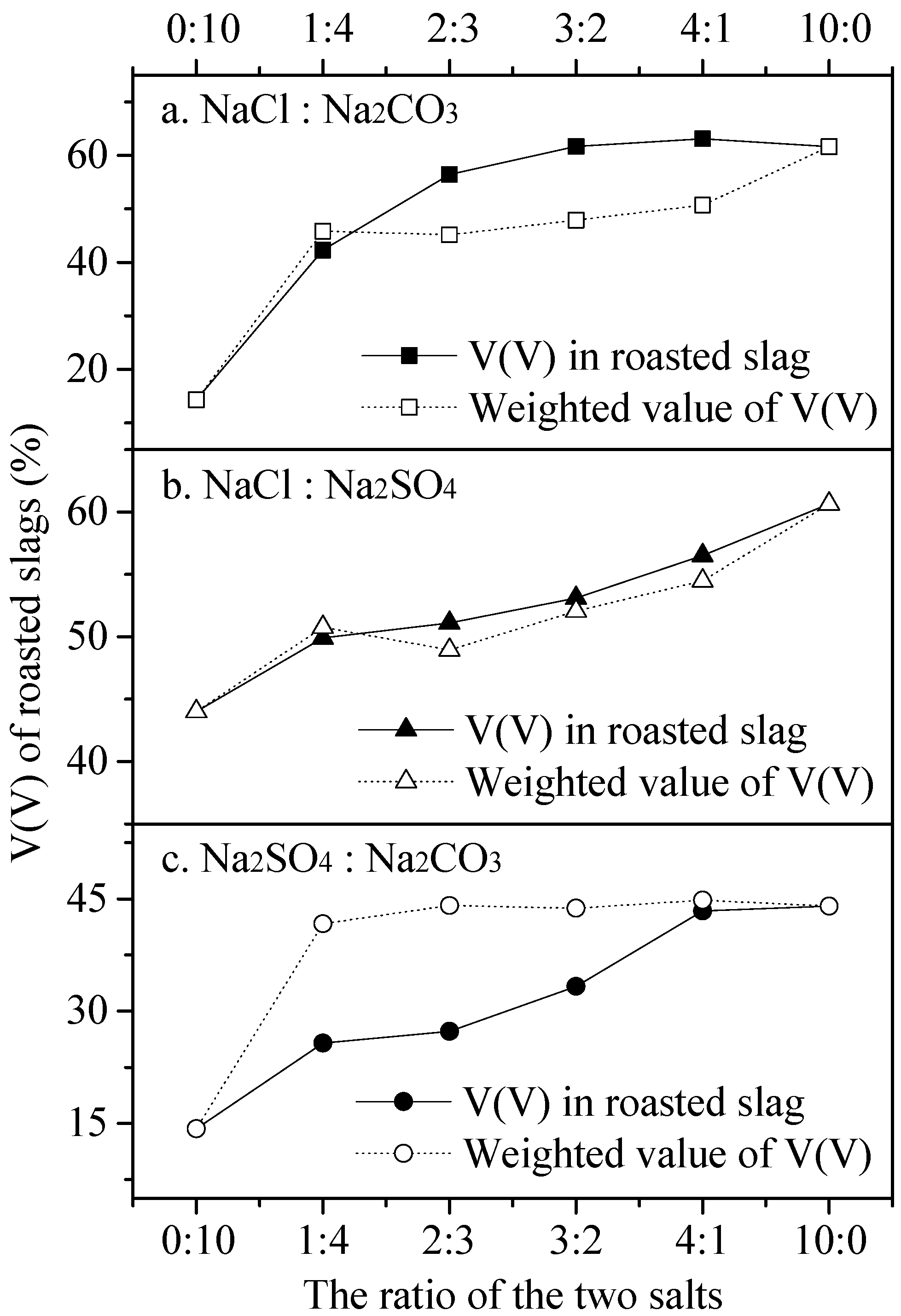
3.2. Synergistic Effect between Two Sodium Salts on the Vanadium Oxidation and Mineral Phase Change
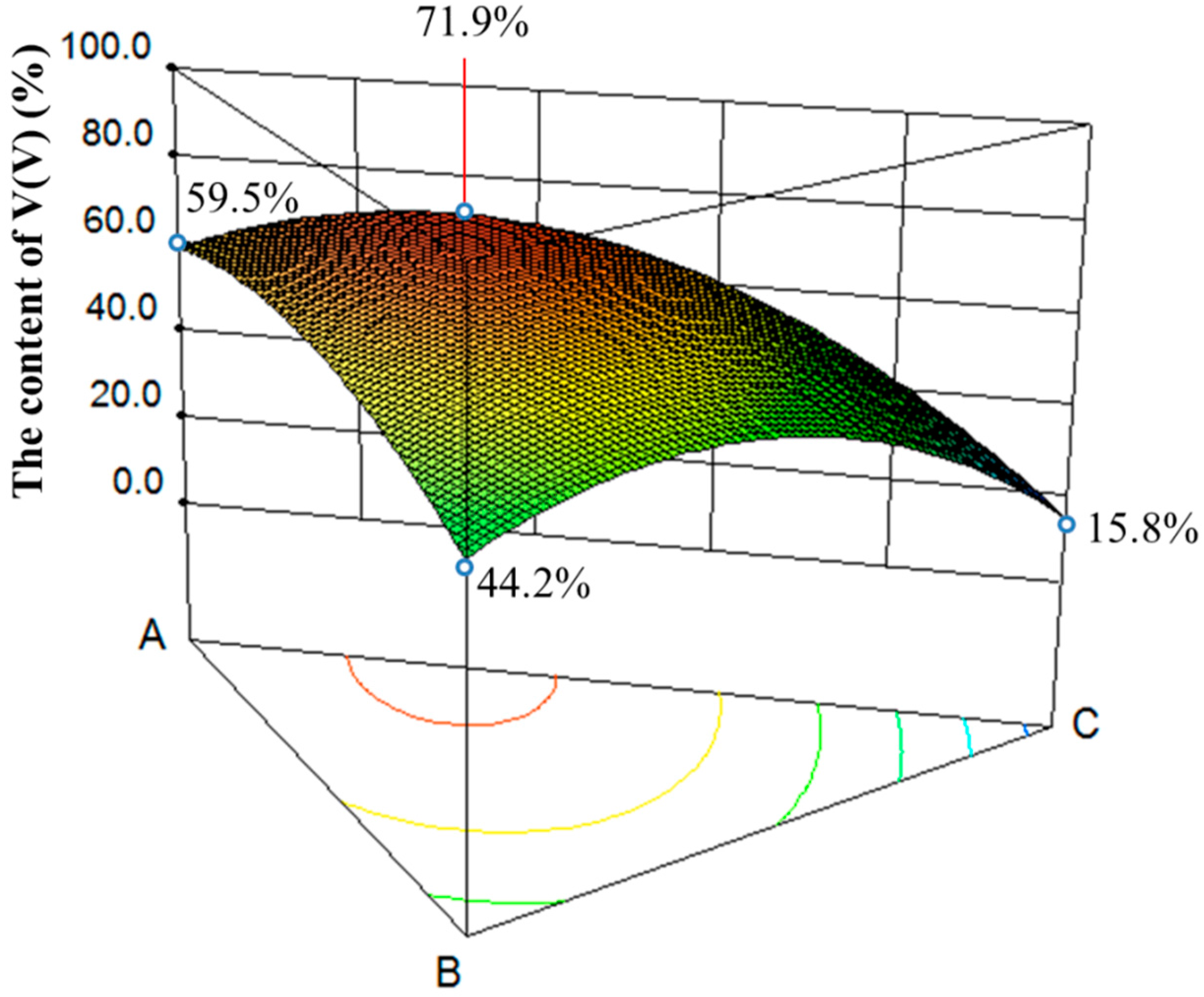
3.2.1. Influence of Composite Additive on the V(V) Content of Roasted Slag
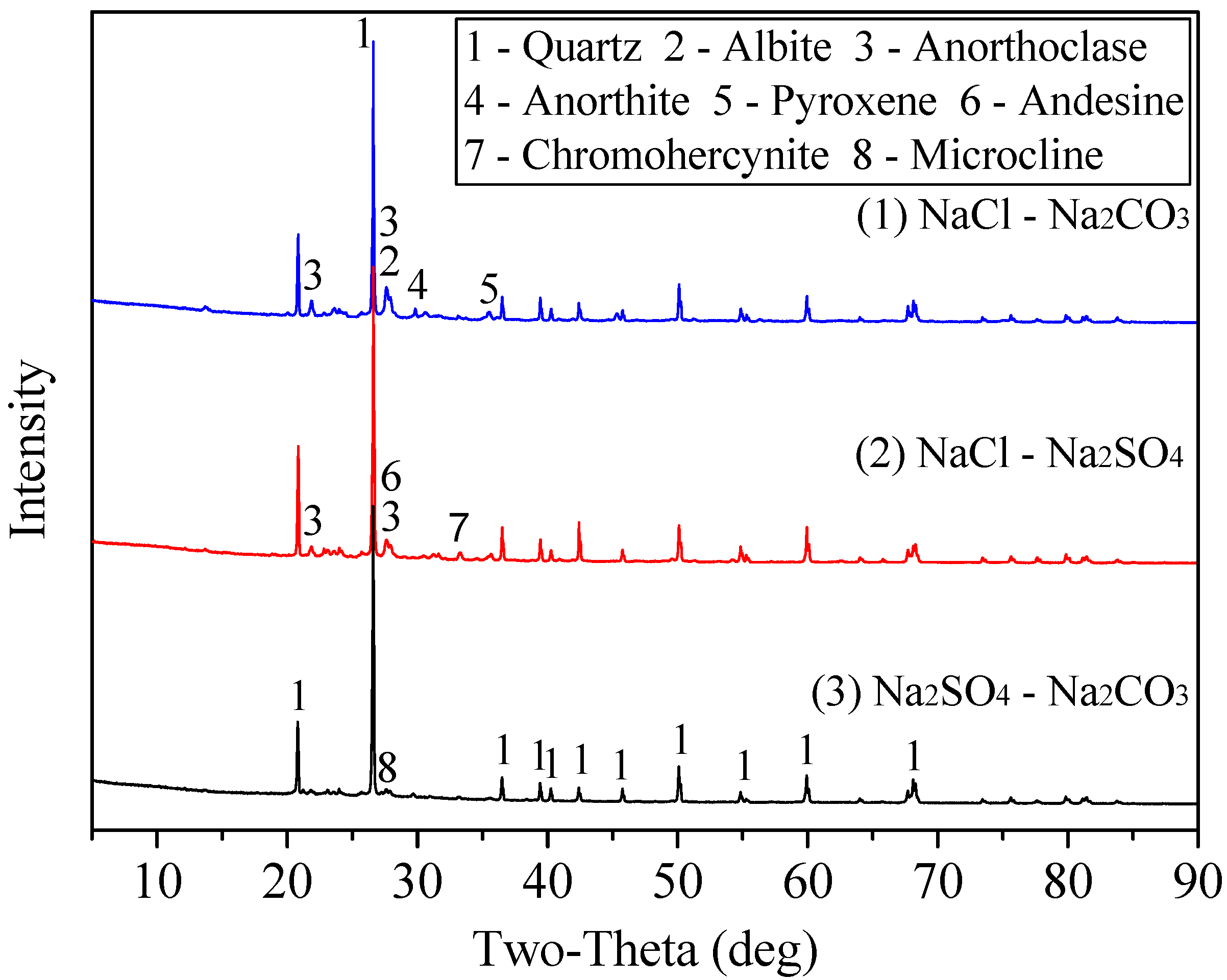
3.2.2. Influence of Composite Additive on the Mineral Phase Change in Roasting
3.3. Influence of a Composite Additive of Three Sodium Salts on the Oxidation Process of Vanadium
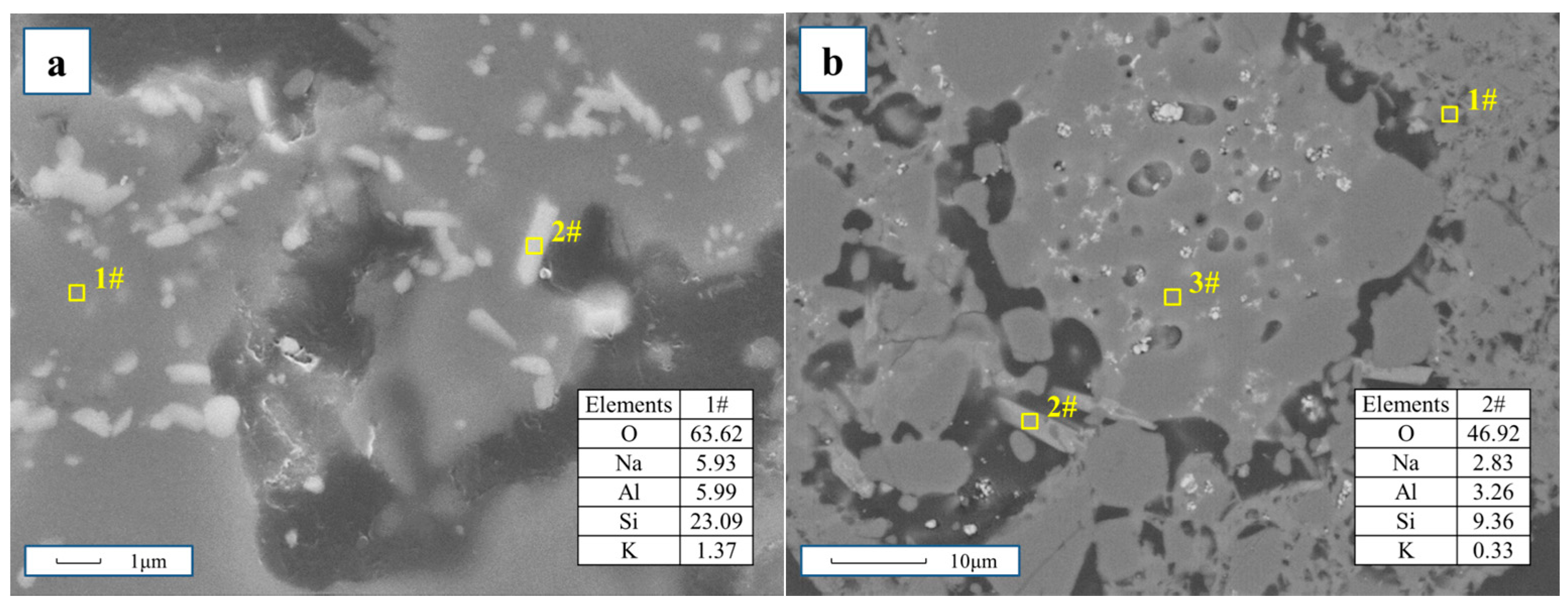
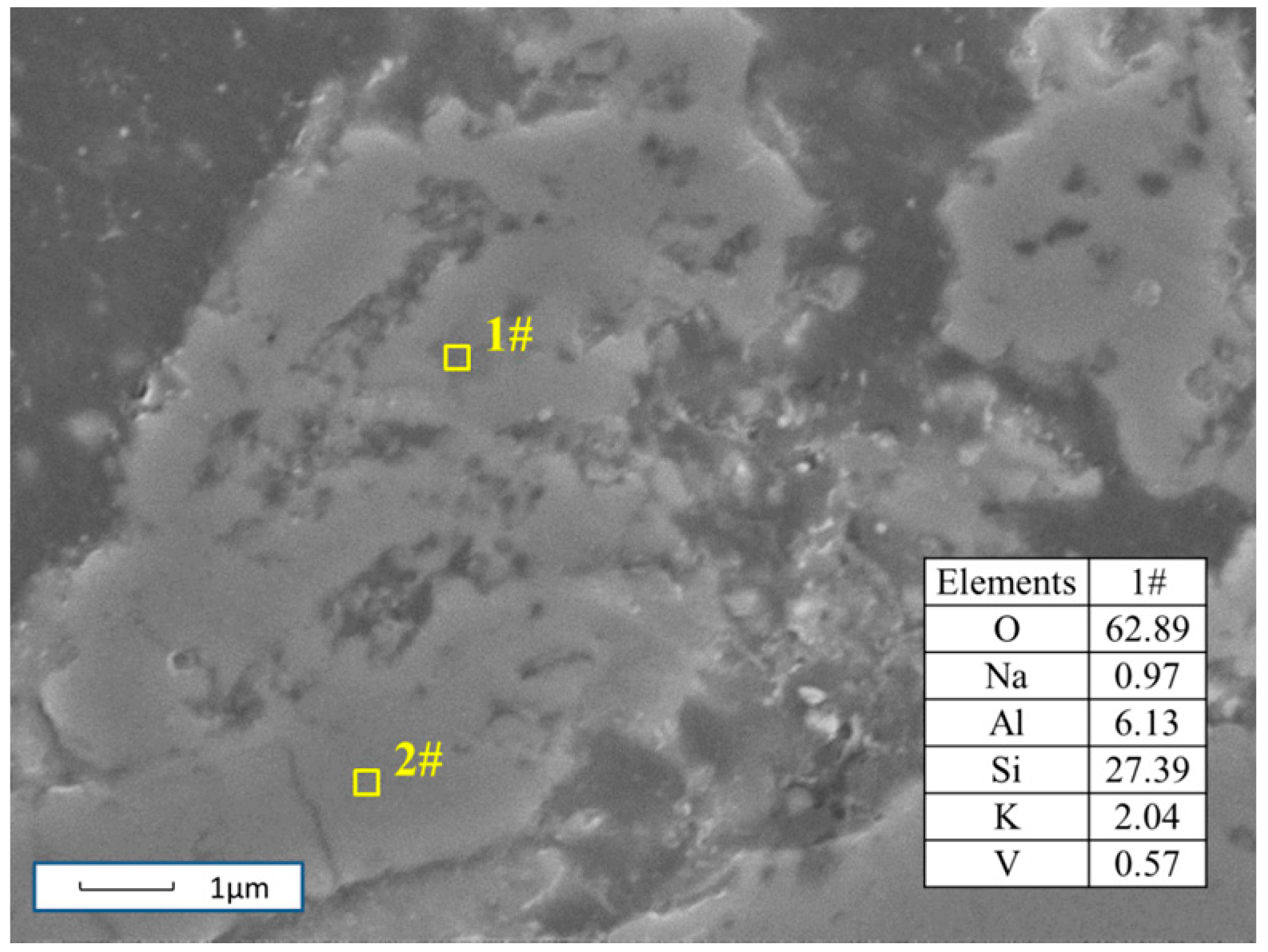
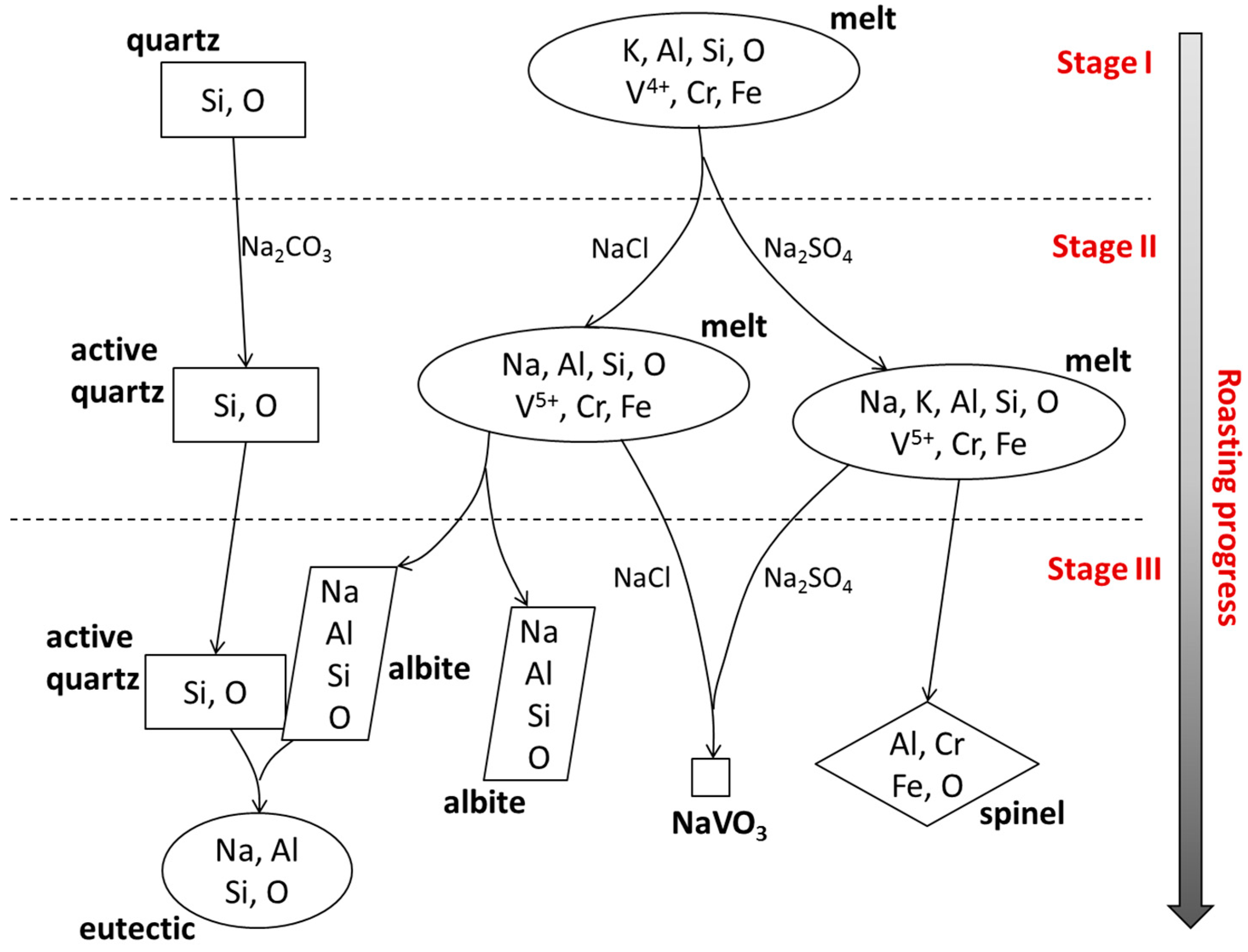
3.4. Reaction Process Between Composite Additive and Siliceous Shale during the Roasting Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ning, P.G.; Lin, X.; Wang, X.Y.; Cao, H.B. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- He, D.S.; Feng, Q.M.; Zhang, G.F.; Ou, L.M.; Lu, Y.P. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Zhang, H.F.; Cui, L.J.; Yu, J.; Xu, G.W. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor. Fuel 2015, 142, 180–188. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Bao, S.X.; Chen, T.J.; Han, J. Calculation of mineral phase and liquid phase formation temperature during roasting of vanadium-bearing stone coal using FactSage software. Int. J. Miner. Process. 2013, 124, 150–153. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Song, S.X.; Chen, T.J.; Bao, S.X. Behaviors of impurity elements Ca and Fe in vanadium-bearing stone coal during roasting and its control measure. Int. J. Miner. Process. 2016, 148, 100–104. [Google Scholar] [CrossRef]
- Shlewit, H.; Alibrahim, M. Extraction of sulfur and vanadium from petroleum coke by means of salt-roasting treatment. Fuel 2006, 85, 878–880. [Google Scholar] [CrossRef]
- Li, X.S.; Xie, B.; Wang, G.E.; Li, X.J. Oxidation process of low-grade vanadium slag in presence of Na2CO3. Trans. Nonferrous Metals Soc. China 2011, 21, 1860–1867. [Google Scholar] [CrossRef]
- Sadykhov, G.B. Oxidation of titanium-vanadium slags with the participation of Na2O and its effect on the behavior of vanadium. Russ. Metall. 2008, 6, 449–458. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Chen, T.J.; Zhang, Y.M.; Song, S.X. Improved extraction of vanadium from a Chinese vanadium-bearing stone coal using a modified roast-leach process. Asia Pac. J. Chem. Eng. 2010, 5, 778–784. [Google Scholar] [CrossRef]
- Bao, S.X.; Zhang, Y.M.; Han, J.; Yang, X.; Hu, Y.J. Determination of vanadium valency in roasted stone coal by separate dissolve-potentiometric titration method. MRS Proc. 2012, 1380, imrc2011-1380. [Google Scholar]
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Xiao, W.D. Mineralogy of stone coal from Shanglin of Guangxi and vanadium extraction with hydrometallurgical process. Nonferrous Metals 2007, 59, 85–90. [Google Scholar]
- Mirazimi, S.M.J.; Abbasalipour, Z.; Rashchi, F. Vanadium removal from LD converter slag using bacteria and fungi. J. Environ. Manag. 2015, 153, 144–151. [Google Scholar] [CrossRef]
- Nazari, E.; Rashchi, F.; Saba, M.; Mirazimi, S.M.J. Simultaneous recovery of vanadium and nickel from power plant fly-ash: Optimization of parameters using response surface methodology. Waste Manag. 2014, 34, 2687–2696. [Google Scholar] [CrossRef]
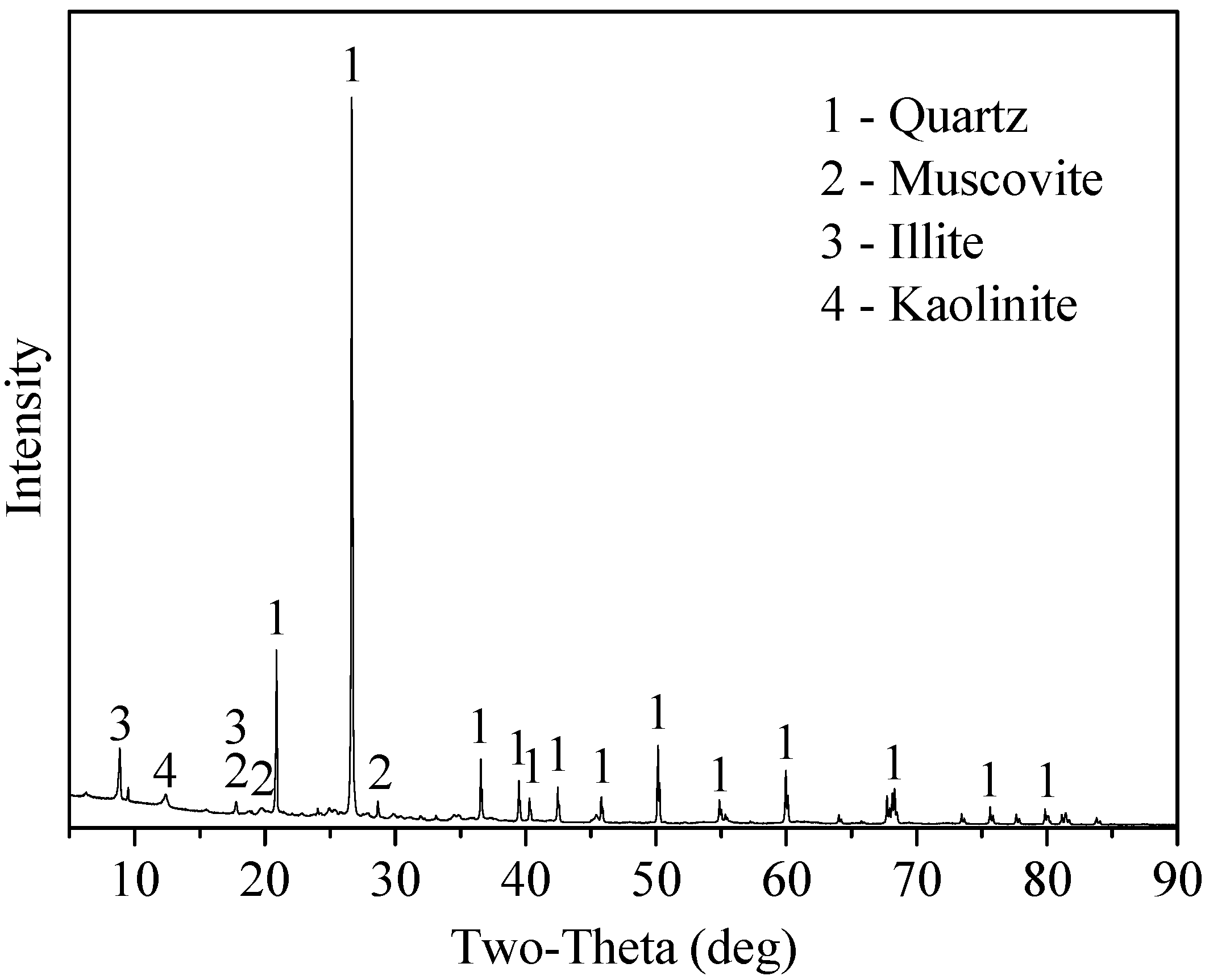



| Chemical Multi-Elemental Analysis | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | V2O5 | P | S |
| 0.13 | 1.36 | 9.63 | 79.16 | 1.24 | 0.69 | 1.72 | 1.37 | 0.28 | |
| As | Ti | Cr | Fe | Zn | Pb | ||||
| 1.32 | 0.26 | 0.25 | 2.18 | 0.06 | 0.01 |
| Vanadium Valence State | V(III) | V(IV) | V(V) |
|---|---|---|---|
| Content | 86.83 | -- | 13.17 |
| Factor | Name | Low Level Values (%) | High Level Values (%) |
|---|---|---|---|
| A | NaCl | 0 | 10 |
| B | Na2SO4 | 0 | 10 |
| C | Na2CO3 | 0 | 10 |
| Source | Std. Dev. | R-Squared | Adjusted R-Squared | Predicted R-Squared | PRESS | |
|---|---|---|---|---|---|---|
| Linear | 14.48 | 0.4340 | 0.2722 | −2.2206 | 8348.68 | |
| Quadratic | 5.35 | 0.9558 | 0.9005 | −0.9683 | 5102.40 | Suggested |
| Special Cubic | 6.17 | 0.9559 | 0.8677 | −2.1540 | 8175.88 | |
| Cubic | 6.92 | 0.9815 | 0.8338 | −221.7276 | 5.774 × 105 |
| Factor | Coefficient Estimate | df (Degree of Freedom) | Standard Error | 95% CI Low | 95% CI High | VIF (Variance Inflation Factor) |
|---|---|---|---|---|---|---|
| A-NaCl | 59.71 | 1 | 5.31 | 44.97 | 74.44 | 1.88 |
| B-Na2SO4 | 43.84 | 1 | 5.31 | 29.10 | 58.57 | 1.88 |
| C-Na2CO3 | 14.98 | 1 | 5.31 | 0.24 | 29.71 | 1.88 |
| AB | 40.29 | 1 | 43.59 | −80.74 | 161.32 | 5.38 |
| AC | 121.70 | 1 | 43.59 | 0.67 | 242.74 | 5.38 |
| BC | 82.39 | 1 | 43.59 | −38.64 | 203.43 | 5.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Feng, Y.; Li, H.; Du, Z. Optimization Mechanism of Additive of Composite Sodium Salts on Vanadium Oxidation of Siliceous Shale. Minerals 2017, 7, 103. https://doi.org/10.3390/min7060103
Yang X, Feng Y, Li H, Du Z. Optimization Mechanism of Additive of Composite Sodium Salts on Vanadium Oxidation of Siliceous Shale. Minerals. 2017; 7(6):103. https://doi.org/10.3390/min7060103
Chicago/Turabian StyleYang, Xinlong, Yali Feng, Haoran Li, and Zhuwei Du. 2017. "Optimization Mechanism of Additive of Composite Sodium Salts on Vanadium Oxidation of Siliceous Shale" Minerals 7, no. 6: 103. https://doi.org/10.3390/min7060103




