Coal-Based Reduction and Magnetic Separation Behavior of Low-Grade Vanadium-Titanium Magnetite Pellets
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Experimental Methods
3. Results and Discussion
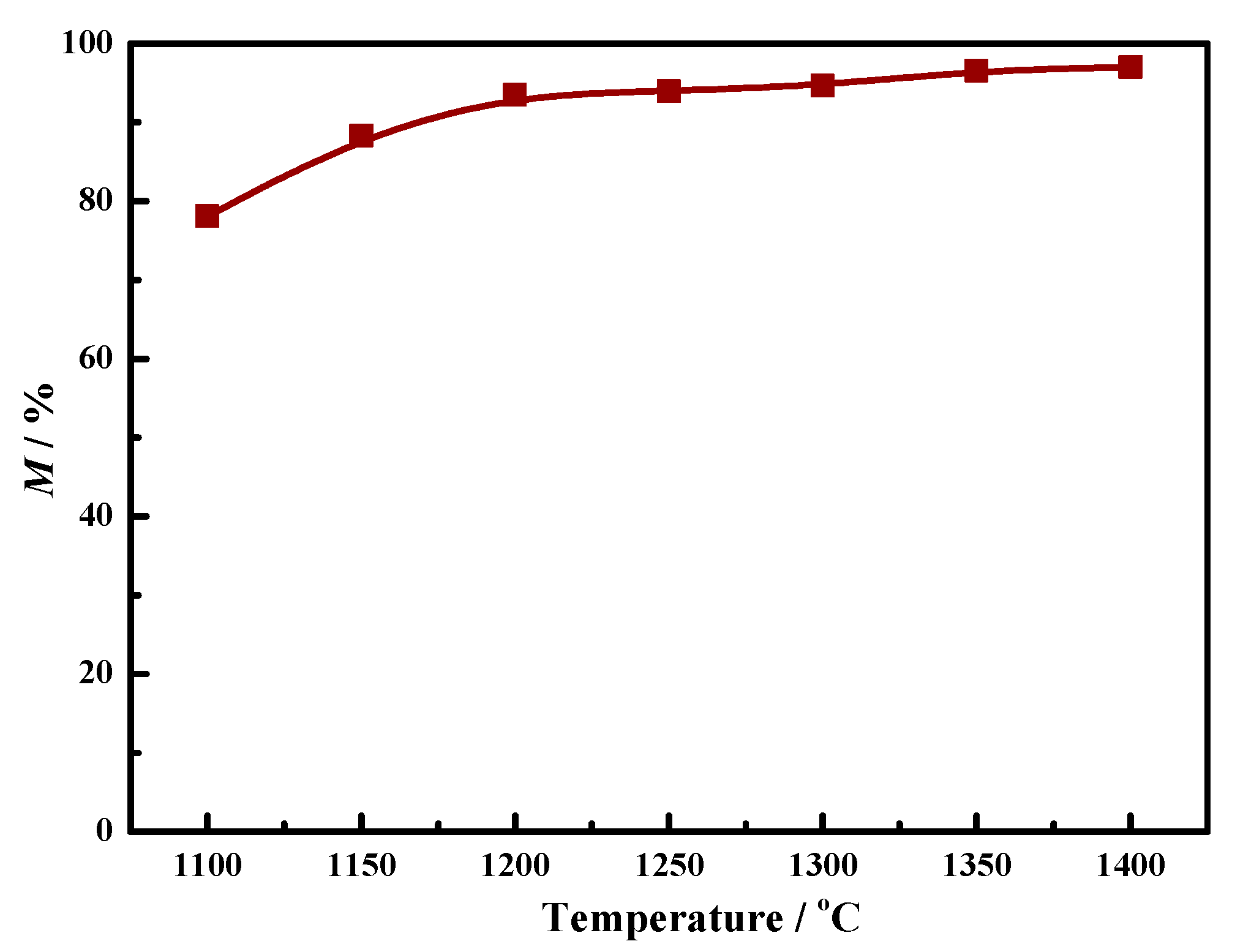
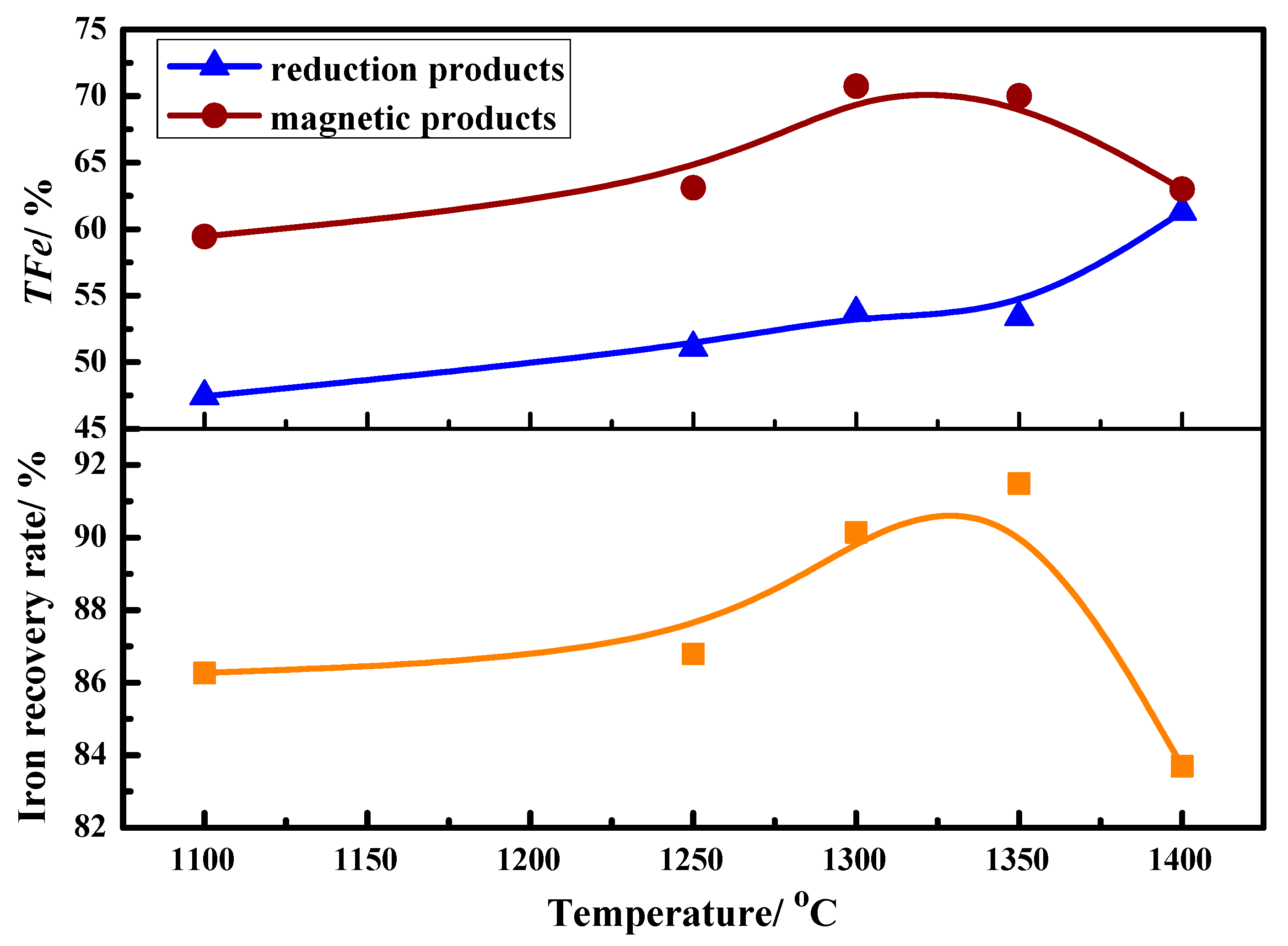
3.1. Metallization Degree
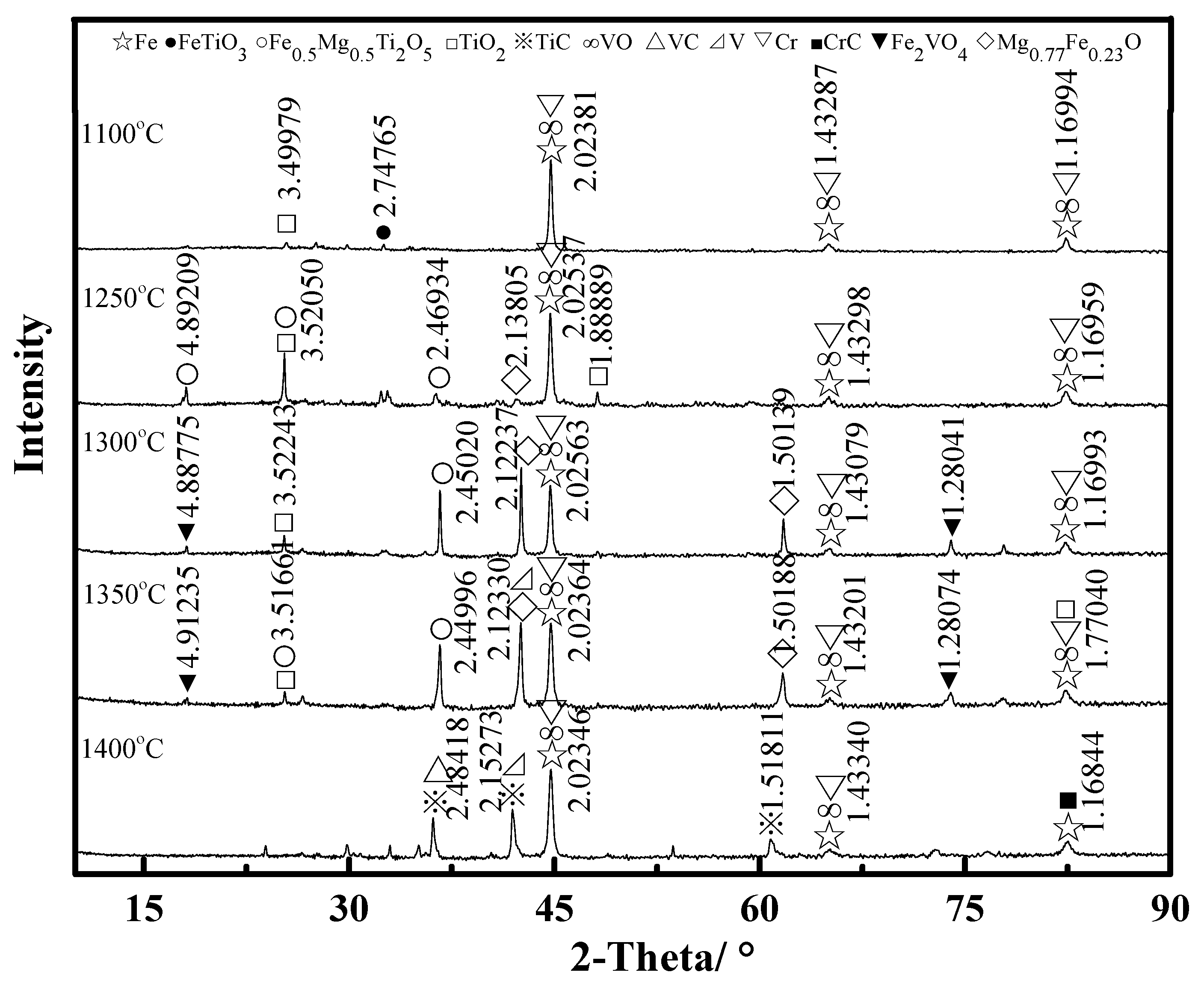
3.2. Phase Composition Transformation
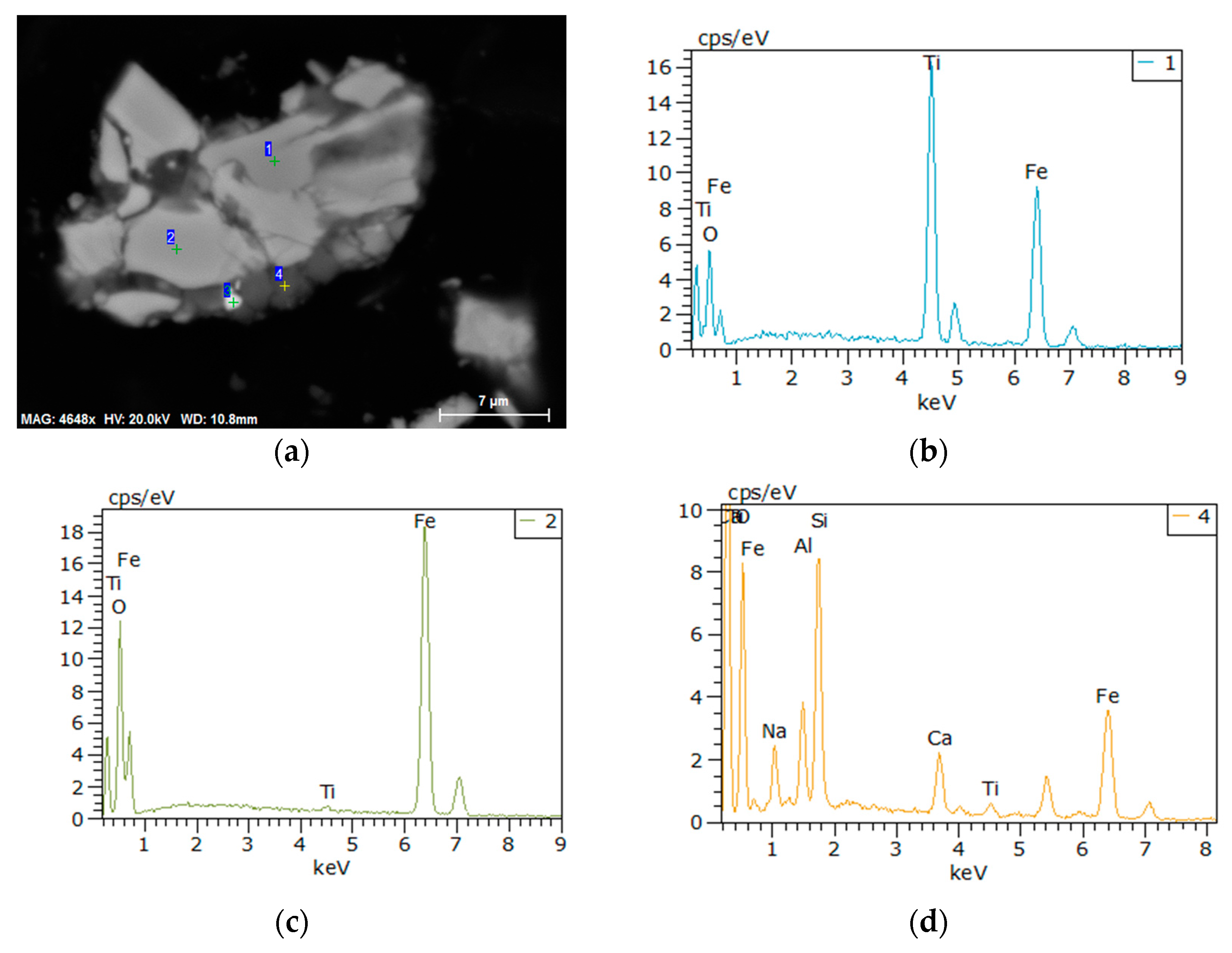
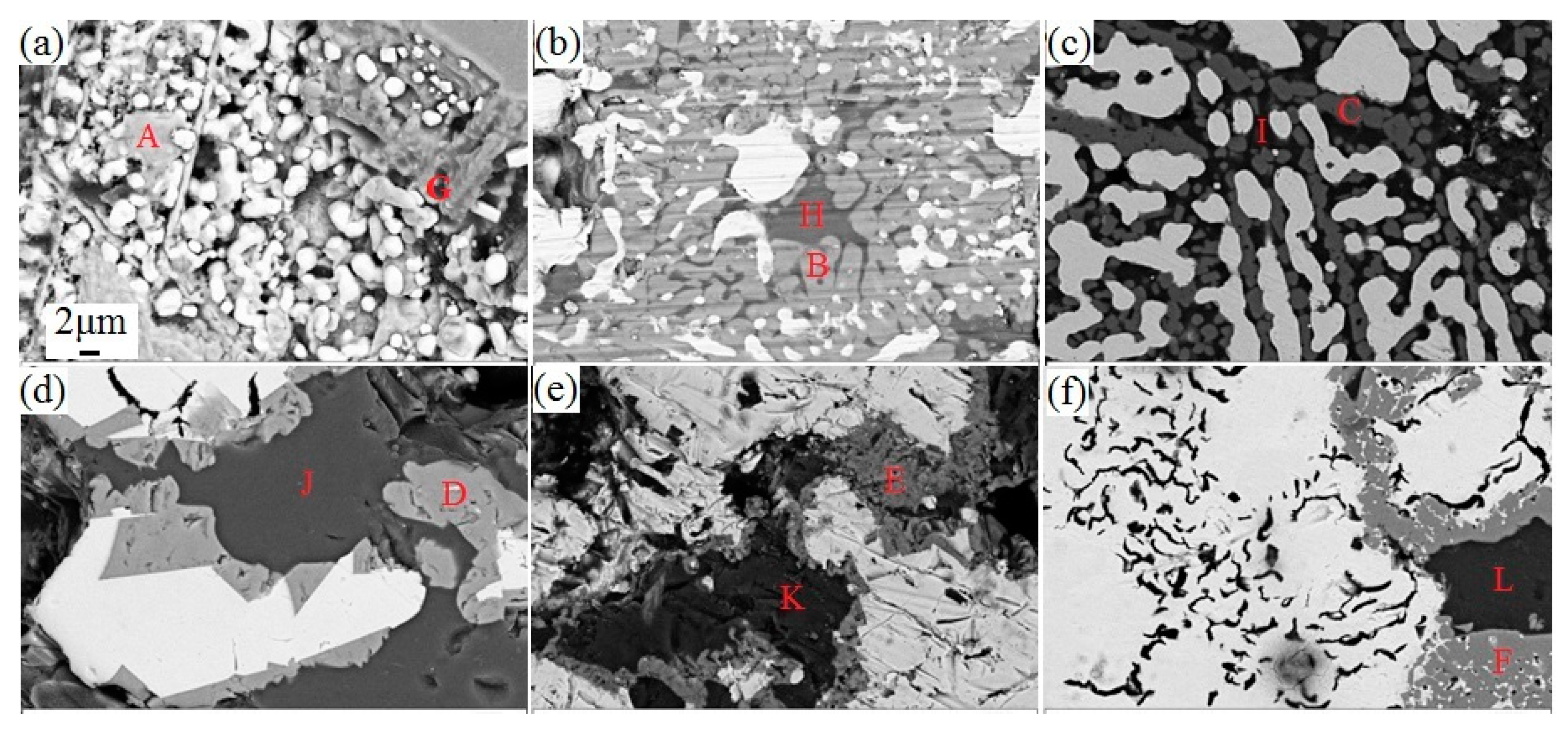
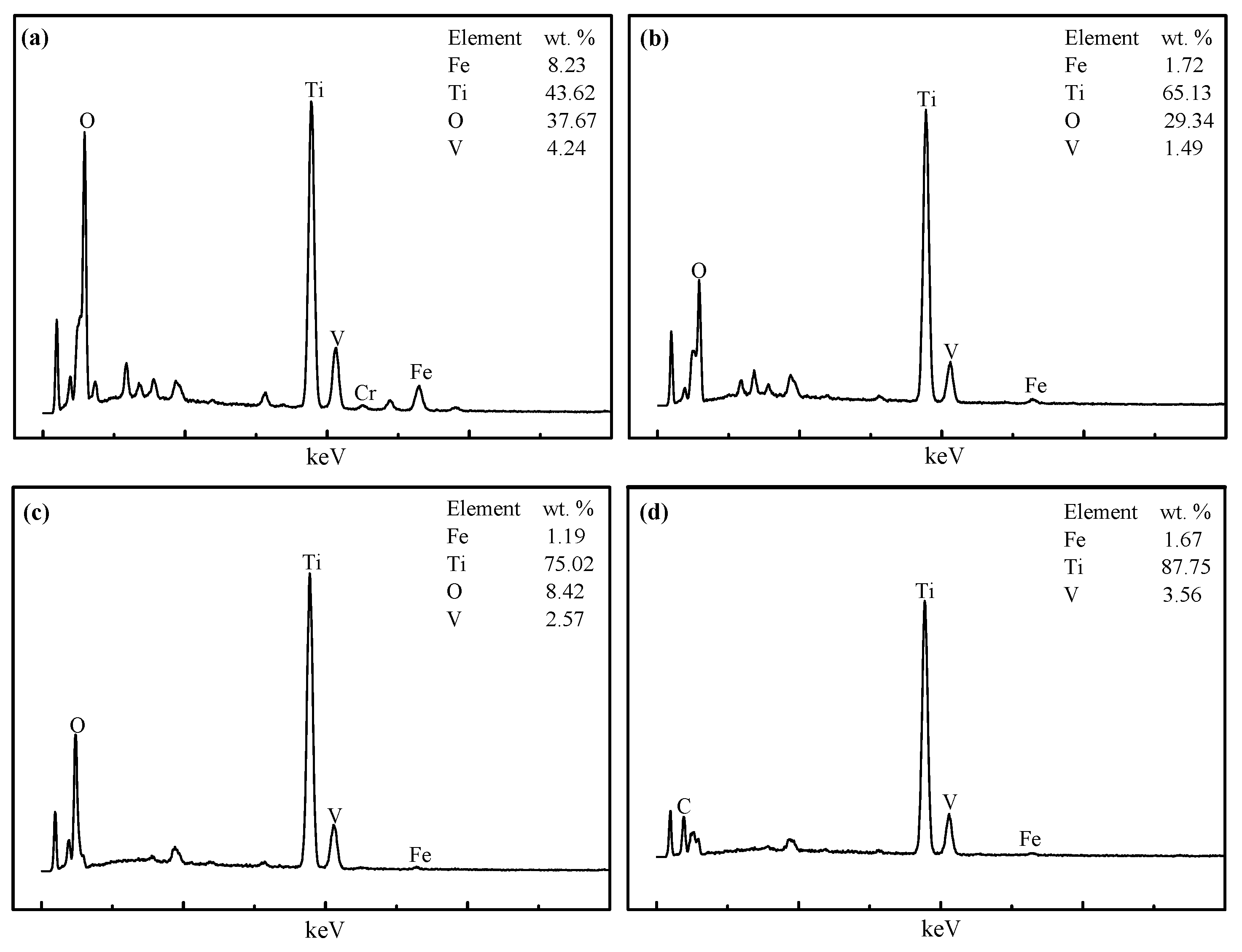
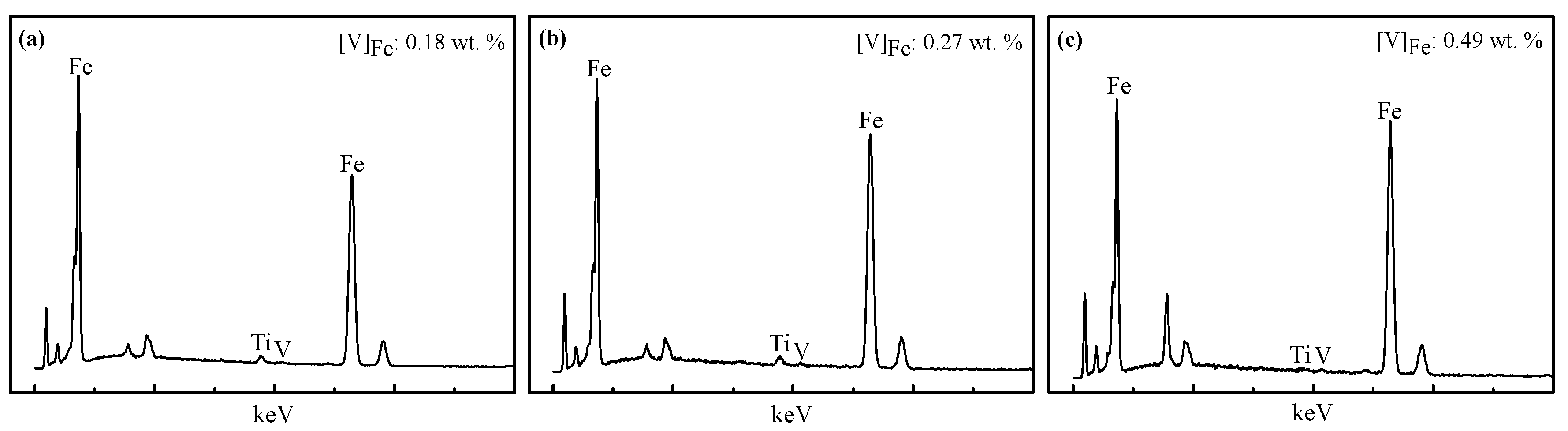
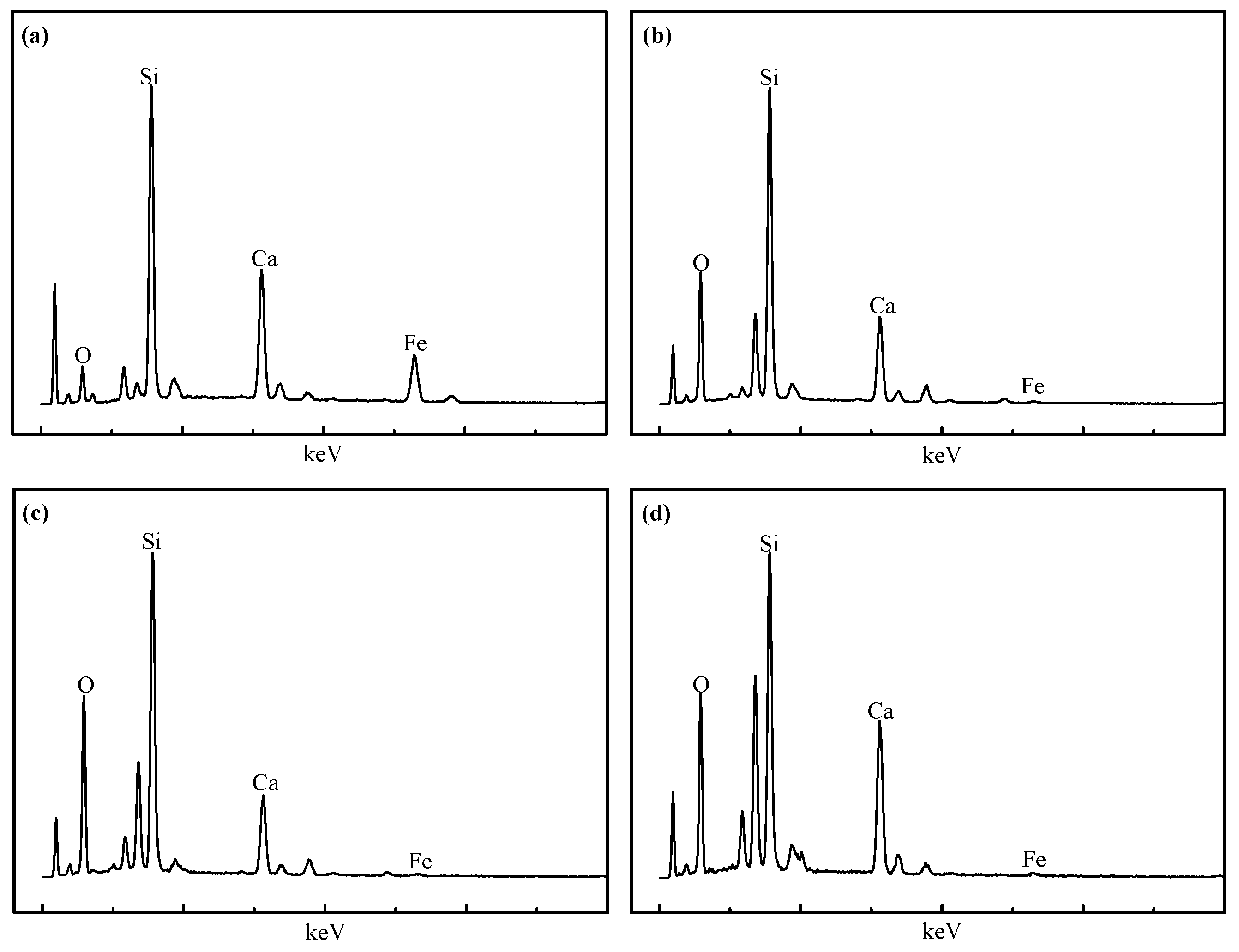
3.3. Microscopic Examination
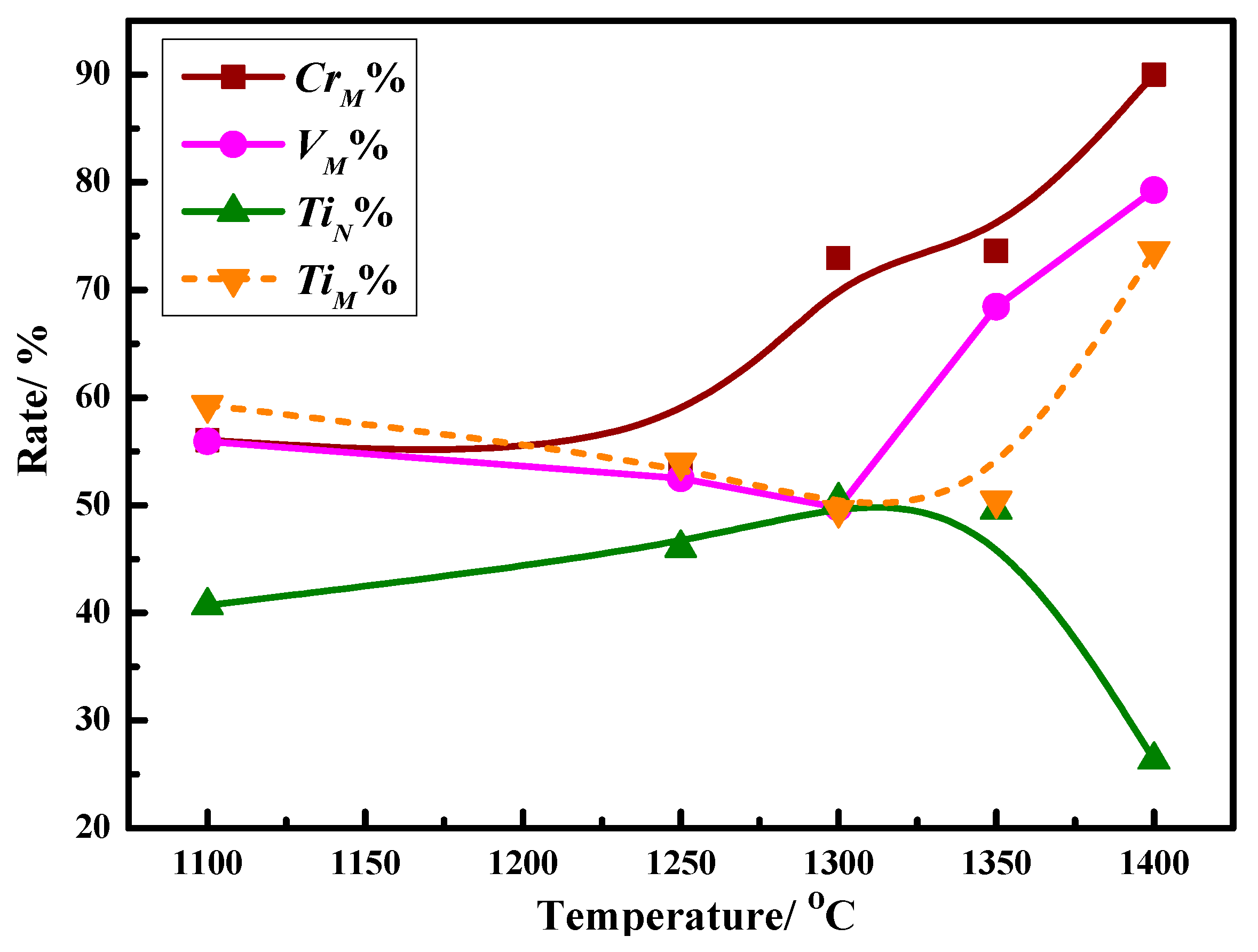
3.4. Magnetic Separation Behavior
4. Conclusions
- Different kinds of metallized pellets were obtained after the coal-based reduction of LGVTMP with appropriate C/O. It is found that the metallization degree increased obviously with increasing the temperature from 1100 °C to 1400 °C.
- From the characterization and analysis of XRF, ICP-AES, XRD, SEM, and EDS, it is observed that the amounts of metallic iron particles obviously increased and the accumulation and growing tendency were gradually facilitated with the increase in the temperature from 1100 °C to 1400 °C. It is also found that the titanium oxides were gradually reduced and separated from titanium-ferrum oxides during reduction. In addition, silicate phases, especially calcium silicate phases, transformed from calcium ferrite at 1100 °C, were observed, and gradually aggregated with the increase in the temperature from 1200 °C to 1350 °C, but some silicate phases infiltrated into metallic iron at 1400 °C, as it appears that carbides, especially TiC, could probably contribute to the sintering phenomenon becoming serious.
- From the comprehensive study, it was obtained that the transformation behavior for valuable elements was as follows: Fe2VO4 → VO → V → VC; FeTiO3 (→ FeTi2O5) → TiO2 → TiC; FeCr2O4 → Cr → CrC; FeTiO3 (→ FeTi2O5) → Fe0.5Mg0.5Ti2O5; (Fe3O4/FeTiO3 →) FeO →Mg0.77Fe0.23O.
- Through the magnetic separation of reduced products, it is demonstrated that the separation of Cr, V, Ti, and non-magnetic phases can be preliminarily realized.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, H.G. Principle of Smelting Vanadium-Titanium Magnetite in the Blast Furnace, 1st ed.; Science Press: Beijing, China, 1996; pp. 1–20. [Google Scholar]
- Wang, X.Q. Smelting of Vanadium-Titanium Magnetite in the Blast Furnace, 1st ed.; Metallurgical Industry Press: Beijing, China, 1994; p. 1. [Google Scholar]
- Li, H.M.; Wang, R.J.; Xiao, K.Y.; Zhang, X.H.; Liu, Y.L.; Sun, L. Characteristics and current utilization status of ultra-low-grade magnetite resource, and suggestion on its exploration and development. Geol. Bull. China 2009, 28, 85–90. [Google Scholar]
- Cheng, G.J.; Xue, X.X.; Jiang, T.; Duan, P.N. Effect of TiO2 on the crushing strength and smelting mechanism of high chromium vanadium-titanium magnetite pellets. Metall. Mater. Trans. B 2016, 47, 1713–1726. [Google Scholar] [CrossRef]
- Cheng, G.J.; Liu, J.X.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Non-isothermal reduction kinetics and mechanism of high chromium vanadium-titanium magnetite pellets. Ironmak. Steelmak. 2015, 42, 17–26. [Google Scholar] [CrossRef]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Reduction process of pellet containing high chromic vanadium–titanium magnetite in cohesive zone. Steel Res. Int. 2015, 86, 808–816. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.T.; Jiang, T.; Xue, X.X. Influence of Basicity on High-Chromium Vanadium-Titanium Magnetite Sinter Properties, Productivity, and Mineralogy. JOM 2015, 67, 1203–1213. [Google Scholar] [CrossRef]
- Sun, H.Y.; Dong, X.J.; She, X.F.; Xue, Q.G.; Wang, J.S. Solid state reduction of titanomagnetite concentrate by graphite. ISIJ Int. 2013, 53, 564–569. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, M.S. Metalizing reduction and magnetic separation of vanadium titano-magnetite based on hot briquetting. Int. J. Miner. Metall. Mater. 2014, 21, 225–233. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, S.; Guo, Y.F.; Chen, F.; Zheng, F.Q. Effects of basicity and MgO in slag on the behaviors of smelting vanadium titanomagnetite in the direct reduction-electric furnace process. Metals 2016, 6, 107–121. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Cui, Q.; Xu, C.; Kou, J. Effect of coal type on the reduction and magnetic separation of a high-phosphorus oolitic hematite ore. ISIJ Int. 2015, 55, 536–543. [Google Scholar] [CrossRef]
- Kasai, E.; Mae, K.; Saito, F. Effect of mixed-grinding on reduction process of carbonaceous material and iron oxide composite. ISIJ Int. 1995, 35, 1444–1451. [Google Scholar] [CrossRef]
- Nascimento, R.C.; Mourao, M.B.; Capocchi, J.D.T. Microstructures of Self-reducing Pellets Bearing Iron Ore and Carbon. ISIJ Int. 1997, 37, 1050–1056. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Liu, Z.; Kou, J.; Xu, C. Effects of particle sizes of iron ore and coal on the strength and reduction of high phosphorus oolitic hematite-coal composite briquettes. ISIJ Int. 2014, 54, 56–62. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Ren, D.Z. Distribution behavior of phosphorus in the coal-based reduction of high-phosphorus-content oolitic iron ore. Int. J. Miner. Metall. Mater. 2014, 21, 331–338. [Google Scholar] [CrossRef]
- Gao, P.; Li, G.F.; Han, Y.X.; Sun, Y.S. Reaction behavior of phosphorus in coal-based reduction of an oolitic hematite ore and pre-dephosphorization of reduced iron. Metals 2016, 6, 82. [Google Scholar] [CrossRef]
- Inaba, S. Overview of new direct reduced iron technology. Tetsu-to-Hagane 2001, 87, 221–227. [Google Scholar] [CrossRef]
- Zhu, D.; Mendes, V.; Chun, T.; Pan, J.; Li, Q.; Li, J.; Qiu, G. Direct reduction behaviors of composite binder magnetite pellets in coal-based grate-rotary kiln process. ISIJ Int. 2011, 51, 214–219. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.; Huang, T.; Zhang, G.; Guo, H.; Shiau, G.H.; Shen, F.M. Effects of reducing time on metallization degree of carbothermic reduction of tall pellets bed. ISIJ Int. 2016, 56, 88–93. [Google Scholar] [CrossRef]
- Jung, S.M. Effects of CaO/CaCO3 on the carbothermic reduction of titanomagnetite ores. Metall. Mater. Trans. B 2015, 46, 1162–1174. [Google Scholar] [CrossRef]
- Jung, S.M. Effects of the content and particle size of char in the composite on the carbothermic reduction of titanomagnetite at 1100 °C. ISIJ Int. 2014, 54, 2933–2935. [Google Scholar] [CrossRef]
- Jung, S.M. Evaluation of reduction progress of raw titanomagnetite and char composite by thermogravimetry. ISIJ Int. 2013, 53, 1487–1489. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.W.; Bai, C.G.; Lun, Z.G.; Qiu, G.B. Carbothermic reduction of titanomagnetite concentrates with ferrosilicon addition. ISIJ Int. 2013, 53, 557–563. [Google Scholar] [CrossRef]
- Yu, S. Research on the Solid-Phase Enhancement Reduction and Magnetic Separation of Vanadium Titanomagnetite. Master’s Thesis, Northeastern University, Shenyang, China, 2013. [Google Scholar]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef]

| TFe | FeO | V | TiO2 | Cr2O3 | CaO | SiO2 | MgO | Al2O3 | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| 43.75 | 18.4 | 0.91 | 21.54 | 0.13 | 3.52 | 7.00 | 0.70 | 1.83 | 0.002 | 0.022 |
| Fixed Carbon | Ash | Volatile Matter | Sulfur (S) |
|---|---|---|---|
| 64.23 | 7.06 | 22.85 | 0.41 |
| Item | TFe | MFe | V | TiO2 | Cr2O3 |
|---|---|---|---|---|---|
| Reduced Products | 53.38 | 51.56 | 1.06 | 24.62 | 0.18 |
| Magnetic Products | 70.01 | 62.59 | 1.04 | 19.67 | 0.19 |
| Non-Magnetic Products | - | - | 1.04 | 34.32 | 0.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Gao, Z.; Lv, M.; Yang, H.; Xue, X. Coal-Based Reduction and Magnetic Separation Behavior of Low-Grade Vanadium-Titanium Magnetite Pellets. Minerals 2017, 7, 86. https://doi.org/10.3390/min7060086
Cheng G, Gao Z, Lv M, Yang H, Xue X. Coal-Based Reduction and Magnetic Separation Behavior of Low-Grade Vanadium-Titanium Magnetite Pellets. Minerals. 2017; 7(6):86. https://doi.org/10.3390/min7060086
Chicago/Turabian StyleCheng, Gongjin, Zixian Gao, Mengyang Lv, He Yang, and Xiangxin Xue. 2017. "Coal-Based Reduction and Magnetic Separation Behavior of Low-Grade Vanadium-Titanium Magnetite Pellets" Minerals 7, no. 6: 86. https://doi.org/10.3390/min7060086





