Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Medium
2.2. Isolation, Identification and Micromorphology of C. israelensis LD532 Bacterium
2.3. Growth of C. israelensis LD532 Bacteria at Different NaCl Concentrations and pH Values
2.4. Growth and pH Curves of LD532 Bacteria at Different Mg/Ca Molar Ratios
2.5. CA Activity of C. israelensis LD532 Bacteria in the Liquid Culture Medium
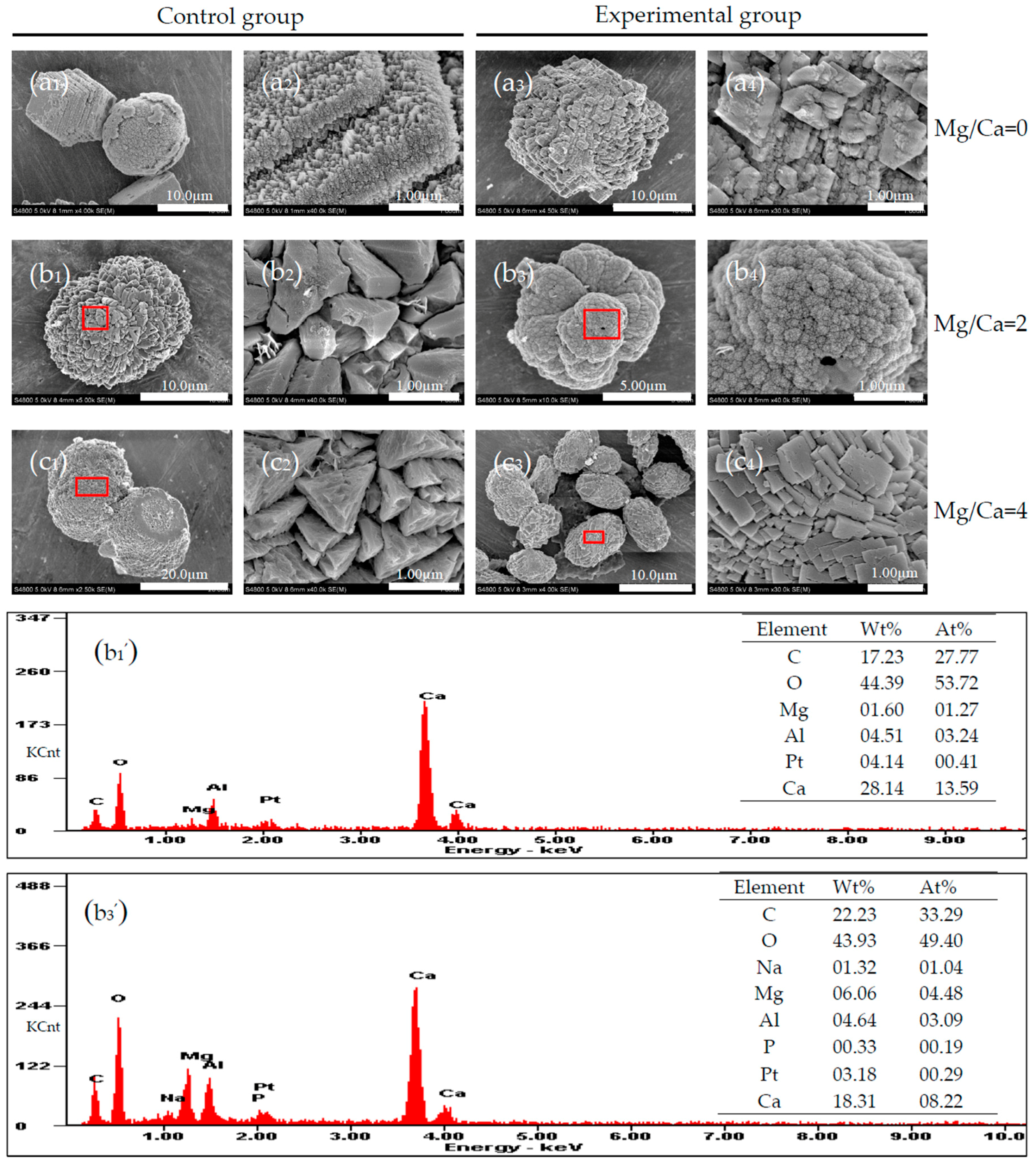
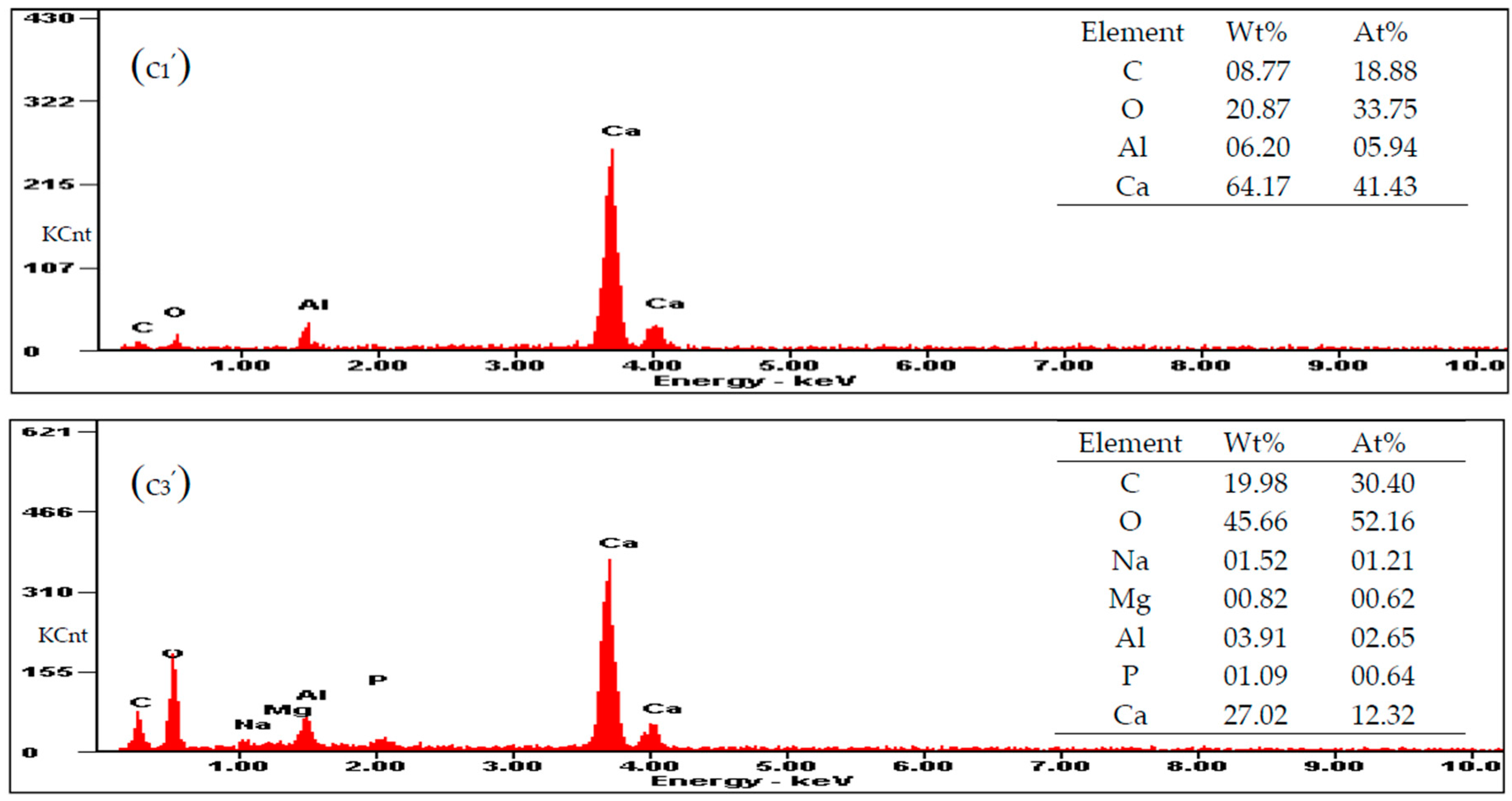
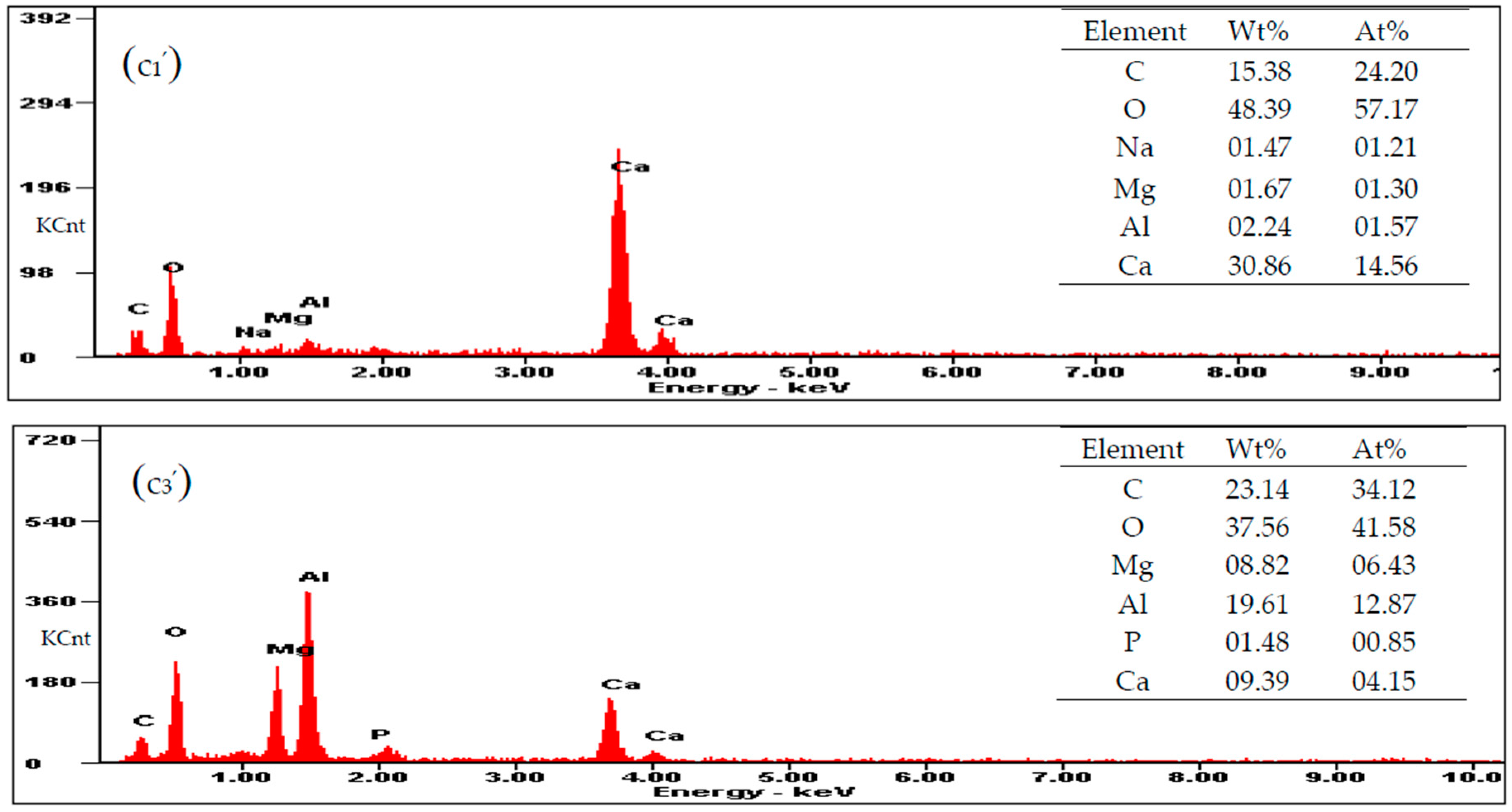
2.6. Characterization of Carbonates Induced by C. israelensis LD532 Bacteria
2.7. Minerals on the Surfaces of C. israelensis LD532 Bacteria
2.8. Analysis of Ultra-Thin Slices of C. israelensis LD532 Bacteria
3. Results
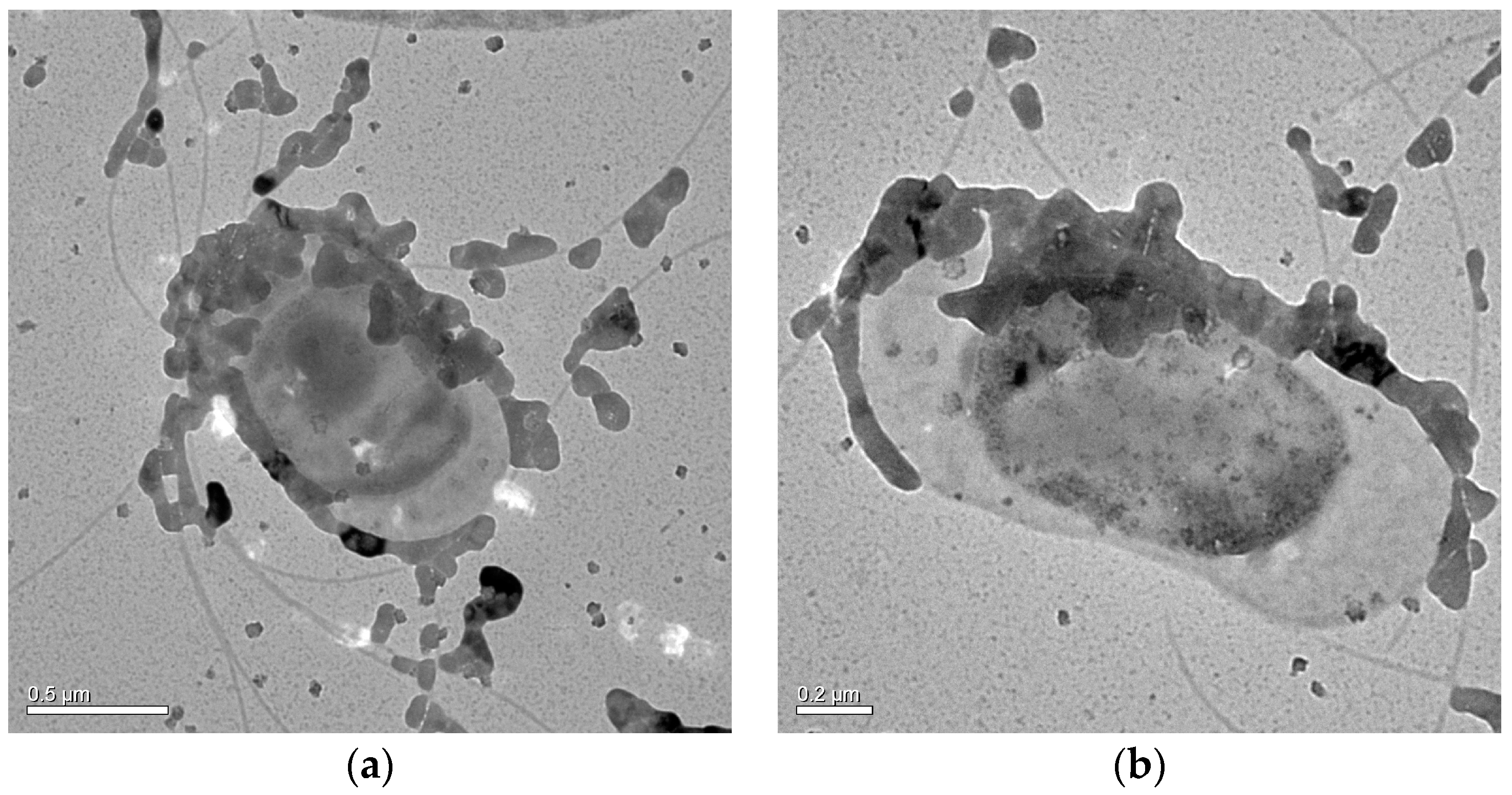
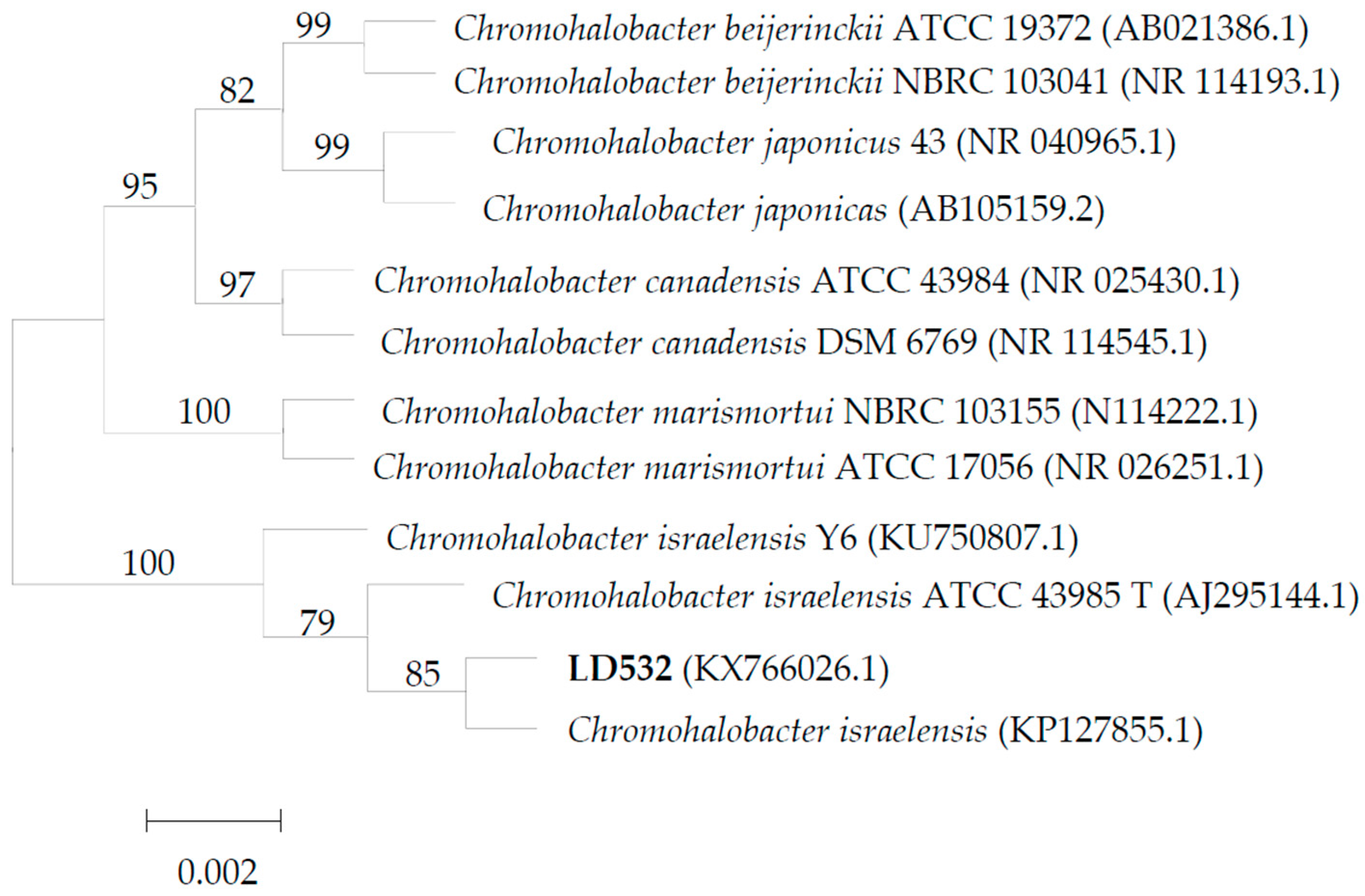
3.1. Identification of C. israelensis LD532 Bacteria
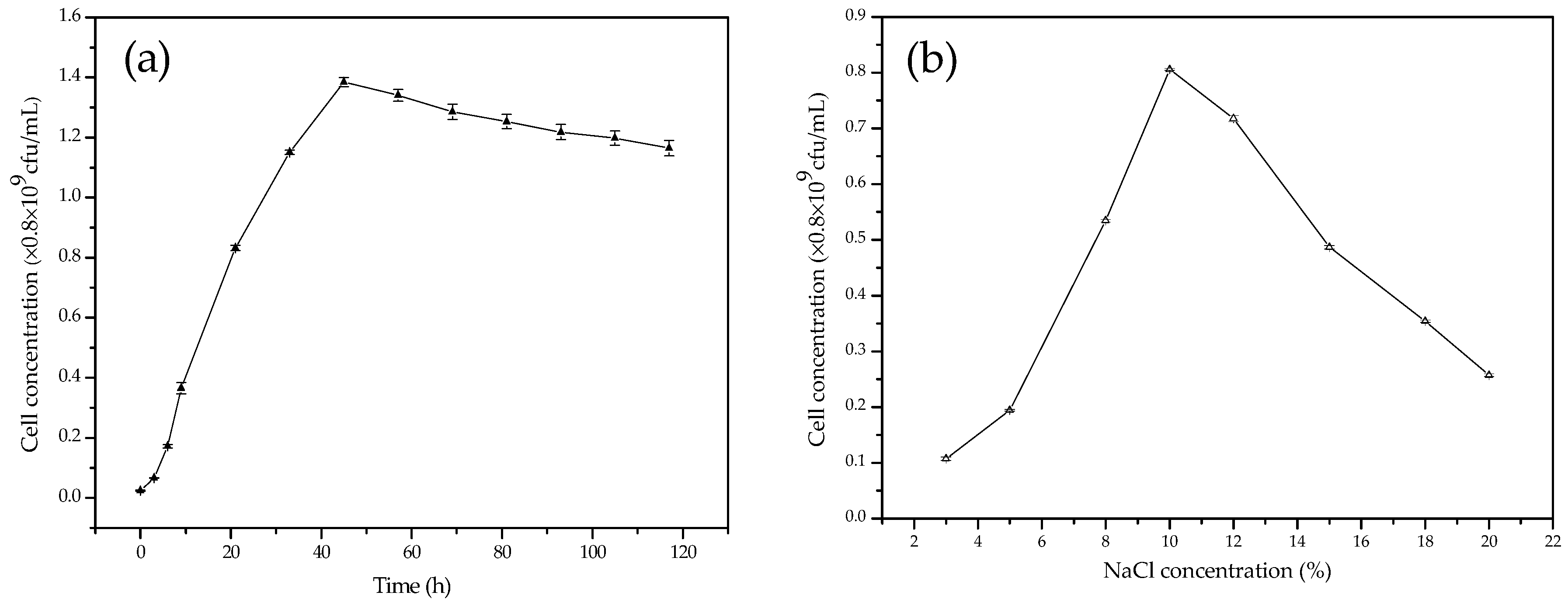
3.2. The Growth Curves of C. israelensis LD532 Bacteria in Liquid Culture Medium at Different Salt Concentrations and pH Values
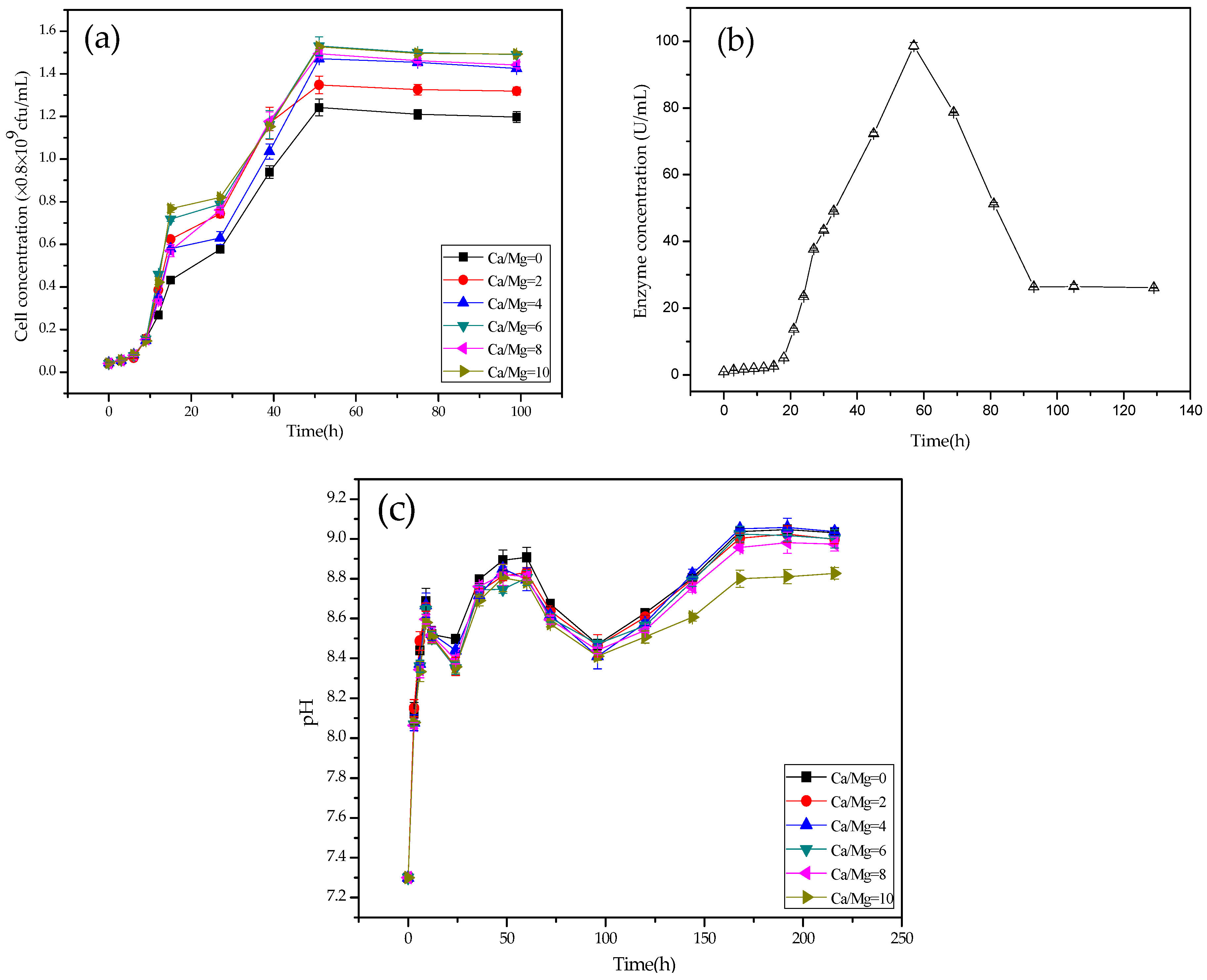
3.3. Growth, pH, and CA Activity Curves of LD532 Bacteria
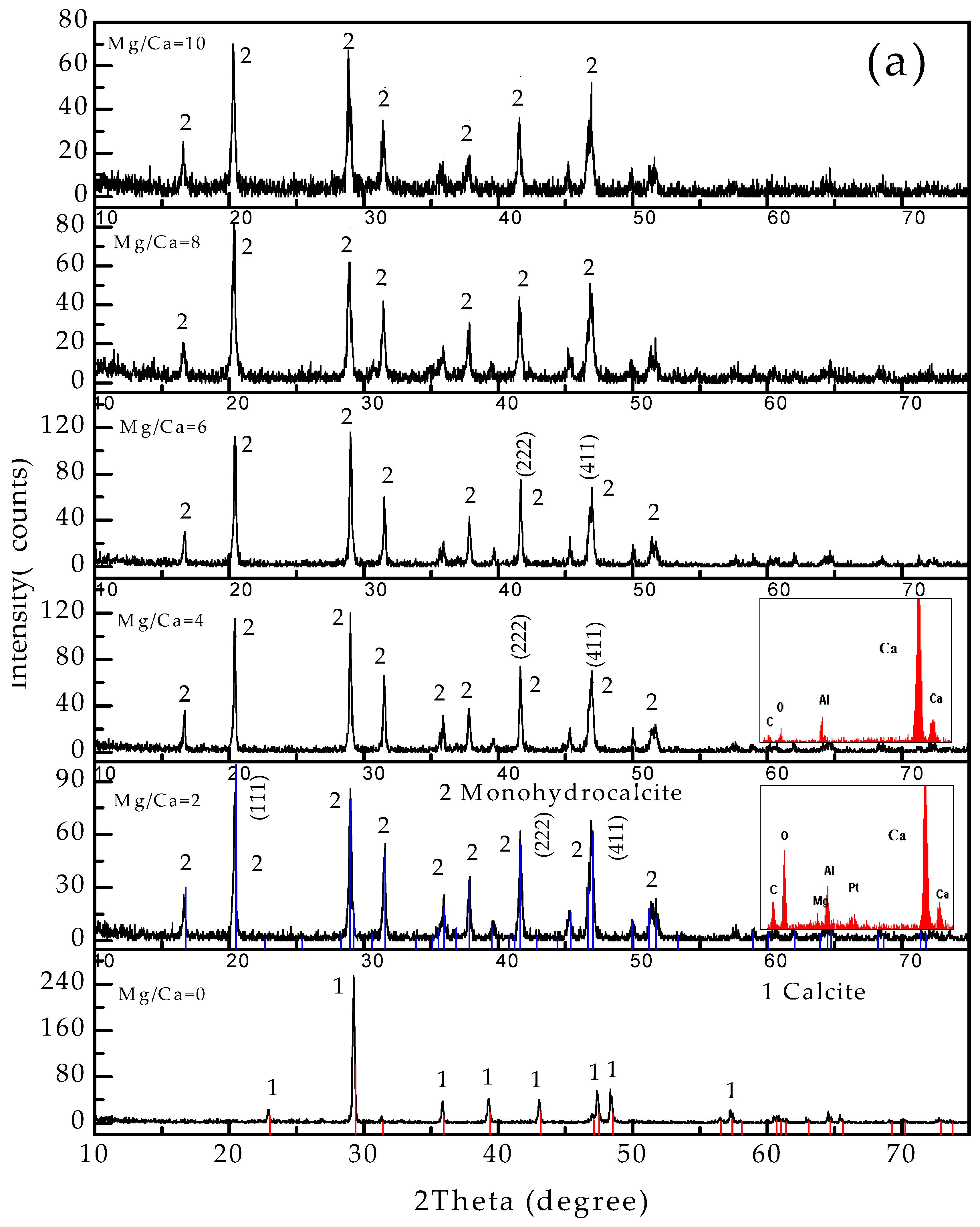
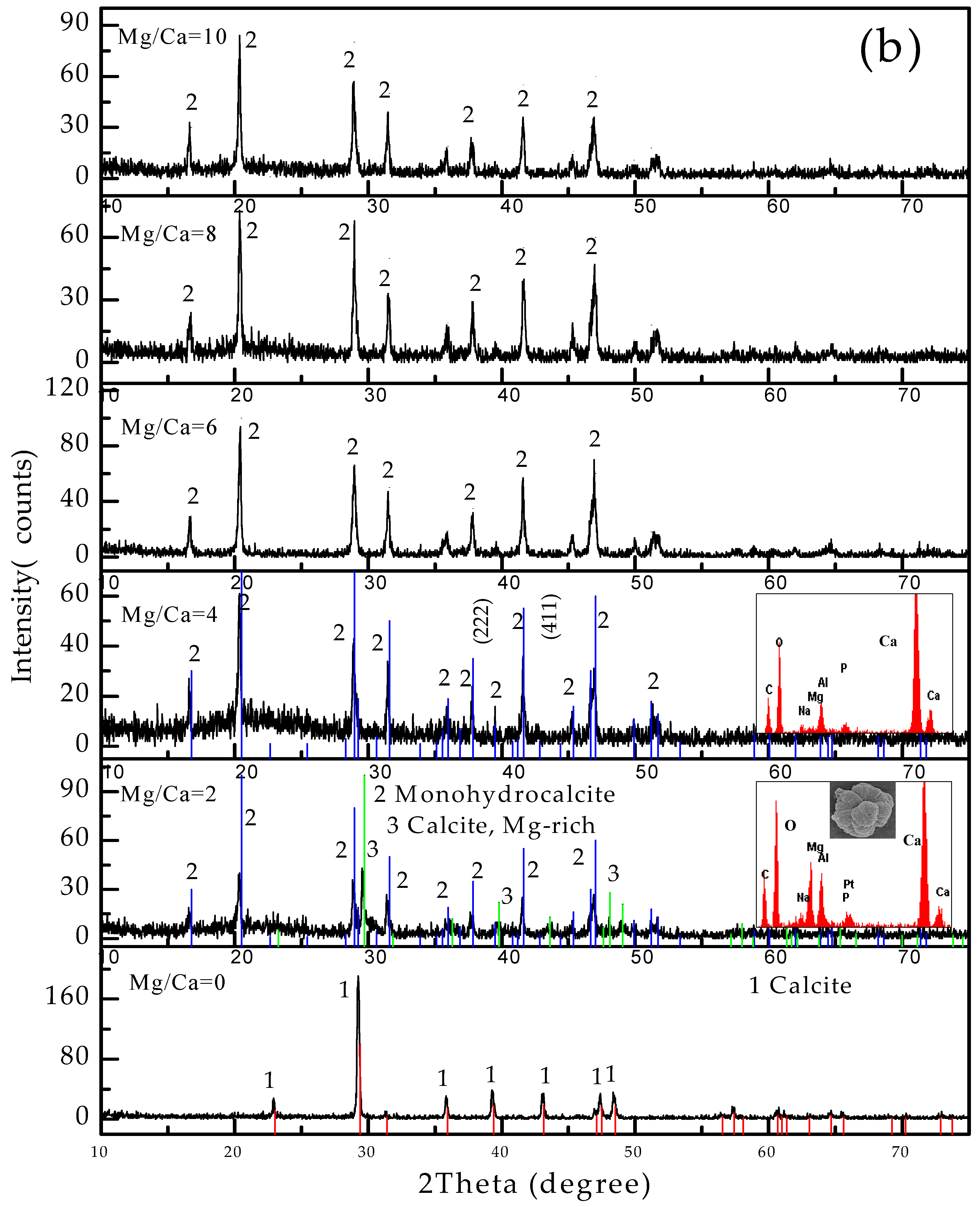
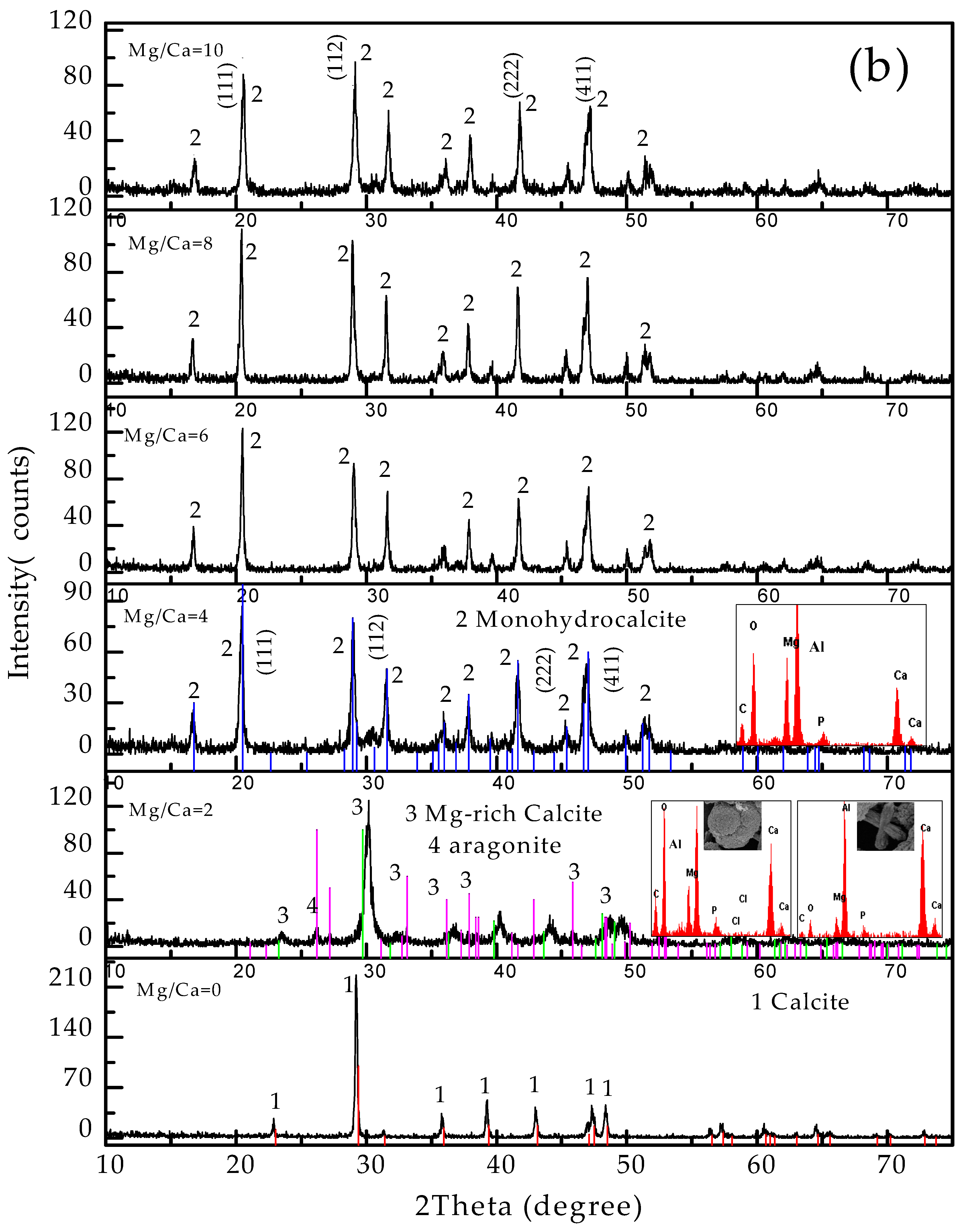
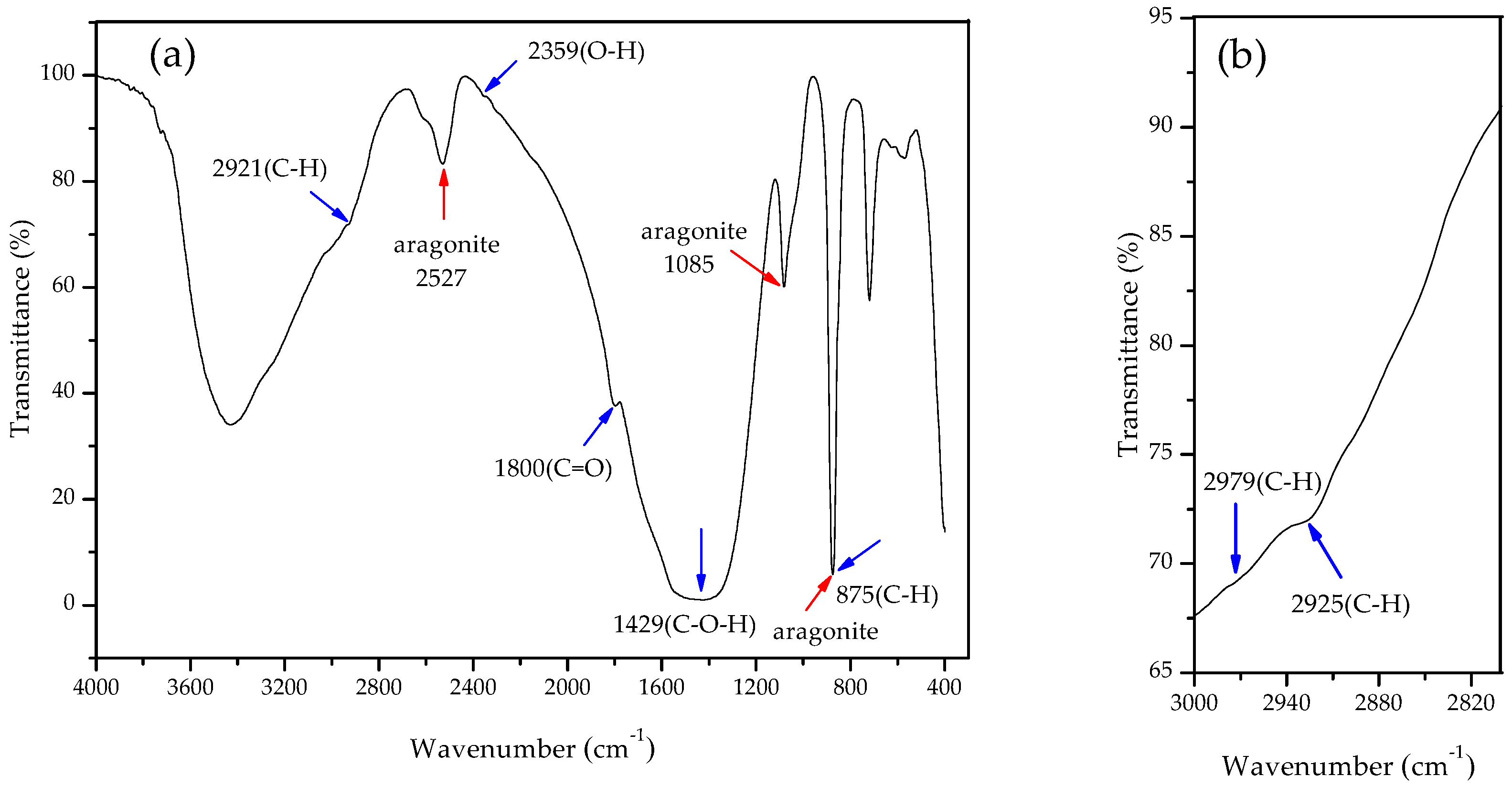
3.4. Carbonate Minerals Analyzed by XRD and FTIR
3.5. Carbonate Minerals Analyzed by SEM and EDS
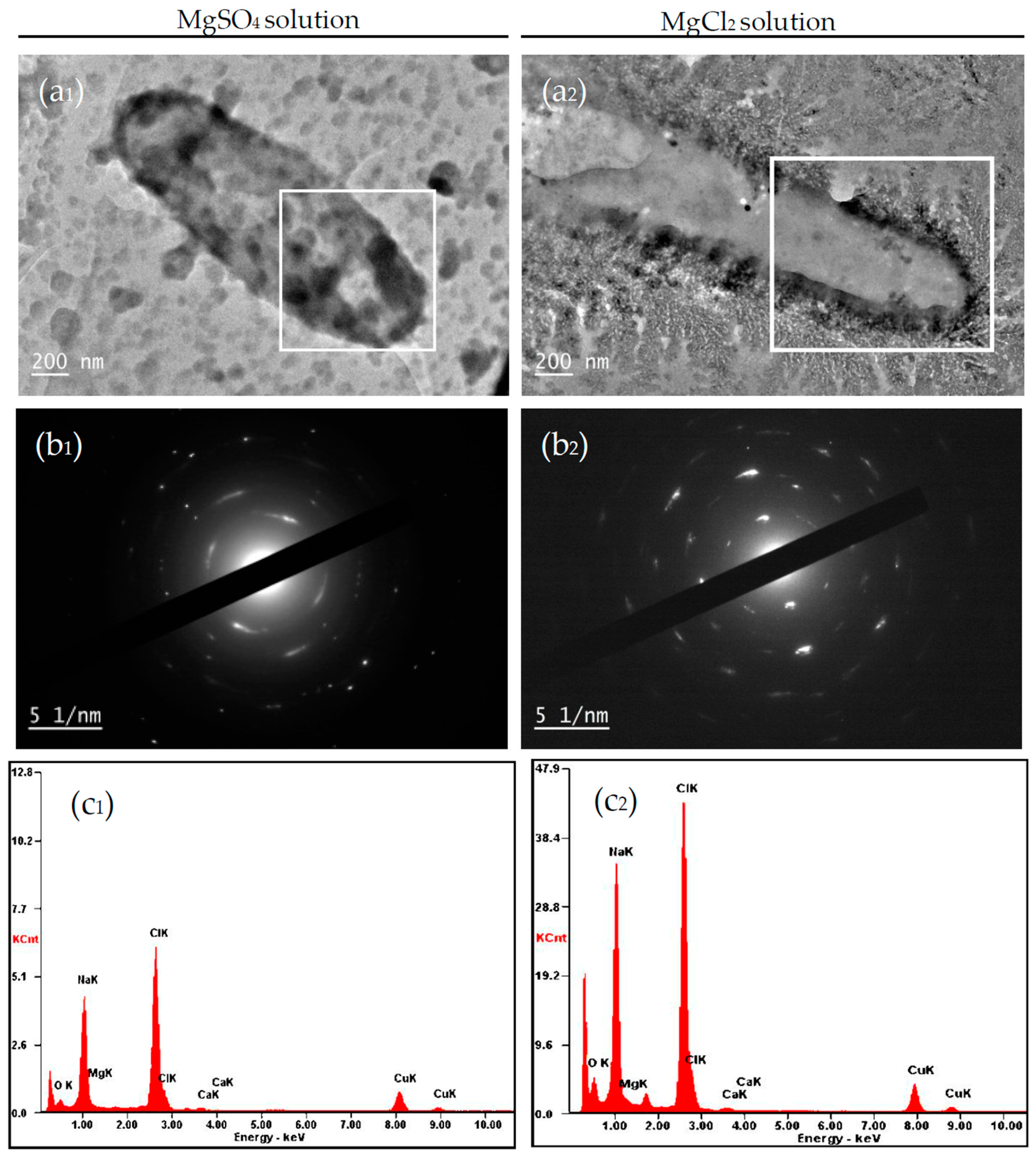
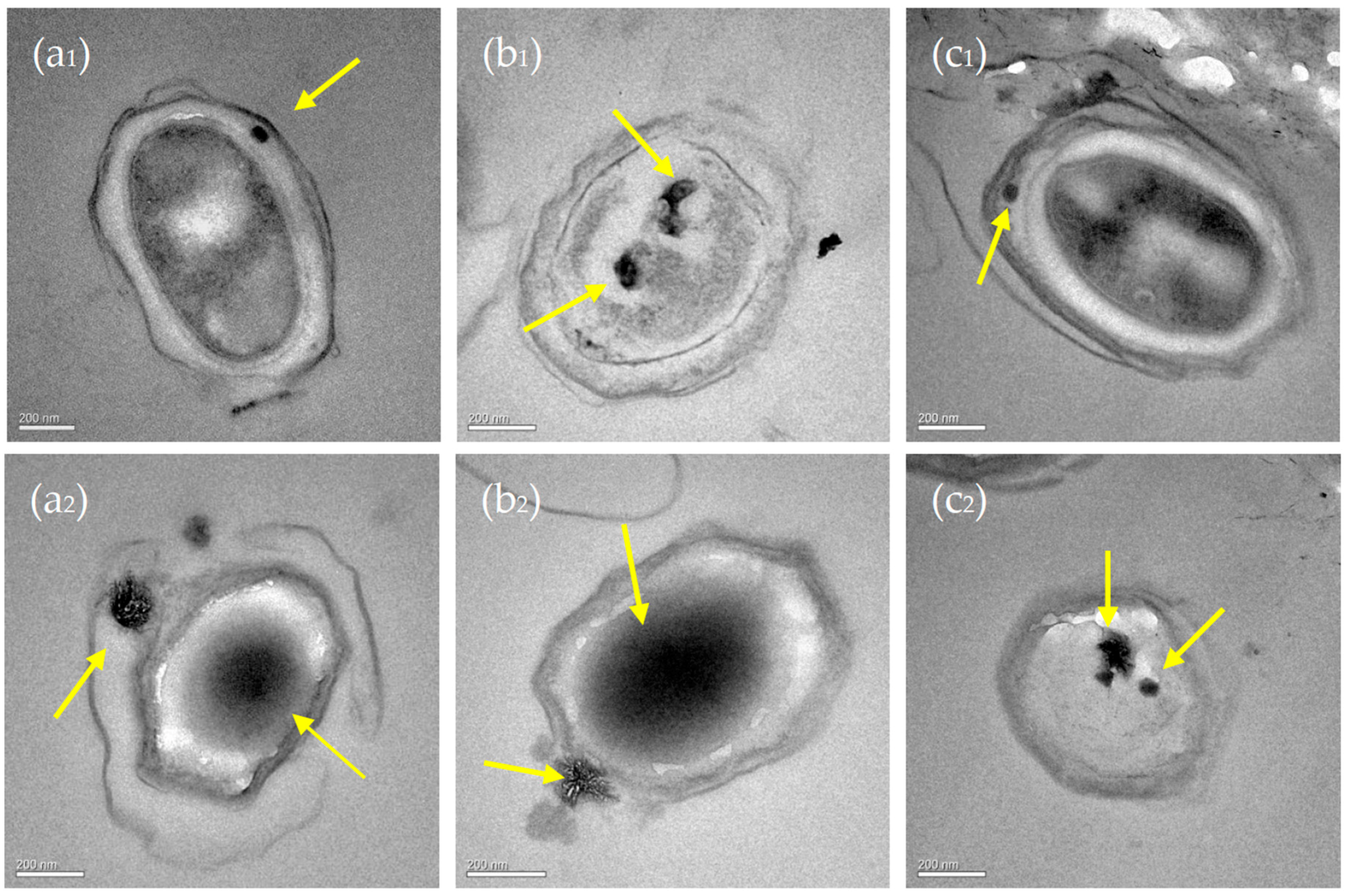
3.6. Nucleation Sites of Minerals on the EPS of C. israelensis LD532 Bacteria
4. Discussion
4.1. The Mechanism of Biomineralization Induced by C. israelensis LD532 Bacteria
4.2. Influence of Mg2+ Source, Concentration and Bacterial Strain on Carbonate Minerals
4.2.1. Carbonate Minerals in MgSO4 and MgCl2 Solutions
4.2.2. Morphology of Carbonate Minerals
4.3. The Formation of Aragonite in the Presence of C. israelensis LD532 Bacteria
4.4. Intracellular and Extracellular Biomineralization of Carbonate Minerals
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spötl, C.; Fairchild, I.J.; Tooth, A.F. Cave air control on dripwater geochemistry, Obir Caves (Austria): Implications for speleothem deposition in dynamically ventilated caves. Geochim. Cosmochim. Acta 2005, 69, 2451–2468. [Google Scholar] [CrossRef]
- Morita, R.Y. Calcite precipitation by marine bacteria. Geomicrobiol. J. 1980, 2, 63–82. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Castanier, S.; Le M’etayer-Levrel, G.; Perthuisot, J.P. Ca-carbonate precipitation and limestone genesis—The microbiologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Párraga, J.; Delgado, R.; Ramos-Cormenzana, A.; Delgado, G. Biomineralization of carbonates by Halobacillus trueperi in solid and liquid media with different salinities. FEMS Microbiol. Ecol. 2004, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, M.A.; Martín-Algarra, A.; Sánchez-Navas, A.; Martín-Ramos, D. Carbonate and phosphate precipitation by Chromohalobacter marismortui. Geomicrobiol. J. 2006, 23, 89–101. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Martín-Algarra, A.; Sánchez-Román, M.; Sánchez-Navas, A.; Martín-Ramos, J.D. Amorphous Ca-phosphate precursors for Ca-carbonate biominerals mediated by Chromohalobacter marismortui. ISME J. 2010, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; Mckenzie, J.A. Sulphate-reducing bacteria induce low-temperature Ca-dolomite and high Mg-calcite formation. Geobiology 2003, 1, 71–79. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Geomicrobiology, 4th ed.; (Revised and Expanded); Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Kranz, S.A.; Wolf-Gladrow, D.; Nehrke, G.; Langer, G.; Rost, B. Calcium carbonate precipitation induced by the growth of the marine cyanobacteria Trichodesmium. Limnol. Oceanogr. 2010, 55, 2563–2569. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Gebiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhao, Y.Y.; Yan, H.X.; Zhao, H.; Han, M.; Sun, B.; Sun, X.Y.; Hou, F.F.; Sun, H.; Han, L.; et al. Struvite Precipitation Induced by a Novel Sulfate-Reducing Bacterium Acinetobacter calcoaceticus SRB4 Isolated from River Sediment. Geomicrobiol. J. 2015, 32, 868–877. [Google Scholar] [CrossRef]
- Ercole, C.; Cacchio, P.; Botta, A.L.; Centi, V.; Lepidi, A. Bacterially induced mineralization of calcium carbonate: The role of exopolysaccharides and capsular polysaccharides. Microsc. Microanal. 2007, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Yan, H.X.; Zhou, S.X.; Zhao, H.; Zhang, Y.; Zhang, N.N.; Yao, C.K.; Zhang, L.; Han, C.Y. Precipitation of calcite induced by Synechocystis sp. PCC6803. World J. Microbiol. Biotechnol. 2013, 29, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.X.; Han, Z.Z.; Zhao, H.; Zhou, S.X.; Chi, N.J.; Han, M.; Kou, X.Y.; Zhang, Y.; Xu, L.L.; Tian, C.C.; et al. Characterization of calcium deposition induced by Synechocystis sp. PCC6803 in BG11 culture medium. Chin. J. Oceanol. Limnol. 2014, 32, 503–510. [Google Scholar] [CrossRef]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Dhami, N.K.; Mukherjee, A.; Reddy, M.S. Micrographical minerological and nano-mechanical characterisation of microbial carbonates from urease and carbonic anhydrase producing bacteria. Ecol. Eng. 2016, 94, 443–454. [Google Scholar] [CrossRef]
- Lian, B.; Hu, Q.N.; Chen, J.; Ji, J.F.; Teng, H.H. Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environment: The role of exopolysaccharides and amino-acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; Mckenzie, J.A. Microbial fossilization in carbonate sediments: A result of the bacterial surface involvement in dolomite precipitation. Sedimentology 2003, 50, 237–245. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhao, Y.Y.; Yan, H.X.; Zhao, H.; Han, M.; Sun, B.; Meng, R.R.; Zhuang, D.X.; Li, D.; Gao, W.J.; et al. The Characterization of Intracellular and Extracellular Biomineralization Induced by Synechocystis sp. PCC6803 Cultured under Low Mg/Ca Ratios Conditions. Geomicrobiol. J. 2016, 34, 362–373. [Google Scholar] [CrossRef]
- Sen, S.; Ingale, S.L.; Kim, Y.W.; Kim, K.H.; Khong, Y.W.; Lohakare, J.D.; Kim, E.K.; Kim, H.S.; Ryu, M.H.; Kwon, I.K.; et al. Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012, 93, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, G.; Braissant, O.; Dupraz, C.; Aragno, M.; Verrecchia, E.P. Biologically induced accumulation of CaCO3 in orthox soils of Biga. Catena 2005, 59, 1–17. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, R.; Parraga, J.; Ramos-Cormenzana, A.; Delgado, G. Precipitation of Minerals by 22 Species of Moderately Halophilic Bacteria in Artificial Marine Salts Media: Influence of Salt Concentration. Folia Microbiol. 2006, 51, 445–453. [Google Scholar] [CrossRef]
- Taylor, S.R.; Mclennan, S.M. The Continental Crust: Its Evolution and Composition; Blackwell Science: Oxford, UK, 1985. [Google Scholar]
- Stanley, S.M.; Ries, J.B.; Hardie, L.A. Low-magnesium calcite produced by coralline algae in seawater of Late Cretaceous composition. Proc. Natl. Acad. Sci. USA 2002, 99, 15323–15326. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallization of monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Ventosa, A.; Gutierrez, M.C.; Garcia, M.T.; Ruiz-Berraquero, F. Classification of “Chomobacterium marismortui” in a New Genus, Chromohalobacter gen. nov., as Chromohalobacter marismortui comb. nov., nom. rev. Int. J. Syst. Bacteriol. 1989, 39, 382–386. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Rivadeneyra, M.A.; Escamilla, C.V.; Algarra, A.M.; Navas, A.S.; Martín-Ramos, J.D. The influence of Salt Concentration on the Precipitation of Magnesium Calcite and Calcium Dolomite by Halomonas Anticariensis. Expert Opin. Environ. Biol. 2016. [Google Scholar] [CrossRef]
- Deng, S.C.; Dong, H.L.; Lv, G.; Jiang, H.C.; Yu, B.S.; Bishop, M.E. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Babavalian, H.; Amoozegar, M.A.; Pourbabaee, A.A.; Moghaddam, M.M.; Shakeri, F. Isolation and identification of moderately halophilic bacteria producing hydrolytic enzymes from the largest hypersaline playa in Iran. Microbiology 2013, 82, 466–474. [Google Scholar] [CrossRef]
- Kim, C.S.; Lee, C.H.; Shin, J.S.; Chung, Y.S.; Hyung, N.I. A simple and rapid method for isolation of high quality genomic DNA from fruit trees and conifers using PVP. Nucleic Acids Res. 1997, 25, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Poonguzhali, S.; Kwon, S.W.; Sa, T.M. Methylobacterium phyllosphaerae sp. nov., a pink-pigmented, facultative methylotroph from rice. Int. J. Syst. Evol. Microbiol. 2009, 59, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 2, 697–703. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. A plant-type (β-class) carbonic anhydrase in the thermophilic methanoarchaeon Methanobacterium thermoautotrophicum. J. Bacteriol. 1999, 181, 6247–6253. [Google Scholar] [PubMed]
- Goldsmith, J.R.; Graf, D.L.; Heard, H.C. Lattice constants of the calcium-magnesium carbonates. Am. Mineral. 1961, 46, 453–457. [Google Scholar]
- Arvidson, R.S.; Mackenzie, F.T. The dolomite problem: Control of precipitation kinetics by temperature and saturation state. Am. J. Sci. 1999, 299, 257–288. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.Z.; Wang, J.; Chen, J.Y. Conformational structure of triblock copolymers by FT-Raman and FTIR spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Liu, H.Z.; Guo, C.; Wang, J. Association behavior of PEO–PPO–PEO block copolymers in water or organic solvent observed by FTIR spectroscopy. Mol. Simul. 2003, 29, 803–808. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.L. Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr. Microbiol. 2011, 62, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Sun, B.; Zhao, H.; Yan, H.X.; Han, M.; Zhao, Y.Y.; Meng, R.R.; Zhuang, D.X.; Li, D.; Ma, Y.T.; et al. Isolation of Leclercia adcarboxglata Strain JLS1 from the dolostone sample and characterization of its induced struvite minerals. Geomicrobiol. J. 2017, 1–11. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhuang, D.X.; Yan, H.X.; Zhao, H.; Sun, B.; Li, D.; Sun, Y.W.; Hu, W.Y.; Xuan, Q.Z.; Chen, J.A.; et al. Thermogravimetric and kinetic analysis of thermal decomposition characteristics of microbial calcites induced by cyanobacteria Synechocystis sp. PCC6803. J. Therm. Anal. Calorim. 2017, 127, 1371–1379. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yao, Q.Z.; Zhou, G.T.; Fu, S.Q. Formation of elongated calcite mesocrystals and implication for biomineralization. Chem. Geol. 2013, 360–361, 126–133. [Google Scholar] [CrossRef]
- Fukushi, K.; Munemoto, T.; Sakai, M.; Yagi, S. Monohydrocalcite: A promising remediation material for hazardous anions. Sci. Technol. Adv. Mater. 2011, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Munemoto, T.; Fukushi, K. Formation condition of monohydrocalcite from CaCl2-MgCl2-Na2CO3 solutions. Geochim. Cosmochim. Acta 2013, 100, 217–231. [Google Scholar] [CrossRef]
- Kimura, T.; Koga, N. Monohydrocalcite in comparisonwith hydrated amorphous calcium carbonate: Precipitation condition and thermal behavior. Cryst. Growth Des. 2011, 11, 3877–3884. [Google Scholar] [CrossRef]
- Han, Z.Z.; Meng, R.R.; Yan, H.X.; Zhao, H.; Han, M.; Zhao, Y.Y.; Sun, B.; Sun, Y.B.; Wang, J.; Zhuang, D.X.; et al. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonate Evaporite 2016, 1–15. [Google Scholar] [CrossRef]
- Porter, S.M. Seawater chemistry and early carbonates biomineralization. Science 2007, 316, 1302. [Google Scholar] [CrossRef] [PubMed]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Dupraz, C.; Bernasconi, S.M.; McKenzie, J.A. Microbes produce nanobacteria-like structures, avoiding entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; Gonzalez, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Q.F.; Zhu, J.Y.; Zhou, S.; Gan, M.; Jiang, H.; Sand, W.G. Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans. Minerals 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Han, Z.Z.; Yan, H.X.; Zhao, H.; Zhou, S.X.; Han, M.; Meng, X.Q.; Zhang, Y.; Zhao, Y.Y.; Sun, B.; Yao, C.K.; et al. Bio-precipitation of Calcite with Preferential Orientation Induced by Synechocystis sp. PCC6803. Geomicrobiol. J. 2014, 31, 884–899. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, D.P.; Dong, H.L.; Kim, J.W.; He, Z.Q.; Morton, J.P. Nontronite particle aggregation induced by microbial Fe(III) reduction and exopolysaccharide production. Clays Clay Miner. 2007, 55, 96–107. [Google Scholar] [CrossRef]
- Von Weymarn, N.; Nyyssölä, A.; Reinikainen, T.; Leisola, M.; Ojamo, H. Improved osmotolerance of recombinant Escherichia coli by de novo glycine betaine biosynthesis. Appl. Microbiol. Biotechnol. 2001, 55, 214–218. [Google Scholar] [CrossRef] [PubMed]



| Test Item | LD532 |
|---|---|
| Ammonia | + |
| V-P | − |
| Catalase | − |
| Sulfureted Hydrogen | − |
| Amylase | + |
| Methyl Red | − |
| Citrate | + |
| Cellulase | − |
| EPS | + |
| Moveability | + |
| Esterase | − |
| Urease | + |
| Triple Sugar Iron Agar | no gas |
| brown yellow | |
| no black precipitates |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Li, D.; Zhao, H.; Yan, H.; Li, P. Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments. Minerals 2017, 7, 95. https://doi.org/10.3390/min7060095
Han Z, Li D, Zhao H, Yan H, Li P. Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments. Minerals. 2017; 7(6):95. https://doi.org/10.3390/min7060095
Chicago/Turabian StyleHan, Zuozhen, Dan Li, Hui Zhao, Huaxiao Yan, and Peiyuan Li. 2017. "Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments" Minerals 7, no. 6: 95. https://doi.org/10.3390/min7060095






