Kinetics of Rare Earth and Aluminum Leaching from Kaolin
Abstract
:1. Introduction
2. Materials and Methods
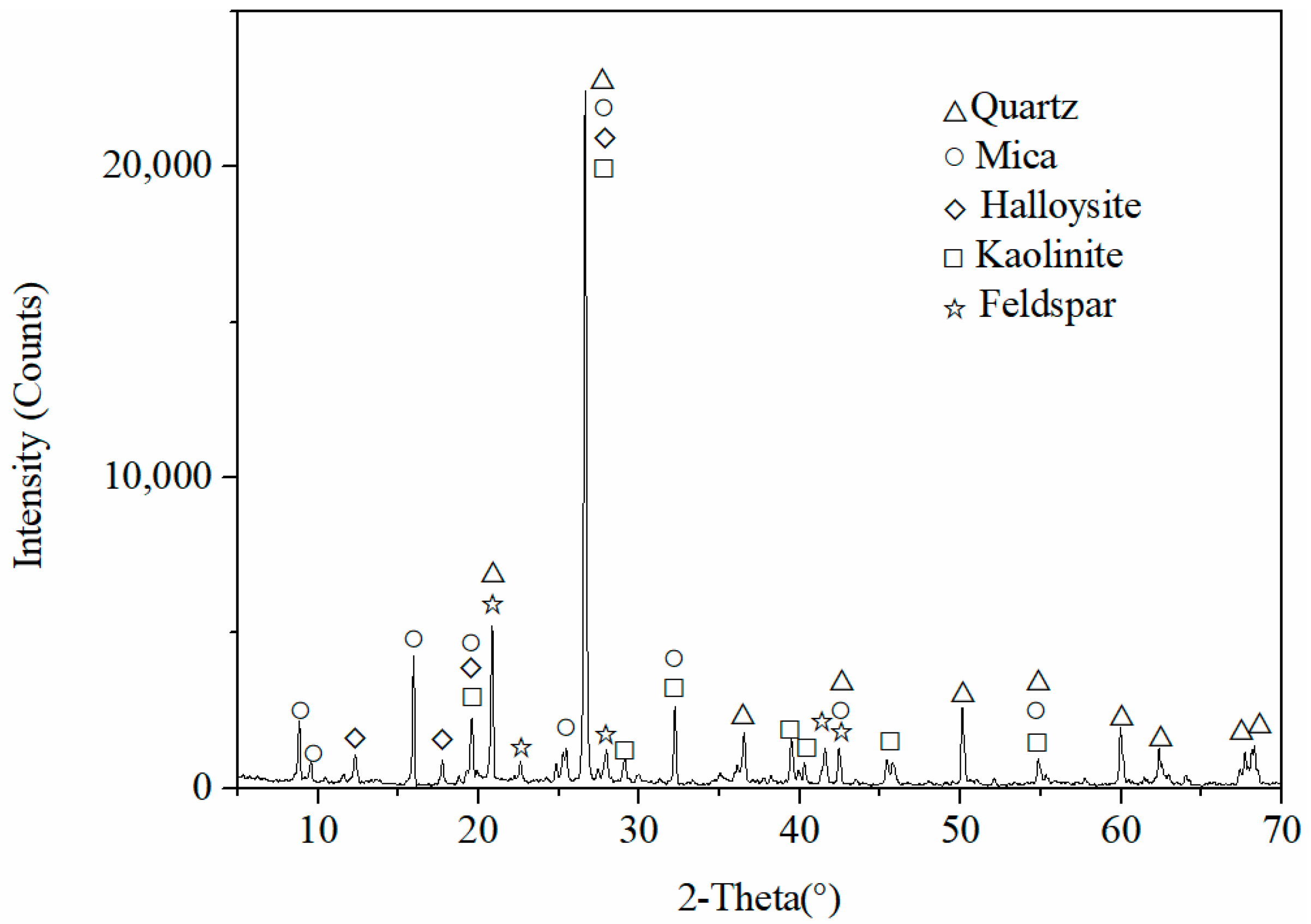
2.1. Mineralogical Characterization and Composition of Rare Earth Ore Samples
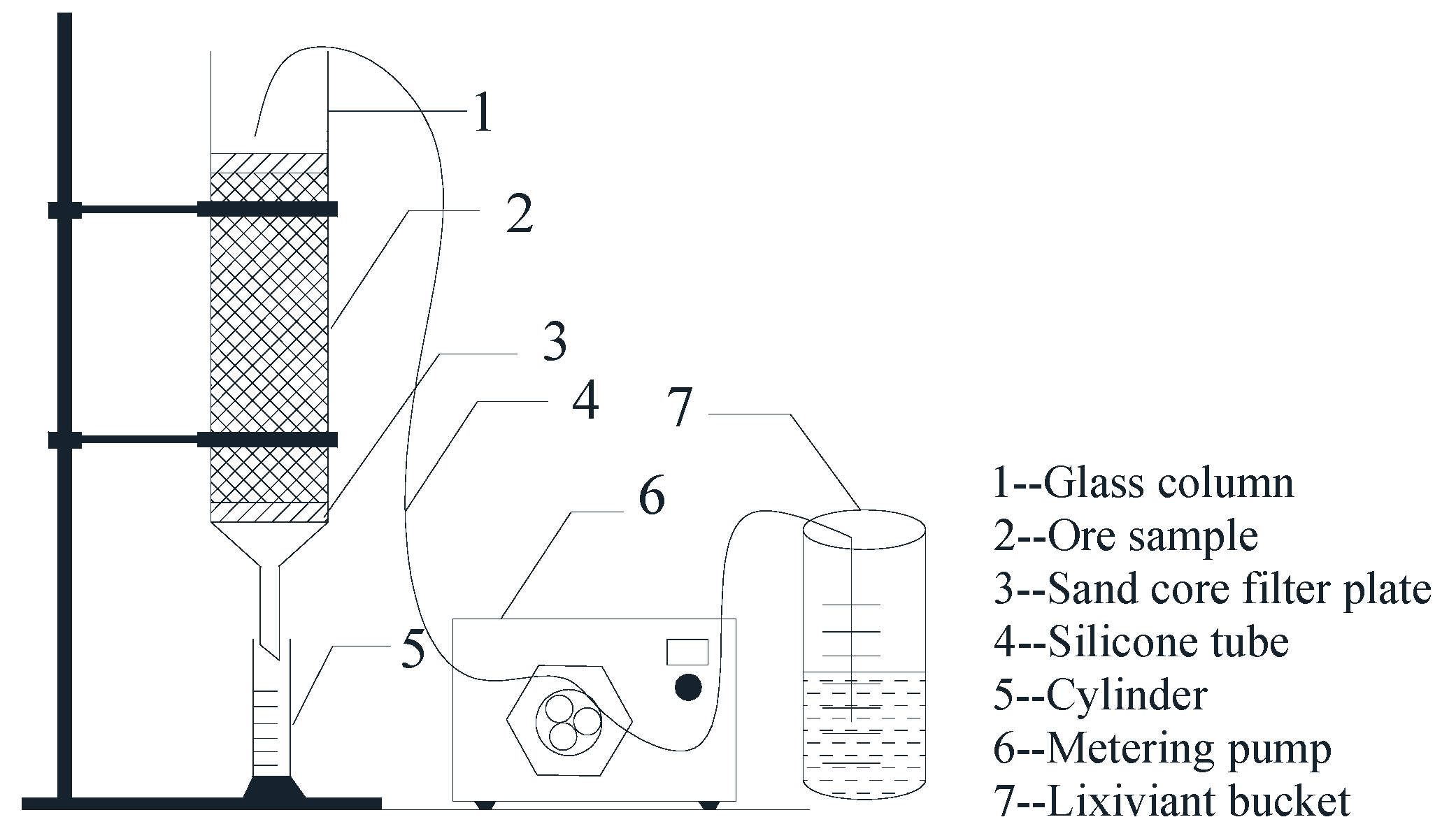
2.2. Column Leaching Experiments
2.3. Analytical Methods
3. Results and Discussion
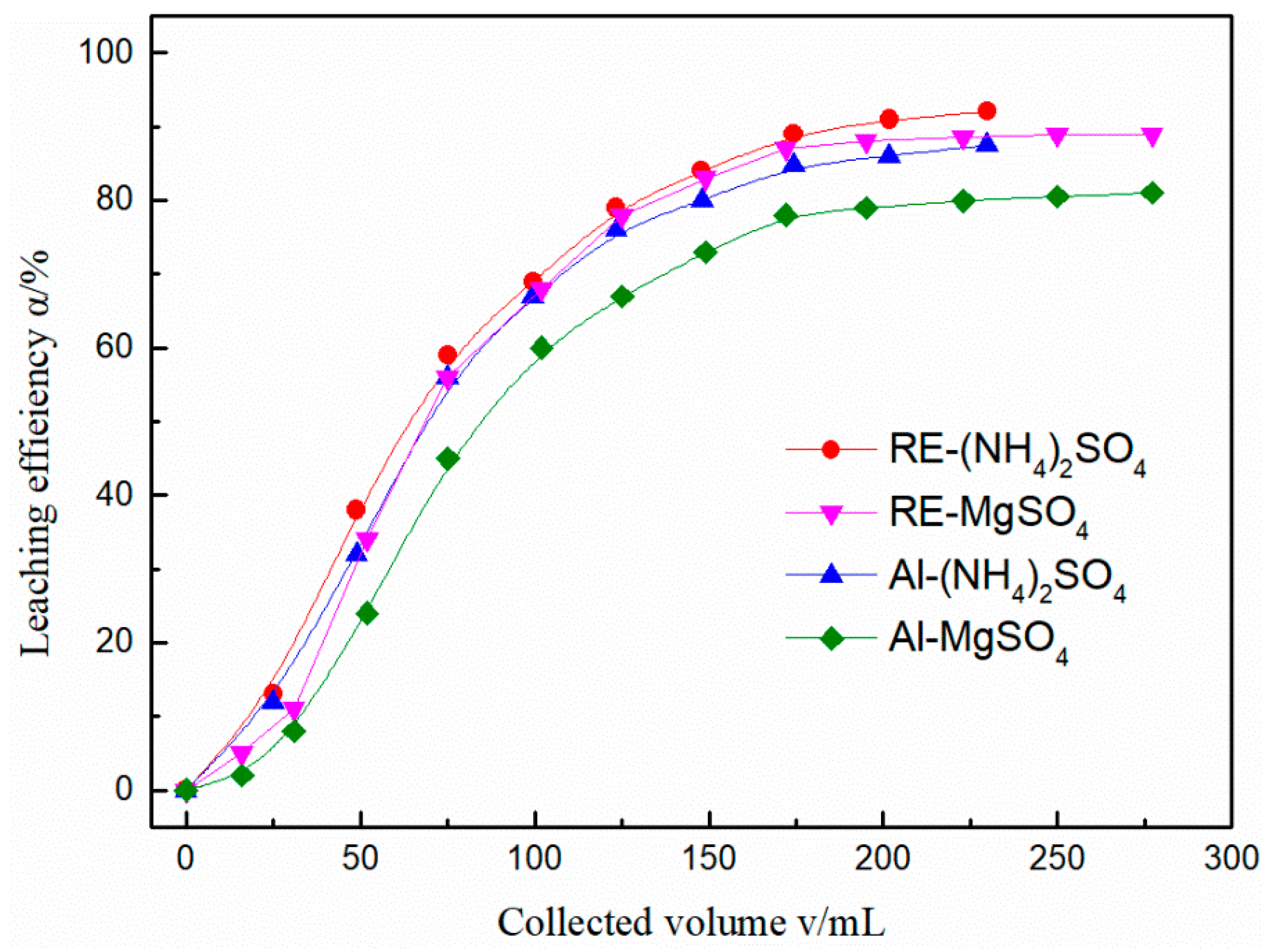
3.1. Leaching with MgSO4 and (NH4)2SO4
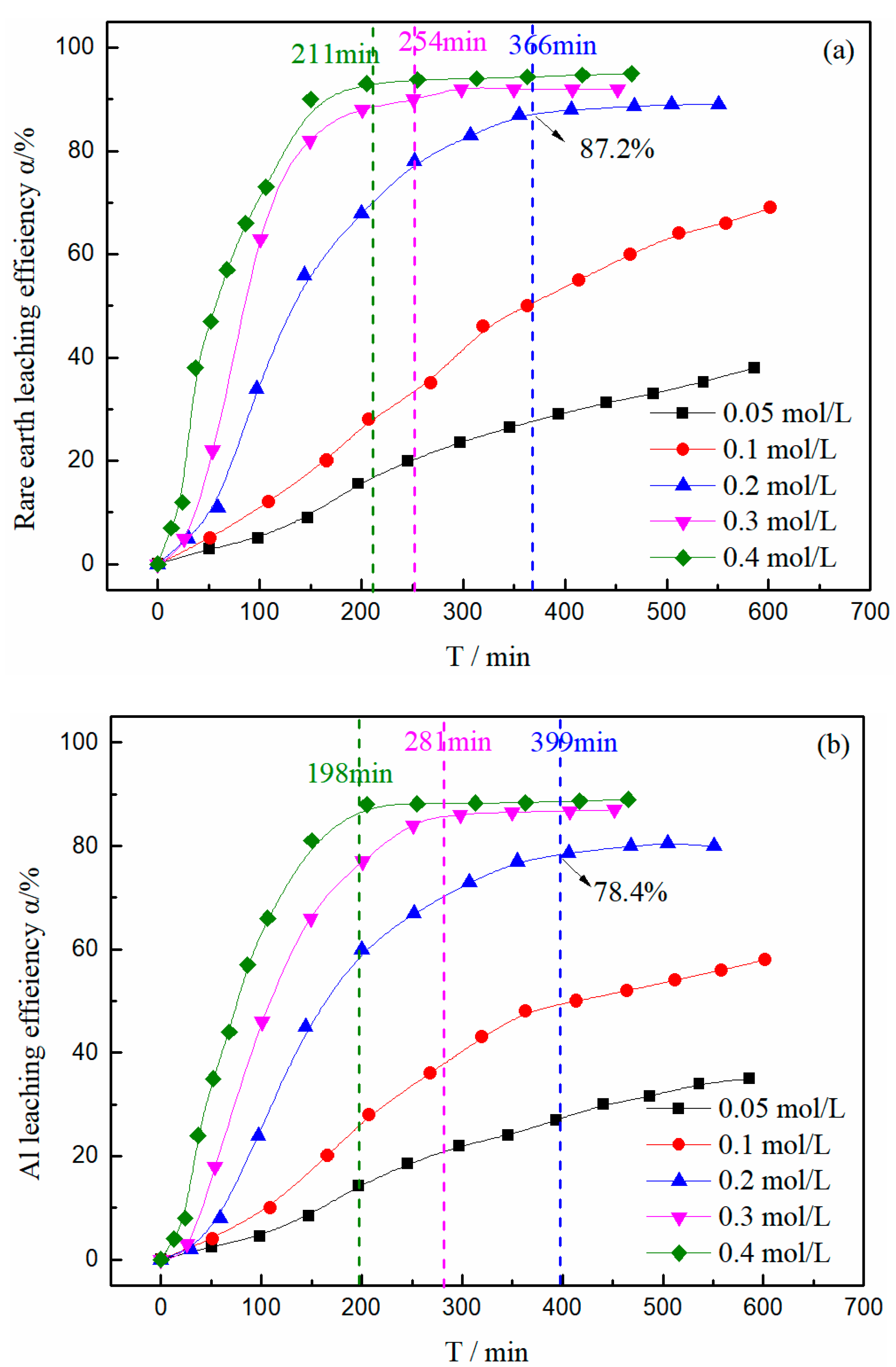
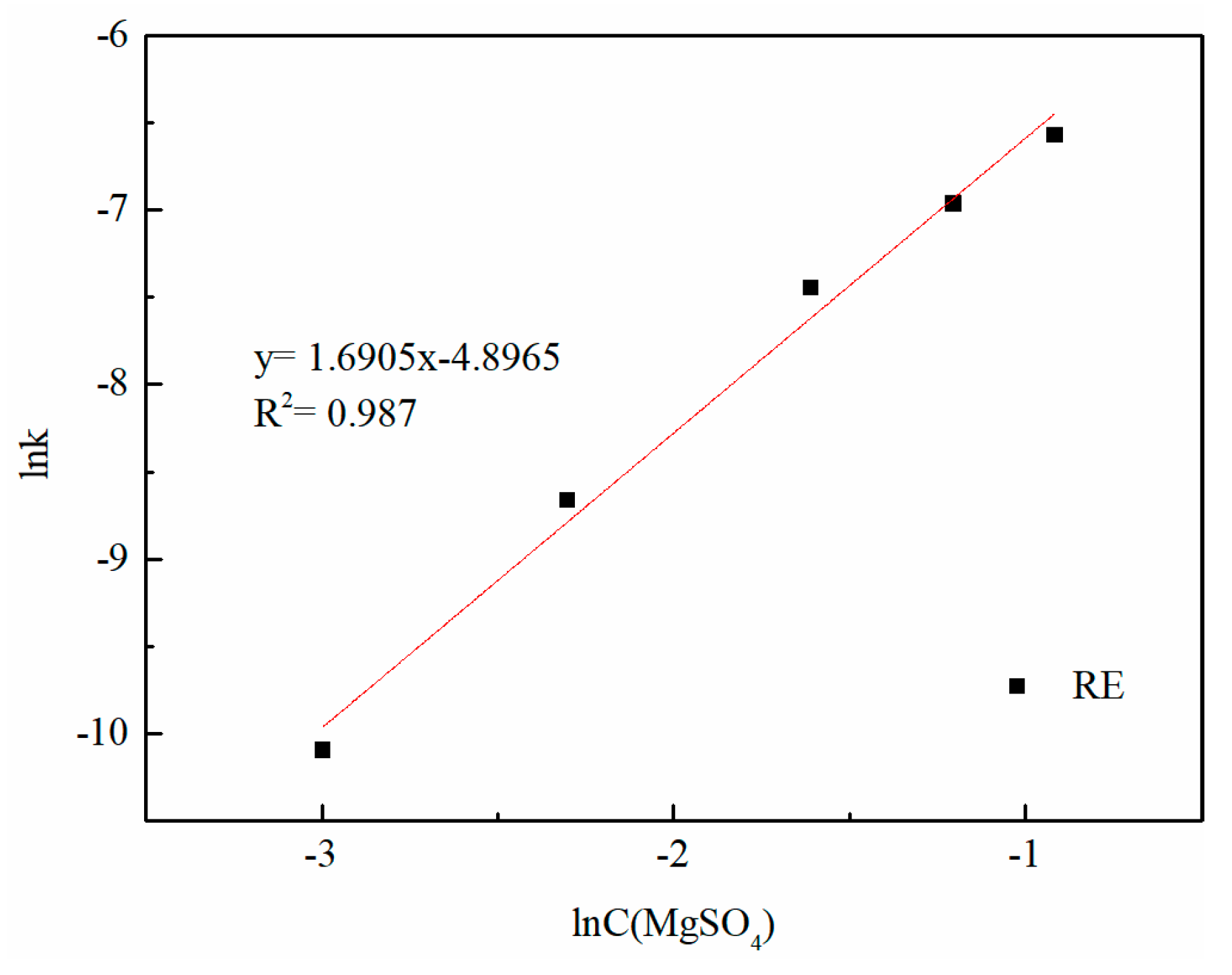
3.2. Effect of Magnesium Concentration on the Leaching Efficiency of Rare Earth and Aluminum
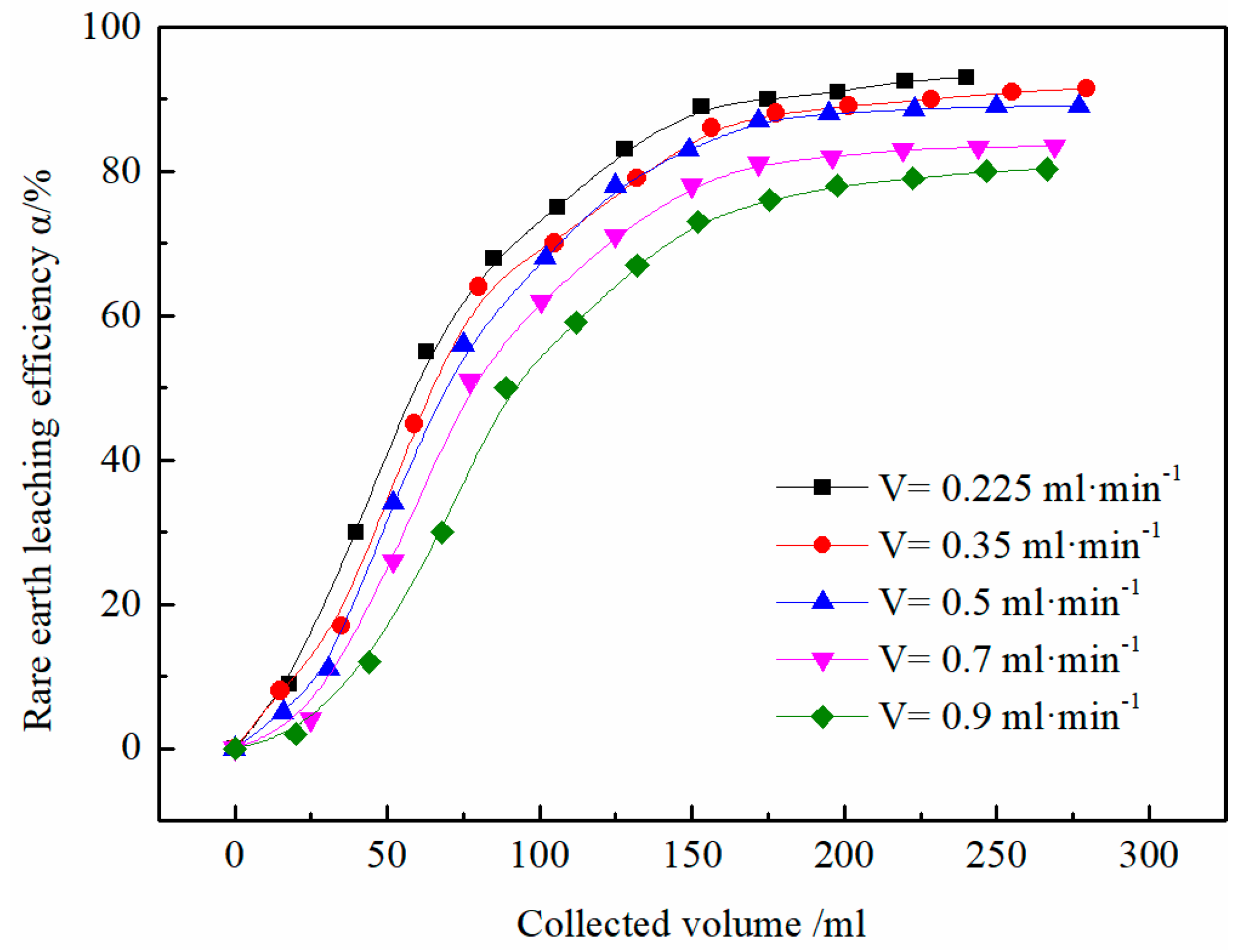
3.3. Effect of Flow Rate on the Leaching Efficiency of Rare Earth
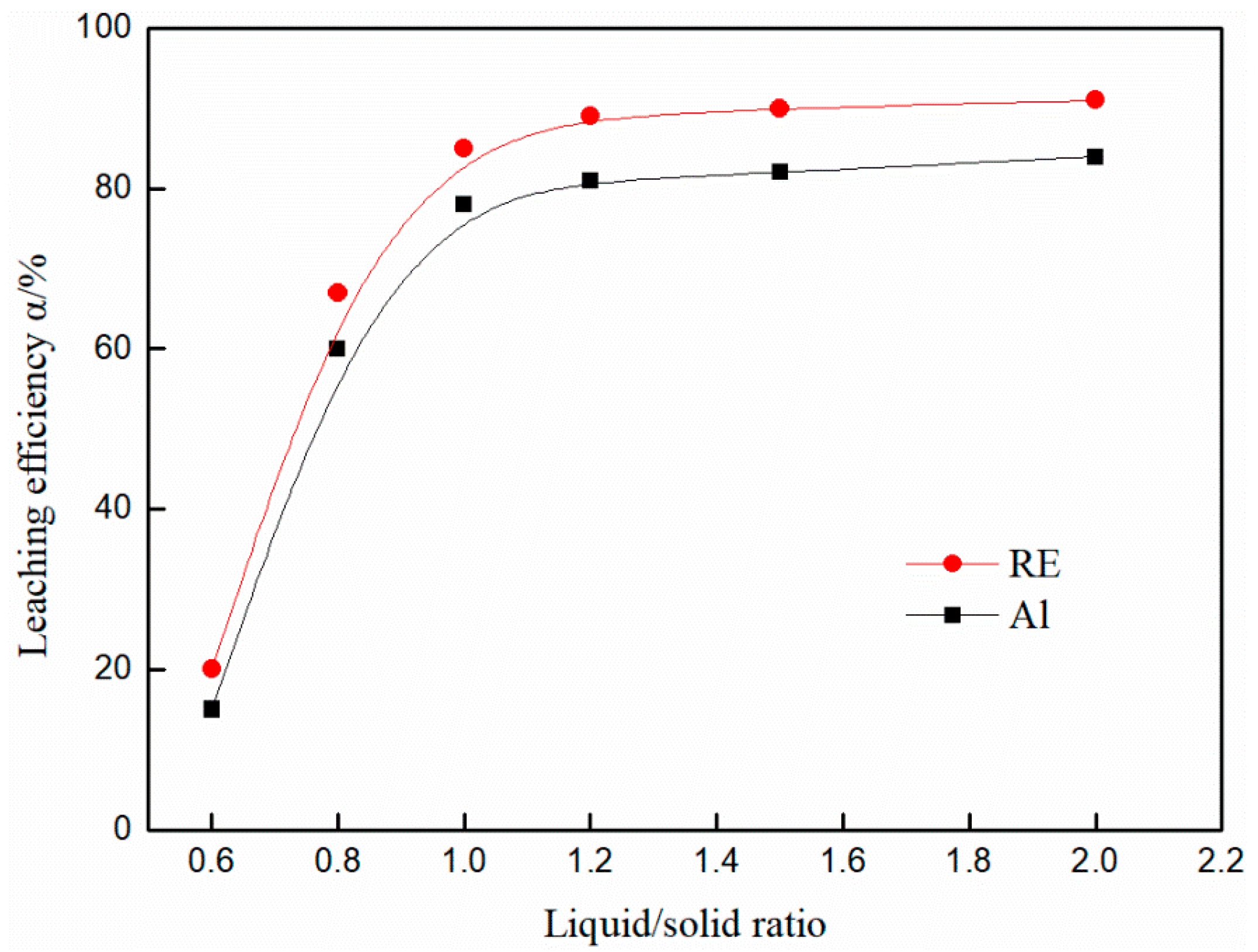
3.4. Effect of Liquid/Solid Ratio on the Leaching Efficiency of Rare Earth and Aluminum
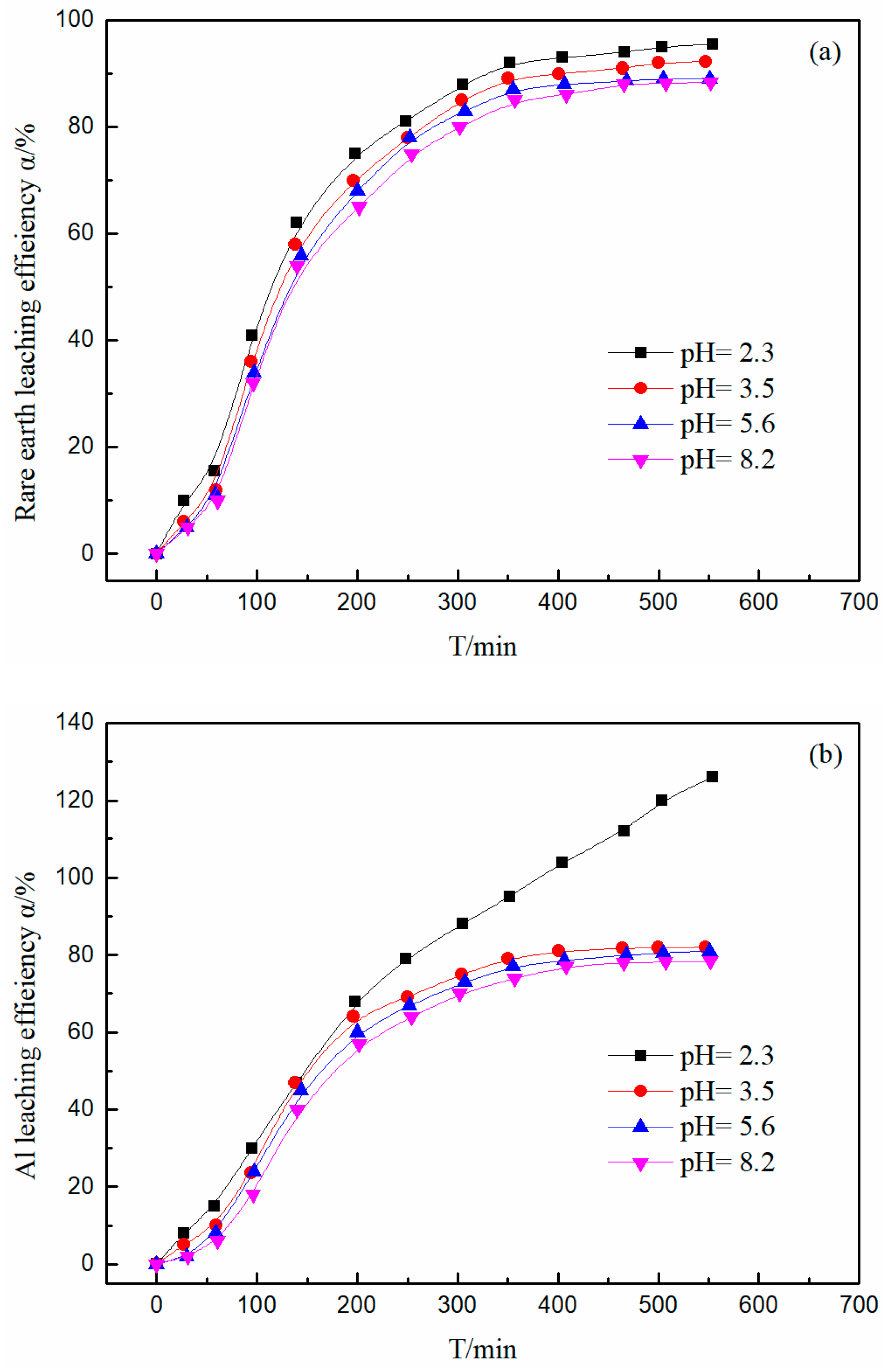
3.5. Effect of pH on the Leaching Efficiency of Rare Earth and Aluminum
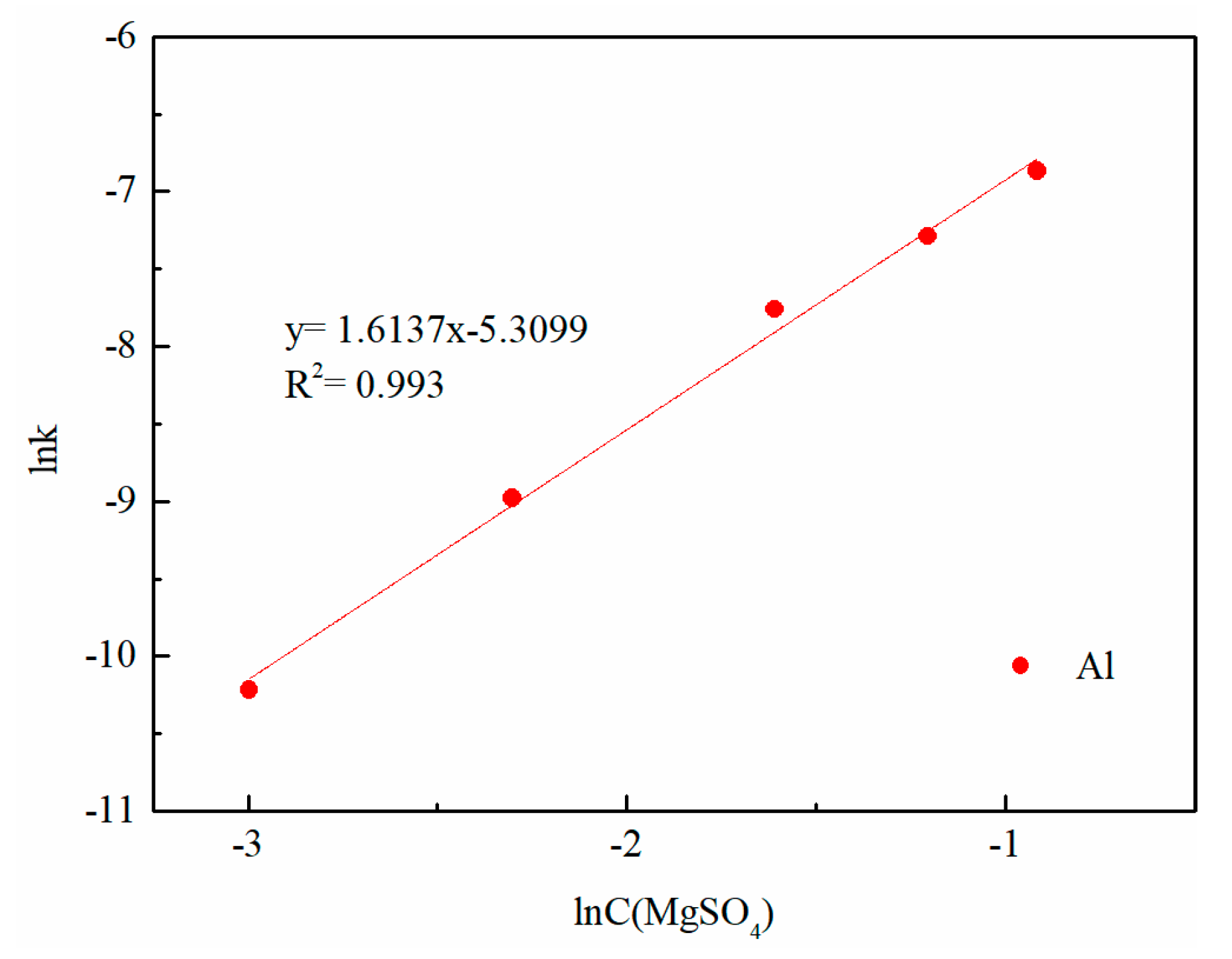
3.6. Kinetic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Z.H. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 2011, 29, 1–6. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Chi, R.; Tian, J.; Luo, X.; Xu, Z.; He, Z. Basic study of weathered crust leaching type rare earth ore. Nonferrous Met. Sci. Eng. 2012, 4, 1–13. [Google Scholar]
- Zang, J.; Edwards, C. A review of rare earth mineral processing technology. In Proceedings of the 44th Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, Canada, 17–19 January 2012; CIM: Montreal, QC, Canada, 2012; pp. 79–102. [Google Scholar]
- Rocha, A.; Schissel, D.; Sprecher, A.; de Tarso, P.; Goode, J. Process development for the Serra Verde weathered crust elution-deposited rare earth deposit in Brazil. In Proceedings of the 52nd Conference of Metallurgists, Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum (MetSoc-CIM), Montreal, QC, Canada, 27–31 October 2013. [Google Scholar]
- Chi, R.; Tian, J.; Li, Z.; Peng, C.; Wu, Y.; Li, S.; Wang, C.; Zhou, Z. Existing state and partitioning of rare earth on weathered ores. J. Rare Earths 2005, 23, 756–759. [Google Scholar]
- Chi, R.; Wang, D. Quantum chemistry calculation of adsorption properties of clay minerals and enrichment of rare earth elements. J. Rare Earths 1993, 3, 199–203. [Google Scholar]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: I. Desorption mechanism. Hydrometallurgy 2012, 117, 71–78. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Feng, Z.; Huang, X.; Huang, L.; Long, Z.; Cui, D. Leaching characteristics of ion-adsorption type rare earths ore with magnesium sulfate. Nonferrous Met. Soc. China 2015, 25, 3784–3790. [Google Scholar] [CrossRef]
- Huang, X.; Wei, Z.; Li, H.; Ying, W.; Zhang, G.; Xue, X. Development of rare earth hydrometallurgy technology in china. J. Rare Earths 2005, 23, 1–4. [Google Scholar] [CrossRef]
- Qiu, T.; Fang, X.; Cui, L.; Fang, Y. Behavior of leaching and precipitation of weathering crust ion-absorbed type by magnetic field. J. Rare Earths 2008, 26, 274. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.; Tang, X.; Chen, J.; Luo, X.; Rao, G. Enhanced leaching process of a low-grade weathered crust elution-deposited rare earth ore with carboxymethyl sesbania gum. Hydrometallurgy 2013, 139, 124–131. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Li, W.; Ding, Y.; Chi, R.A.; Zuo, X. Kinetics of weathered-crust elution-deposited rare-earth ore in a leaching process. Mater. Tehnol. 2013, 47, 145–148. [Google Scholar]
- Luo, X.-P.; Feng, B.; Wang, P.-C.; Zhou, H.-P.; Chen, X.-M. The effect of fulvic acid on the leaching of a weathered rare-earth ore. Metall. Mater. Trans. B 2015, 46, 2405–2407. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J. Recovery of rare earth from ion-adsorption rare earth ores with a compound lixiviant. Sep. Purif. Technol. 2015, 142, 203–208. [Google Scholar] [CrossRef]
- Qiu, T.; Fang, X.; Wu, H.; Zeng, Q.; Zhu, D. Leaching behaviors of iron and aluminum elements of ion-absorbed-rare-earth ore with a new impurity depressant. Nonferrous Met. Soc. China 2014, 24, 2986–2990. [Google Scholar] [CrossRef]
- Qiu, T.; Zhu, D.; Fang, X.; Zeng, Q.; Gao, G.; Zhu, H. Leaching kinetics of ionic rare-earth in ammonia-nitrogen wastewater system added with impurity inhibitors. J. Rare Earths 2014, 32, 1175–1183. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Hu, G.; Huang, L.; Huang, X.; Chen, Y.; Long, Z. Reduction leaching of rare earth from ion-adsorption type rare earths ore with ferrous sulfate. J. Rare Earths 2016, 34, 917–923. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Yang, B.; Nie, H.; Ye, X.; Liao, C.; Yu, P.; Shen, W. A Kind of Method for Extraction of Rare Earth from Ion-Adsorption Type Rare Earth. China Patent CN 201,310,199,034.3, 28 August 2013. [Google Scholar]
- Xiao, Y.F.; Feng, Z.Y.; Hu, G.H.; Huang, L.; Huang, X.W.; Chen, Y.Y.; Li, M.L. Leaching and mass transfer characteristics of elements from ion-adsorption type rare earth ore. Rare Met. 2015, 34, 357–365. [Google Scholar] [CrossRef]
- Huang, X.W.; Long, Z.Q.; Wang, L.S.; Feng, Z.Y. Technology development for rare earth cleaner hydrometallurgy in china. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Wang, R.X.; Xie, B.Y.; Yu, P.; Zhang, Z.X.; Mao, J.Y.; Xiong, J.C. Selection of leaching agent for ion type rare earth ore and optimization of column leaching process. Rare Met. 2015, 39, 1060–1064. [Google Scholar]
- Tian, J.; Yin, J.Q.; Chi, R.; Rao, G.H.; Jiang, M.T.; Ouyang, K.X. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Xu, P. Handbook of Practical Chemical Analysis of Factory, 1st ed.; Jiangsu Institute of Mechanical Engineering Physical and Chemical Inspection Branch Press: Nanjing, China, 1980; Volume 1, pp. 237–240. [Google Scholar]
- Tian, J.; Tang, X.; Yin, J.; Luo, X.; Rao, G.; Jiang, M. Process optimization on leaching of a lean weathered crust elution-deposited rare earth ores. Int. J. Miner. Process. 2013, 119, 83–88. [Google Scholar] [CrossRef]
- Jun, T.; Jingqun, Y.; Kaihong, C.; Guohua, R.; Mintao, J.; Ruan, C. Optimisation of mass transfer in column elution of rare earths from low grade weathered crust elution-deposited rare earth ore. Hydrometallurgy 2010, 103, 211–214. [Google Scholar] [CrossRef]
- Chi, R.; Zhu, G.; Xu, S.; Tian, J.; Liu, J.; Xu, Z. Kinetics of manganese reduction leaching from weathered rare-earth mud with sodium sulfite. Metall. Mater. Trans. B 2002, 33, 41–46. [Google Scholar] [CrossRef]
- Shi, F. Rare Earth Metallurgy Technology, 1st ed.; Metallurgy Industry Press: Beijing, China, 2009. [Google Scholar]
- Shao, Z.C.; He, Q.; Wang, W. Forms of aluminum in red soils. ACTA Pedol. Sin. 1998, 35, 38–48. [Google Scholar]
- Li, T.; Tu, A.; Zhang, Y.; Zhang, M.; Chi, R. Kinetic study on leaching of rare earth from weathered crust Leaching Rare Earth Ore with mixed ammonium salts. Ind. Miner. Process 2009, 38, 19–24. [Google Scholar]
- Li, M.; Zhang, X.; Liu, Z.; Hu, Y.; Wang, M.; Liu, J.; Yang, J. Kinetics of leaching fluoride from mixed rare earth concentrate with hydrochloric acid and aluminum chloride. Hydrometallurgy 2013, 140, 71–76. [Google Scholar] [CrossRef]

| Particle Size/mm | +0.5 | −0.5 + 0.1 | −0.1 + 0.045 | −0.045 |
|---|---|---|---|---|
| Mass distribution/% | 52.34 | 23.16 | 5.9 | 18.59 |
| Y2O3 | Al2O3 | MnO2 | Na2O | CaO | MgO | K2O | SiO2 |
| 0.023 | 17.665 | 0.022 | 0.487 | 0.058 | 0.051 | 4.269 | 73.048 |
| SO3 | TiO2 | Fe2O3 | Rb2O | ZrO2 | CuO | Nb2O5 | Loss |
| 0.017 | 0.019 | 0.624 | 0.037 | 0.011 | 0.004 | 0.006 | 2.98 |
| REO | Al | Ca | Mg | Others |
|---|---|---|---|---|
| 0.109 | 0.0088 | 0.024 | 0.00592 | <0.001 |
| Element | La2O3 | CeO2 | Pr6O11 | Nd2O3 | Eu2O3 | Sm2O3 | Tb4O7 | Gd2O3 |
| Content/% | 6.17 | 0.88 | 2.83 | 9.67 | 0.18 | 4.92 | 1.28 | 6.52 |
| Element | Ho2O3 | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 | Y2O3 | Dy2O3 | |
| Content/% | 1.46 | 3.98 | 0.65 | 3.66 | 0.52 | 49.97 | 7.31 |
| Concentration (mol/L) | Second Period | |||
|---|---|---|---|---|
| 1 − 2/3α − (1 − α)2/3 | ||||
| Rare Earth | Aluminum | |||
| k/min−1 | R2 | k/min−1 | R2 | |
| 0.05 | 4.12 × 10−5 | 0.997 | 3.65 × 10−5 | 0.995 |
| 0.1 | 1.73 × 10−4 | 0.985 | 1.26 × 10−4 | 0.982 |
| 0.2 | 5.82 × 10−4 | 0.987 | 4.26 × 10−4 | 0.982 |
| 0.3 | 9.44 × 10−4 | 0.959 | 6.82 × 10−4 | 0.981 |
| 0.4 | 1.4 × 10−3 | 0.969 | 1.04 × 10−3 | 0.971 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, X.; Ren, Z.; Gao, H.; Zheng, R.; Jin, J. Kinetics of Rare Earth and Aluminum Leaching from Kaolin. Minerals 2017, 7, 152. https://doi.org/10.3390/min7090152
Ran X, Ren Z, Gao H, Zheng R, Jin J. Kinetics of Rare Earth and Aluminum Leaching from Kaolin. Minerals. 2017; 7(9):152. https://doi.org/10.3390/min7090152
Chicago/Turabian StyleRan, Xiuchuan, Zijie Ren, Huimin Gao, Renji Zheng, and Junxun Jin. 2017. "Kinetics of Rare Earth and Aluminum Leaching from Kaolin" Minerals 7, no. 9: 152. https://doi.org/10.3390/min7090152





