The Role of Water Glass in the Flotation Separation of Fine Fluorite from Fine Quartz
Abstract
:1. Introduction
2. Materials and Methods
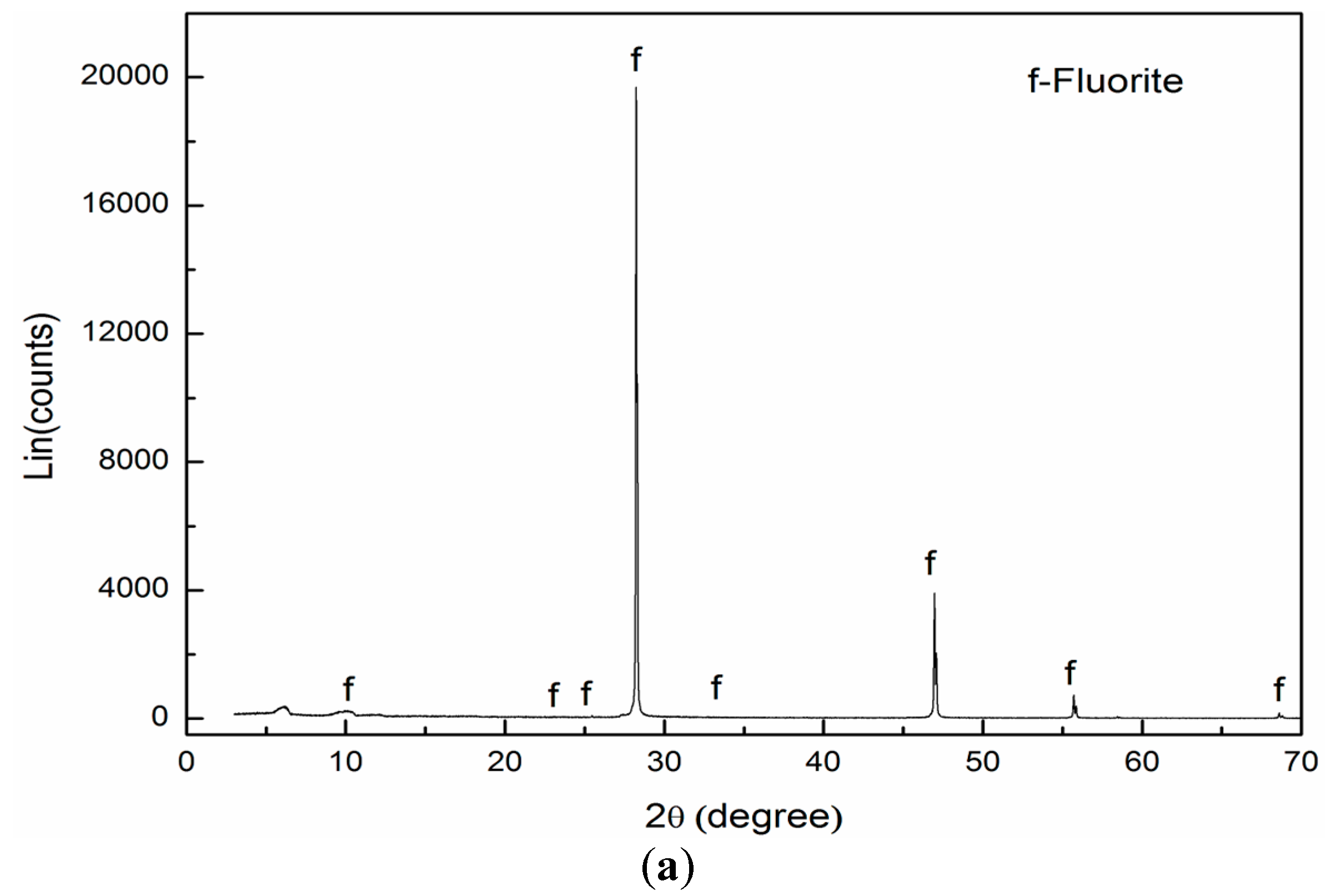
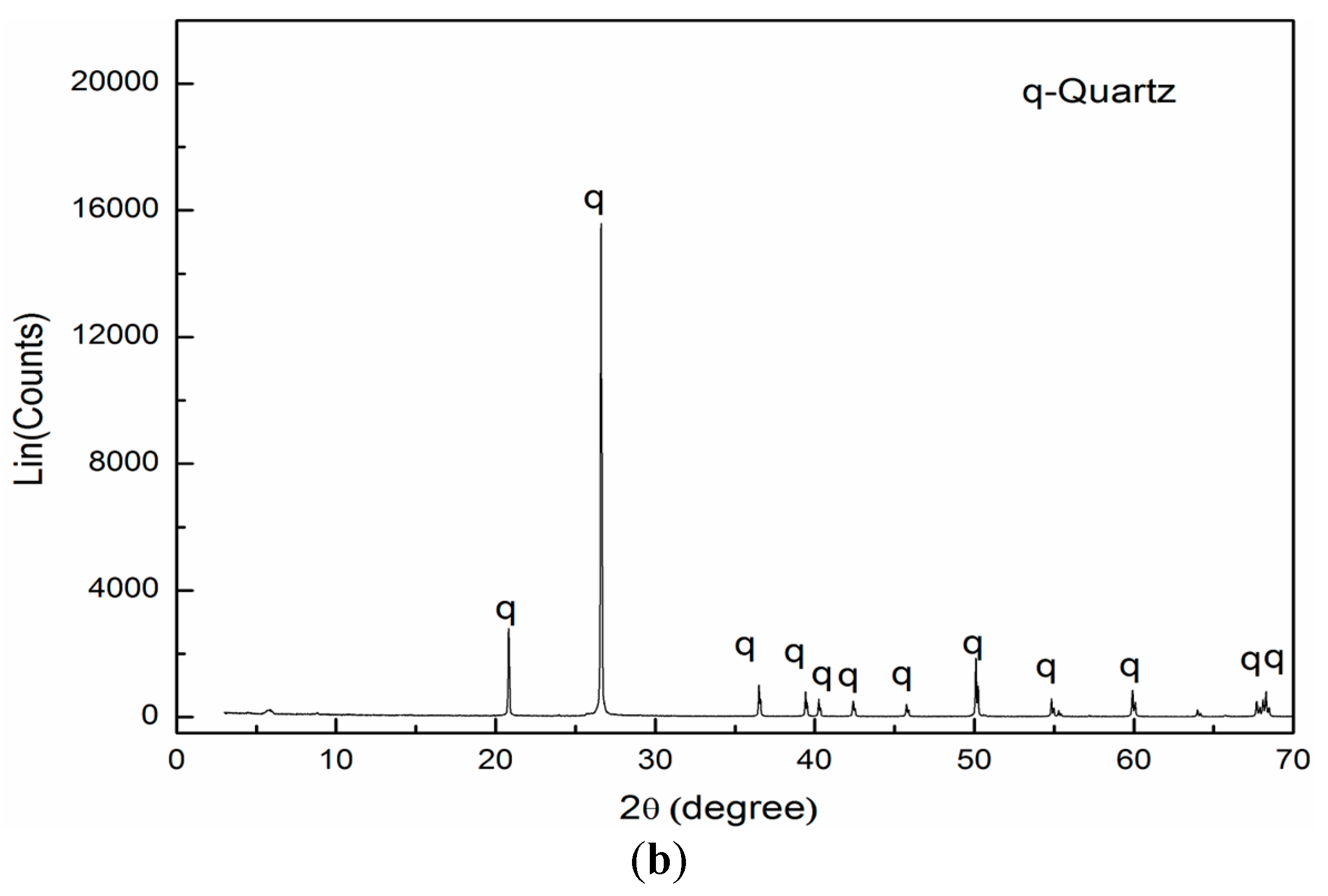
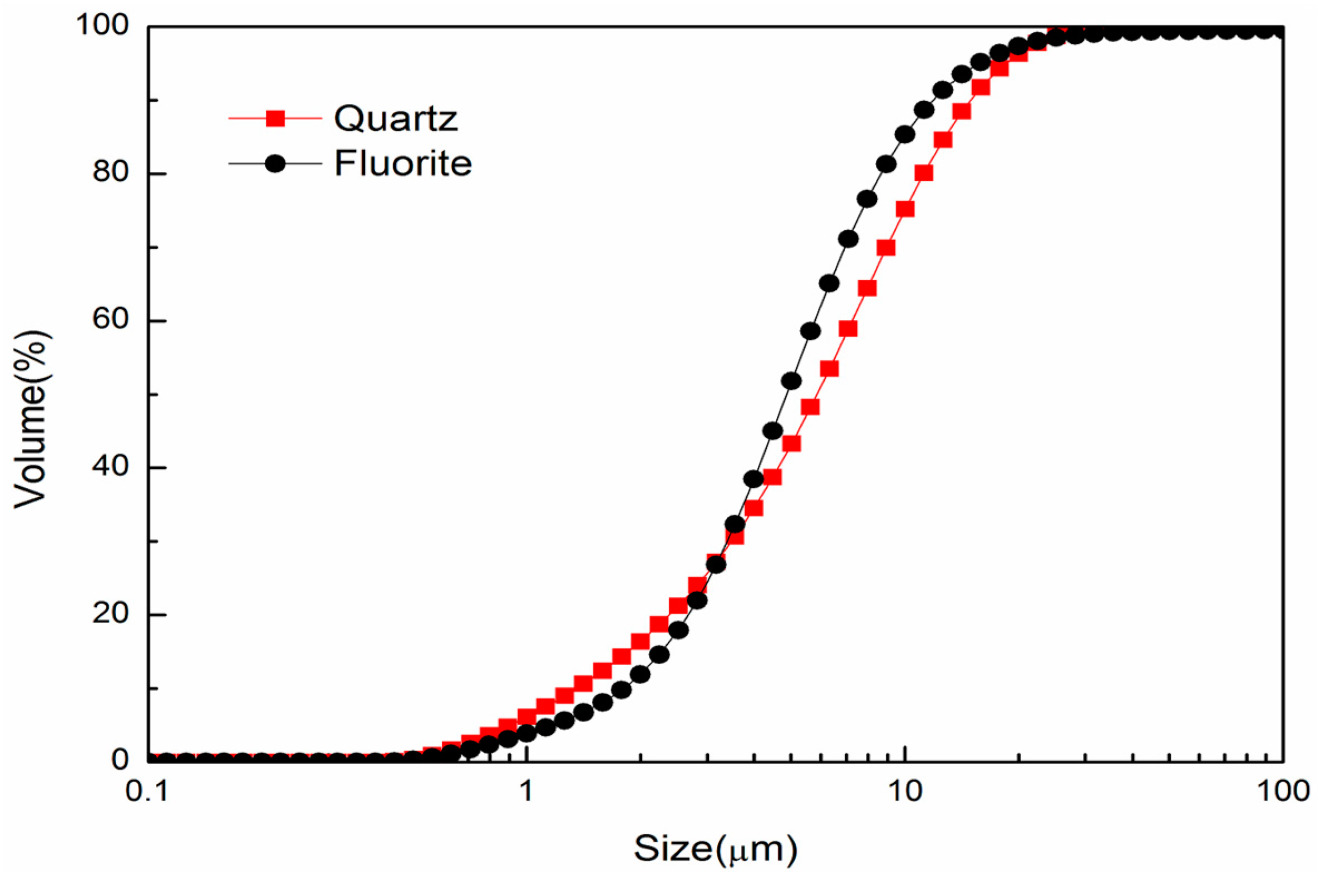
2.1. Materials and Regents
2.2. Flotation Tests
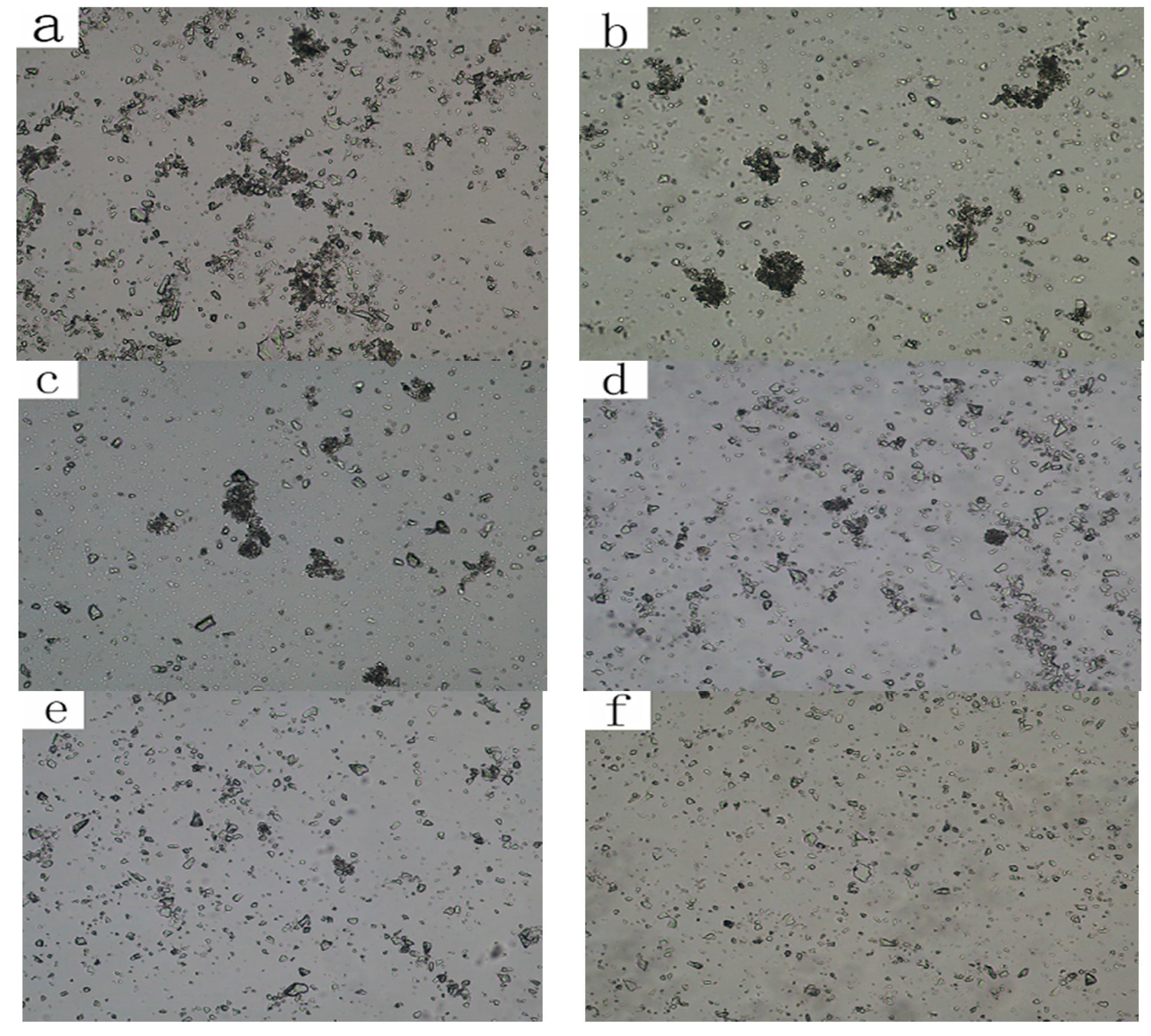
2.3. Observations of Mineral Pulp
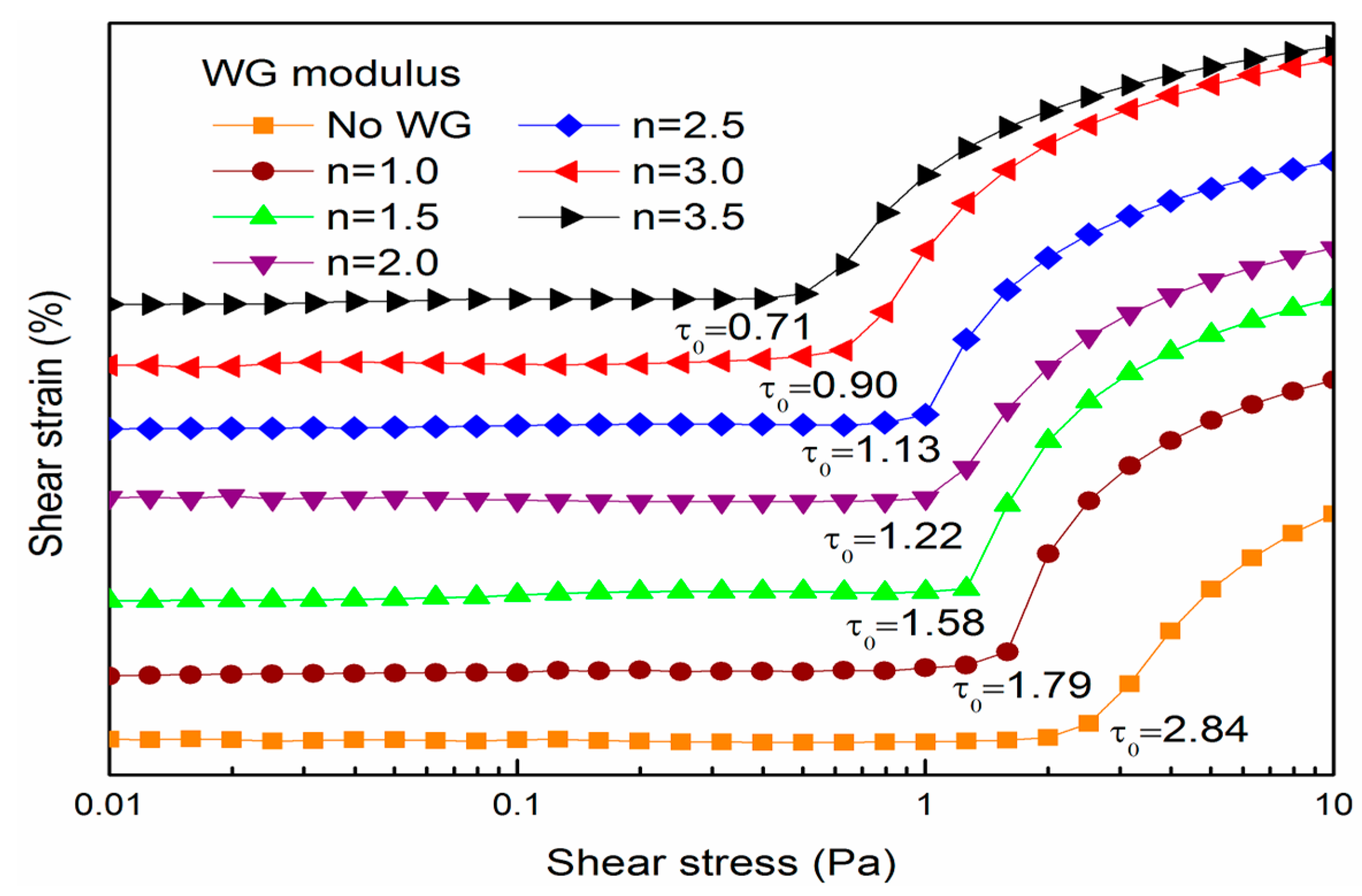
2.4. Shear Yield Stress Measurements
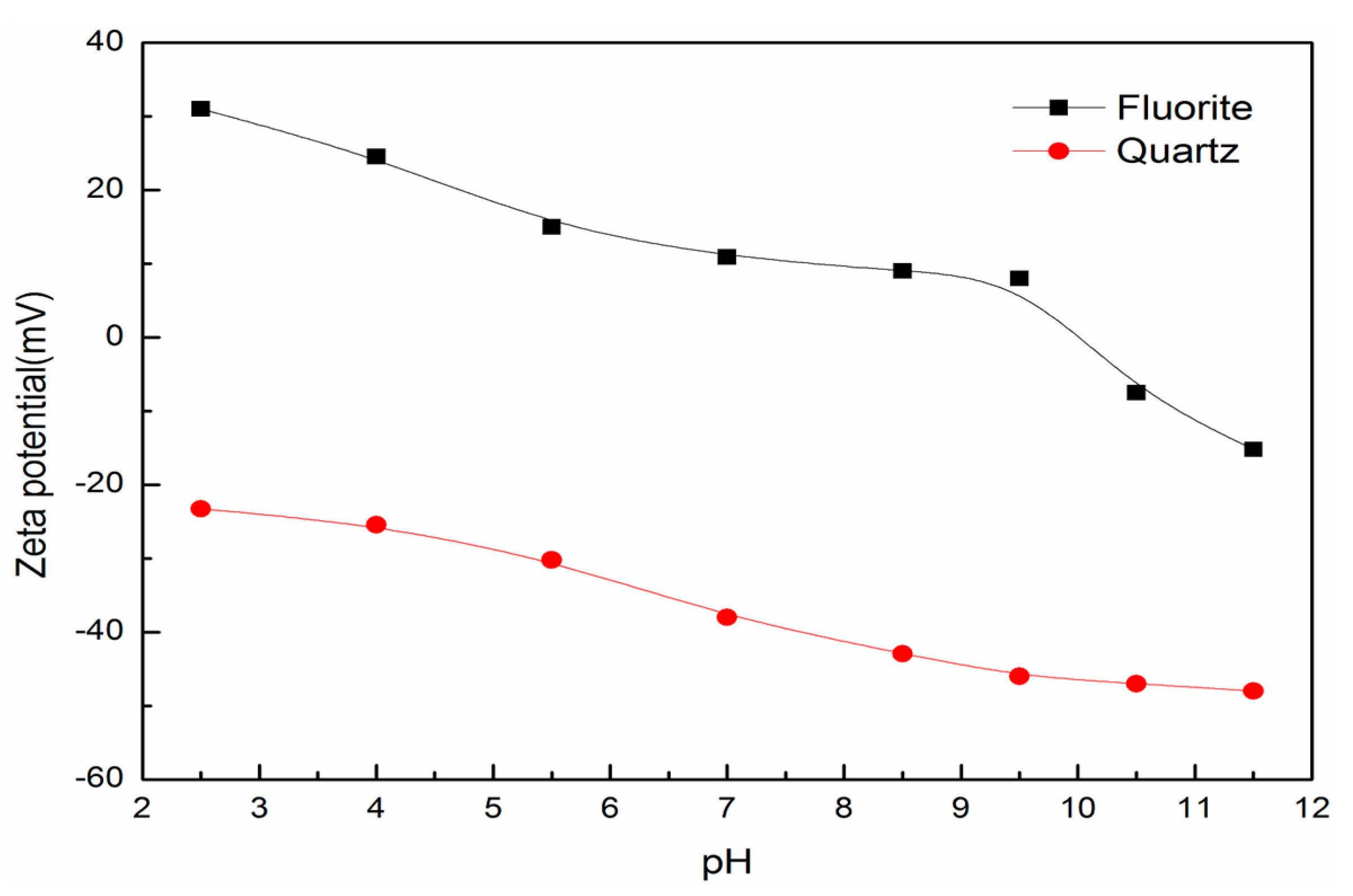
2.5. Zeta Potential Measurements
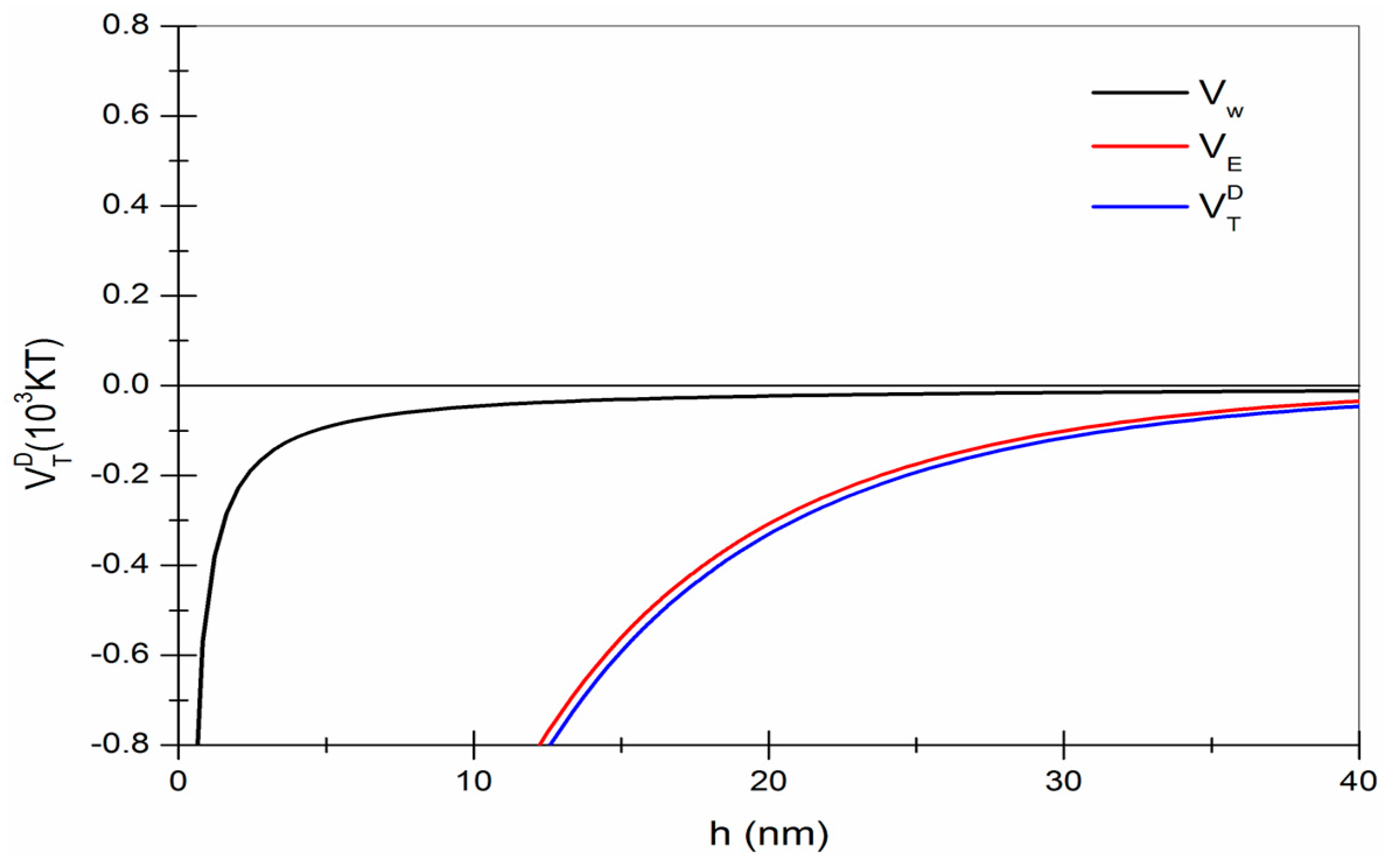
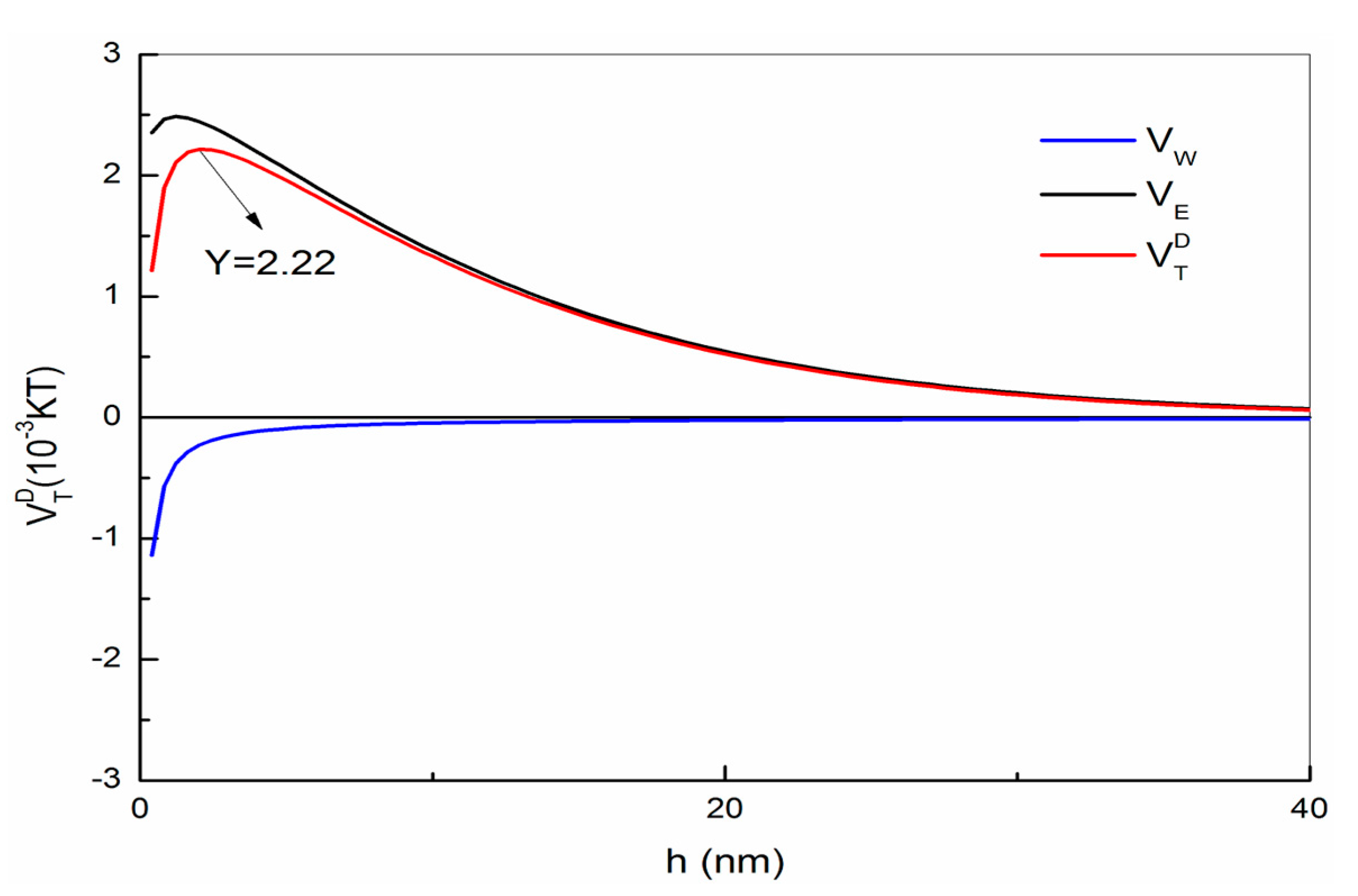
2.6. Particle Interaction Calculations—Derjguin-Landau-Verwey-Overbeek (DLVO) Theory
3. Results and Discussion
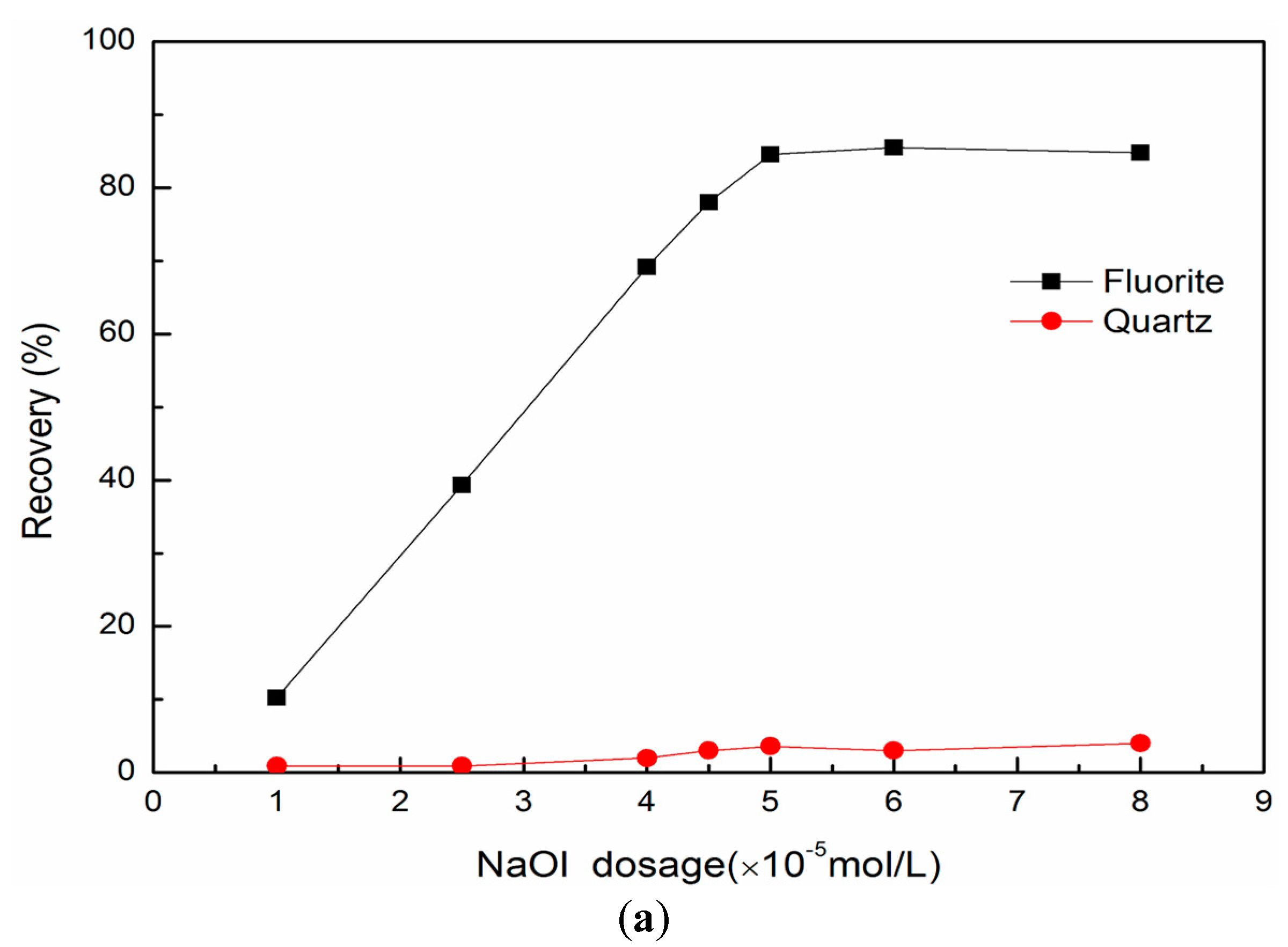
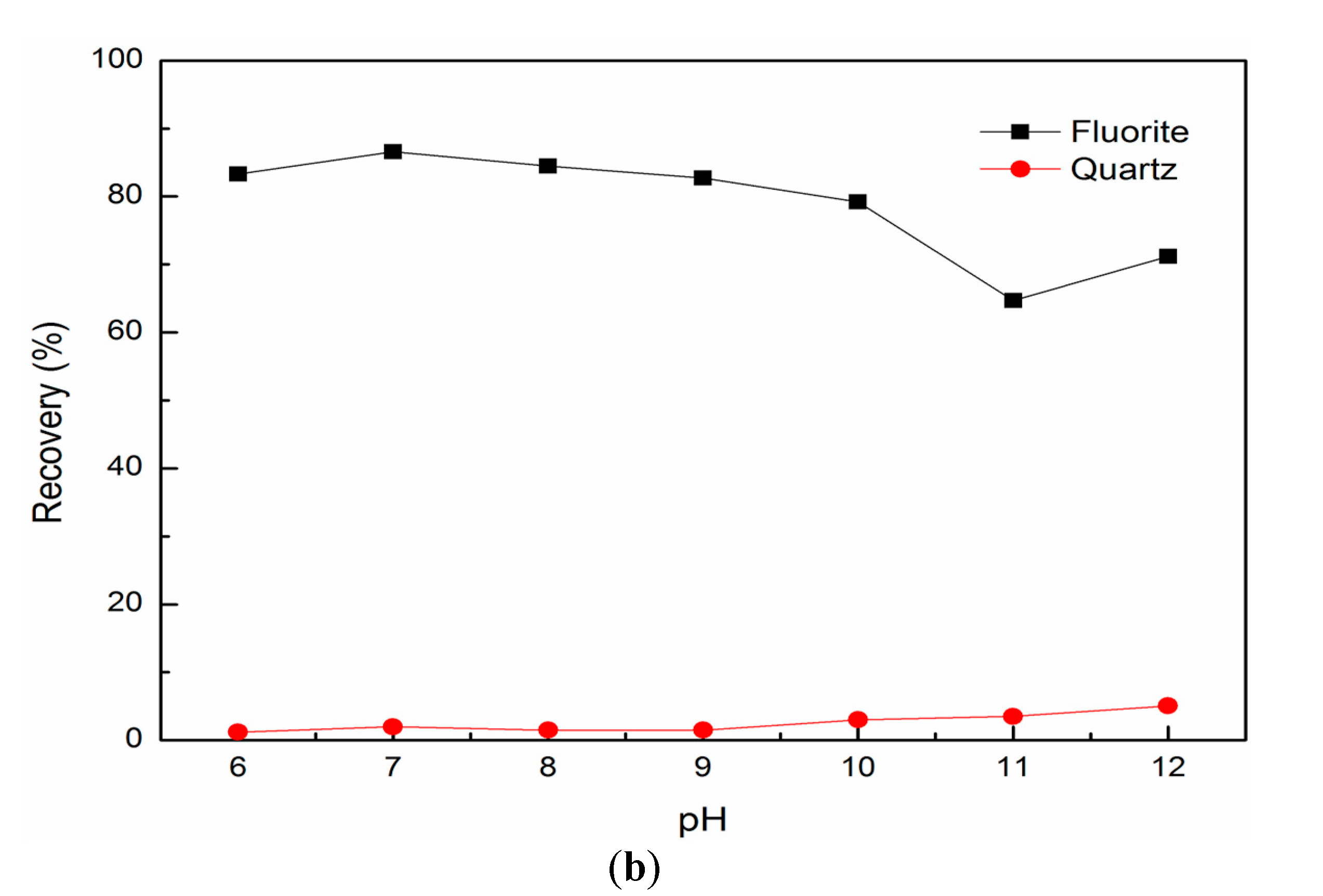
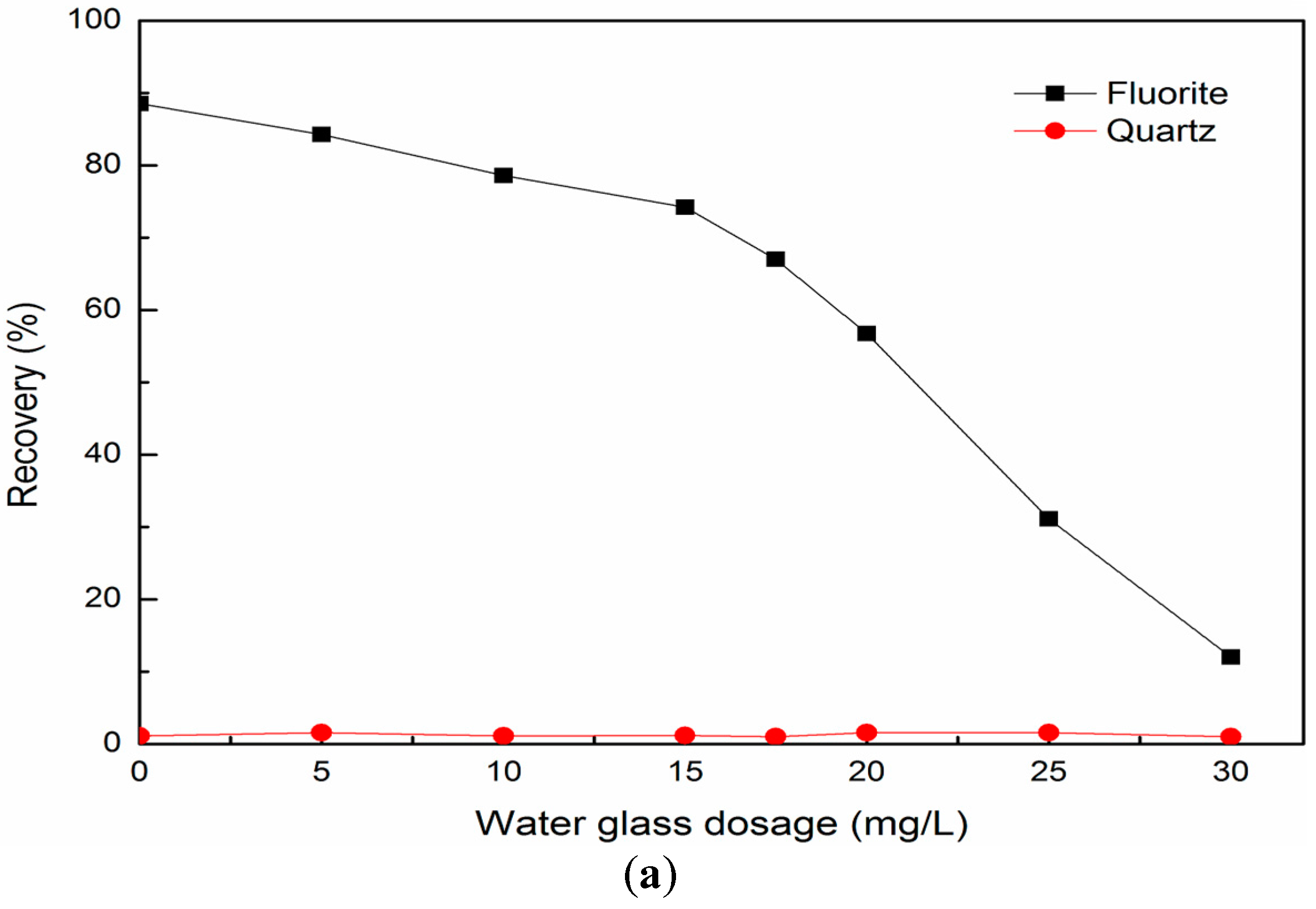
3.1. Single Mineral Flotation Results
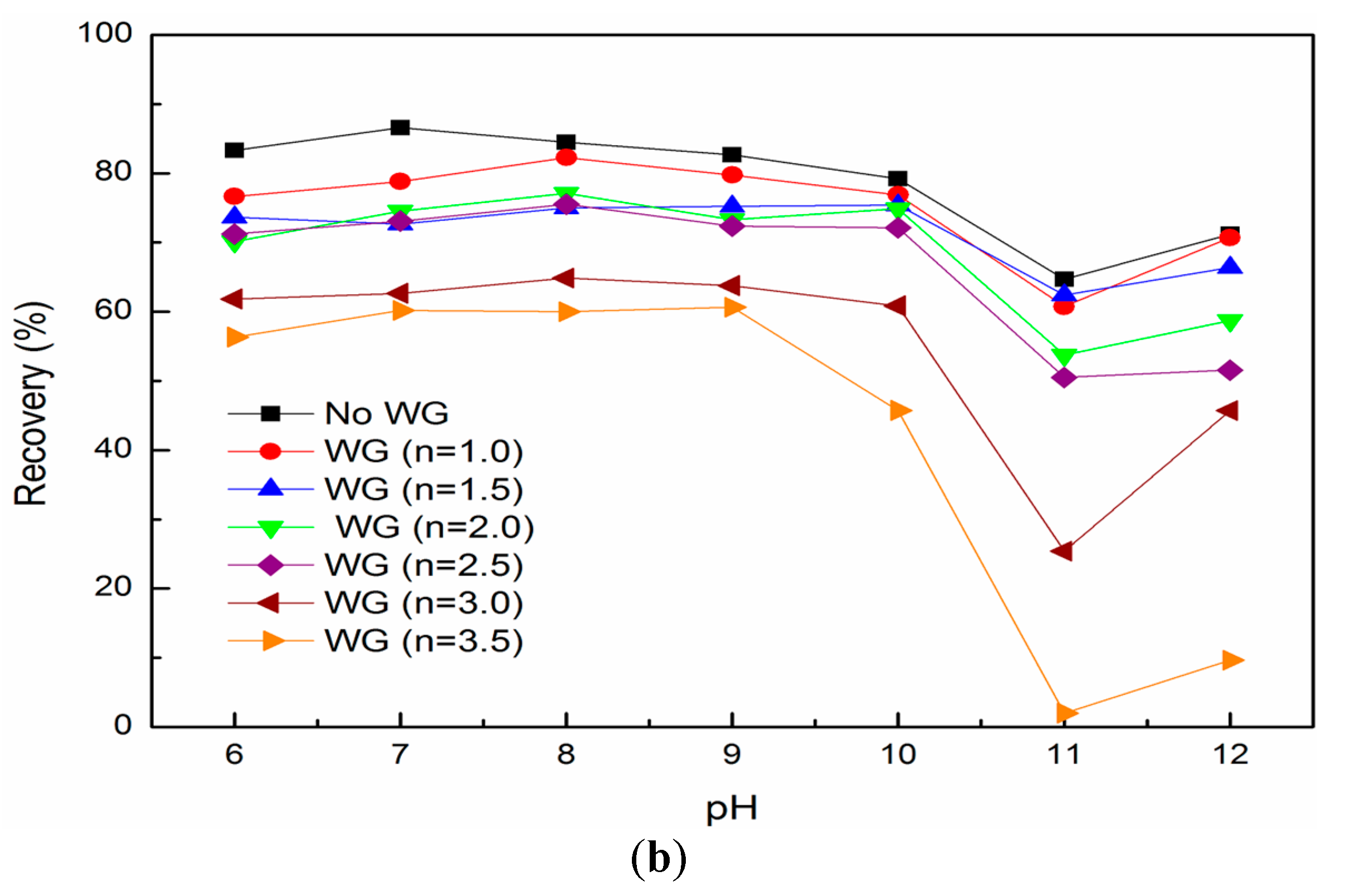
3.2. Mixed Binary Mineral Flotation Results
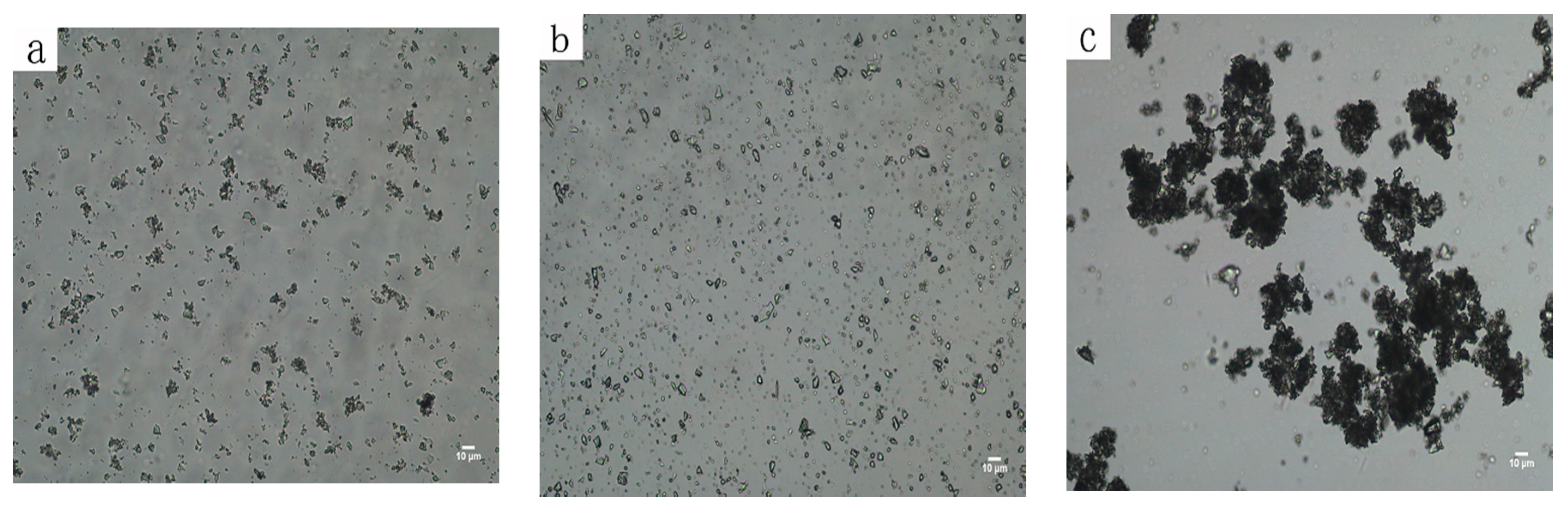
3.3. The Dispersion and Aggregation State of Mineral Particles in Pulp
3.4. The Hetero-Coagulation between Fine Fluorite and Quartz in Pulp
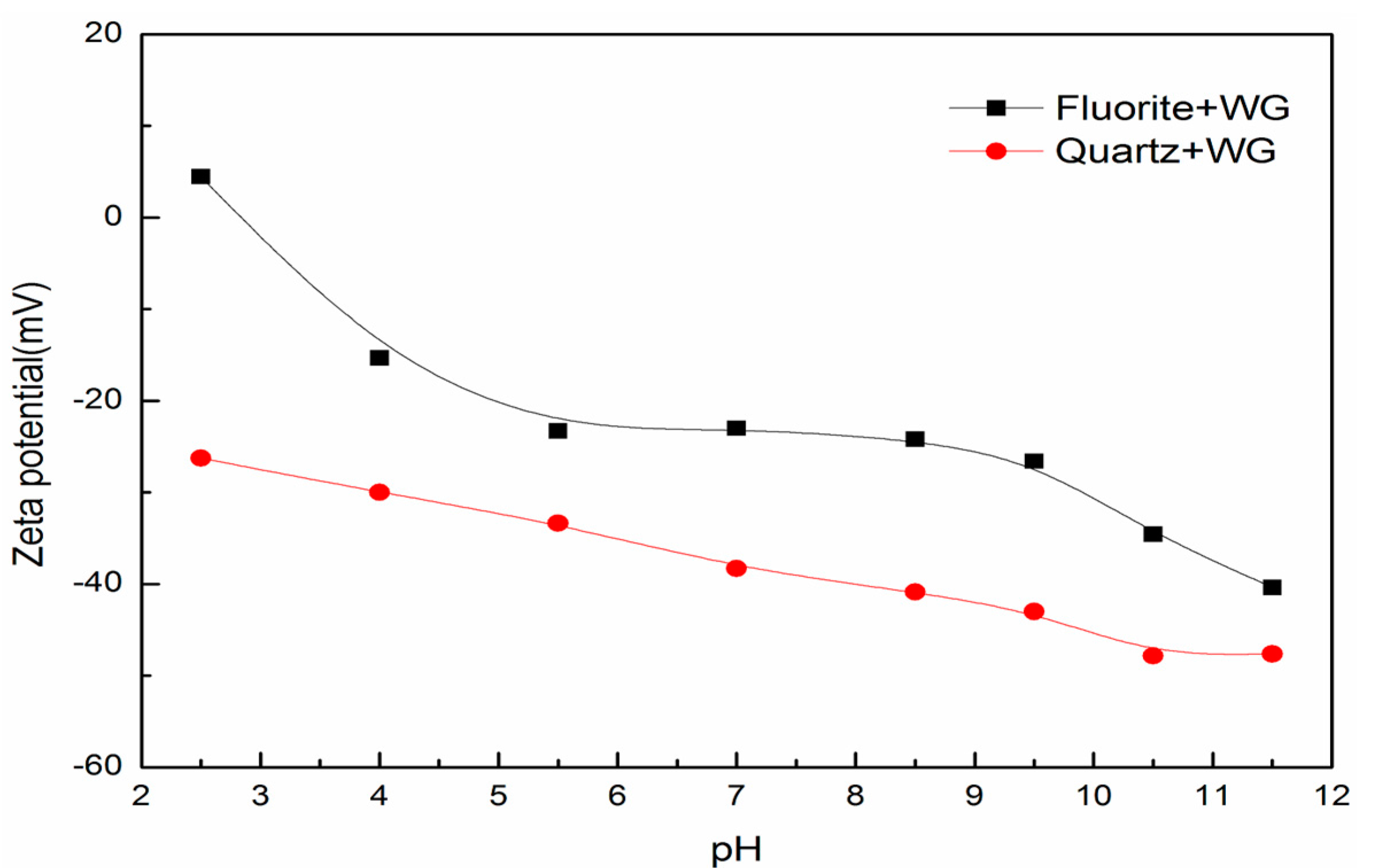
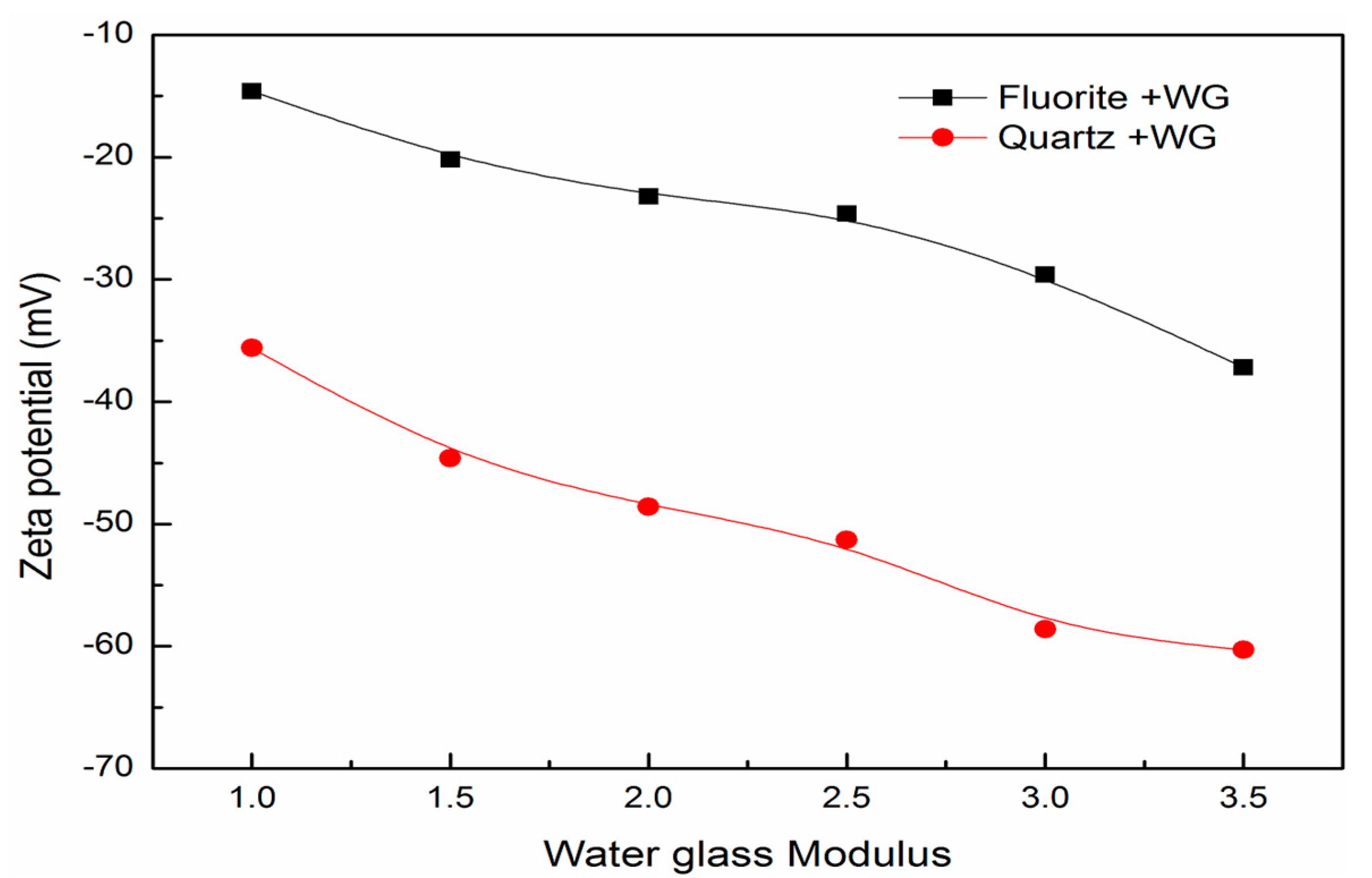
3.5. Surface Charge and Particle Interactions between Fluorite and Quartz
4. Conclusions
- (1)
- Fluorite readily floats but quartz does not float using sodium oleate as collector;
- (2)
- There exists strong hetero-coagulation between fine fluorite and quartz which decreases the flotation selectivity to a large extent;
- (3)
- The addition of water glass helps to depress the hetero-coagulation and then improves the separation efficiency in flotation of mixed binary minerals;
- (4)
- Water glass with higher modulus eliminates the hetero-coagulation more thoroughly through increasing the energy barrier between fine fluorite and quartz.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beger, J.; Schmidt, C.; Szymanowskib, J.; Barhoum, R. Surface activity and fluorite flotation with myristic acid derivatives containing oxygen and sulphur atoms. Colloids Surf. A Physicochem. Eng. Asp. 1993, 80, 197–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Free, M.L.; Miller, J.D. The significance of collector colloid adsorption phenomena in the fluorite/oleate flotation system as revealed by FTIR/IRS and solution chemistry analysis. Int. J. Miner. Process. 1996, 48, 197–216. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.V.; Miller, J.D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation. Int. J. Miner. Process. 2006, 81, 166–177. [Google Scholar] [CrossRef]
- Schubert, H.; Baldauf, H.; Kramer, W.; Schoenherr, J. Further development of fluorite flotation from ores containing higher calcite contents with oleoylsarcosine as collector. Int. J. Miner. Process. 1990, 30, 185–193. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Zhou, W.; Moreno, J.; Torres, R.; Valle, H.; Song, S. Flotation of fluorite from ores by using acidized water glass as depressant. Miner. Eng. 2013, 45, 142–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, J. Chemical Principle of Flotation Regents; Central South University of Technology Press: Changsha, China, 1996. [Google Scholar]
- Nordström, J.; Sundblom, A.; Jensen, G.V.; Pedersen, J.S.; Palmqvist, A.; Matic, A. Silica/alkali ratio dependence of the microscopic structure of sodium silicate solutions. J. Colloid Interface Sci. 2013, 397, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Arantes, R.S.; Lima, R.M.F. Influence of sodium silicate modulus on iron ore flotation with sodium oleate. Int. J. Miner. Process. 2013, 125, 157–160. [Google Scholar] [CrossRef]
- Zhang, G.F.; Cui, M.M.; Zhu, Y.G.; Shi, Q.; Luo, N. Effect of water glass on flotation separation of smithsonite and quartz. Chin. J. Nonferr. Met. 2012, 22, 3535–3540. [Google Scholar]
- Al-Thyabat, S. Empirical evaluation of the role of sodium silicate on the separation of silica from Jordanian siliceous phosphate. Sep. Purif. Technol. 2009, 67, 289–294. [Google Scholar] [CrossRef]
- Ou, L.M.; Huang, S.J.; Zhu, Y.G. Influence of metal ions on floatability of quartz in flotation of sulfide ores. J. Cent. South Univ. Sci. Technol. 2012, 43, 3–7. [Google Scholar]
- Kou, J.; Xu, S.; Sun, T.; Sun, C.; Guo, Y.; Wang, C. A study of sodium oleate adsorption on Ca2+ activated quartz surface using quartz crystal microbalance with dissipation. Int. J. Miner. Process. 2016, 154, 24–34. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite. Int. J. Miner. Process. 2005, 76, 75–81. [Google Scholar] [CrossRef]
- GÜler, T.; Akdemir, Ü. Statistical evaluation of flotation and entrainment behavior of an artificial ore. Trans. Nonferr. Met. Soc. China 2012, 22, 199–205. [Google Scholar] [CrossRef]
- Gong, J.; Peng, Y.; Bouajila, A.; Ourriban, M.; Yeung, A.; Liu, Q. Reducing quartz gangue entrainment in sulphide ore flotation by high molecular weight polyethylene oxide. Int. J. Miner. Process. 2010, 97, 44–51. [Google Scholar] [CrossRef]
- Kirjavainen, V.M. Review and analysis of factors controlling the mechanical flotation of gangue minerals. Int. J. Miner. Process. 1996, 46, 21–34. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y. Rheology measurements for flotation slurries with high clay contents—A critical review. Miner. Eng. 2016, 98, 137–150. [Google Scholar] [CrossRef]
- Liang, L.; Peng, Y.; Tan, J.; Xie, G. A review of the modern characterization techniques for flocs in mineral processing. Miner. Eng. 2015, 84, 130–144. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.; Li, Y.; Chen, J. The importance of mechanical scrubbing in magnetite-concentrate reverse-flotation. Miner. Eng. 2014, 69, 133–136. [Google Scholar] [CrossRef]


| Modulus | SiO2 | Na2O | Solids (wt %) | H2O (wt %) | Viscosity (mPa·s) |
|---|---|---|---|---|---|
| 1.0 | 23.8 | 23.8 | 47.6 | 52.4 | 2.06 |
| 1.5 | 23.04 | 15.36 | 38.4 | 61.6 | 4.62 |
| 2.0 | 21.6 | 10.8 | 32.4 | 67.6 | 21.46 |
| 2.5 | 28.93 | 11.57 | 40.5 | 59.5 | 127.57 |
| 3.0 | 20.67 | 6.92 | 27.68 | 72.32 | 10.15 |
| 3.5 | 27.9 | 8.1 | 34.6 | 65.4 | 75.16 |
| WG Modulus | No WG | n = 1.0 | n = 1.5 | n = 2.0 | n = 2.5 | n = 3.0 | n = 3.5 |
|---|---|---|---|---|---|---|---|
| Fluorite grade (%) | 61.80 | 65.12 | 68.40 | 70.08 | 73.56 | 77.01 | 79.10 |
| Fluorite recovery (%) | 79.12 | 78.34 | 77.00 | 76.62 | 76.69 | 74.00 | 69.64 |
| Concentration ratio | 1.24 | 1.31 | 1.38 | 1.42 | 1.47 | 1.54 | 1.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Gao, Y.; Chen, W.; Liu, D. The Role of Water Glass in the Flotation Separation of Fine Fluorite from Fine Quartz. Minerals 2017, 7, 157. https://doi.org/10.3390/min7090157
Zhang G, Gao Y, Chen W, Liu D. The Role of Water Glass in the Flotation Separation of Fine Fluorite from Fine Quartz. Minerals. 2017; 7(9):157. https://doi.org/10.3390/min7090157
Chicago/Turabian StyleZhang, Guofan, Yawen Gao, Wei Chen, and Dezhi Liu. 2017. "The Role of Water Glass in the Flotation Separation of Fine Fluorite from Fine Quartz" Minerals 7, no. 9: 157. https://doi.org/10.3390/min7090157





