Metal Recovery from the Mobile Phone Waste by Chemical and Biological Treatments
Abstract
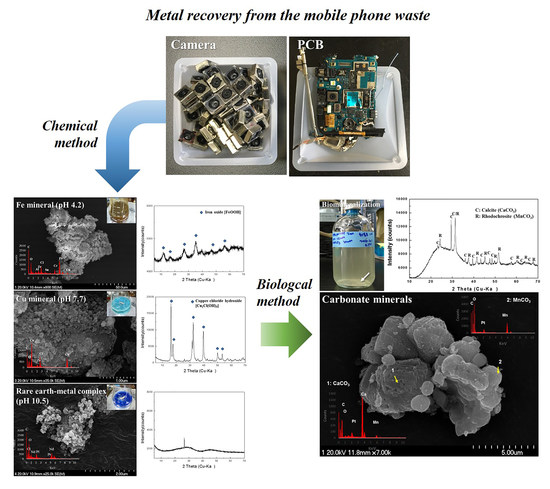
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Metal Solution Using Aqua Regia
2.2. Chemical Method for Metal Recovery
2.3. Biological Method for Metal Recovery
2.4. Analytical Methods
3. Results
3.1. Dissolution of Metals in Aqua Regia
3.2. Chemical Recovery of Copper, Iron, and Rare Earth-Metal Complex
3.3. Biological Recovery of Manganese and Calcium
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Union (EU). Waste Electrical & Electronic Equipment (WEEE). Available online: http://ec.europa.eu/environment/waste/weee/index_en.htm (accessed on 20 November 2017).
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.; Lee, J.; Pandey, B.D. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Ono, T.; Tsubota, M.; Kitagawa, J. Improved room-temperature-selectivity between Nd and Fe in Nd recovery from Nd-Fe-B magnet. AIP Adv. 2015, 5, 117212. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A. Study of pH influence on selective precipitation of heavy metals from acid mine drainage. Chem. Eng. Trans. 2011, 25, 345–350. [Google Scholar]
- Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Lin, C.S.; Hui, C.W.; Mckay, G. Waste printed circuit board recycling techniques an product utilization. J. Hazard. Mater. 2015, 283, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.A.; Ting, Y.P. Gold bioleaching of electronic waste by cyanogenic bacteria and its enhancement with bio-oxidation. Adv. Mater. Res. 2009, 71–73, 661–664. [Google Scholar] [CrossRef]
- Chi, T.D.; Lee, J.; Pandey, B.D. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Brandl, H.; Faramarzi, M.A. Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuology 2006, 4, 93–97. [Google Scholar] [CrossRef]
- Madrigal-Arias, J.E.; Argumedo-Delira, R.; Alarcón, A.; Mendoza-López, M.; García-Barradas, O.; Cruz-Sánchez, J.S.; Ferrera-Cerrato, R.; Jiménez-Fernández, M. Bioleaching of gold, copper and nickel from waste cellular phone PCBs and computer goldfinger motherboards by two Aspergillus nigerstrains. Braz. J. Microbiol. 2015, 46, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computermunching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.; Suh, Y.; Roh, Y. Characterization of magnetite-organic complex nanoparticles by metal-reducing bacteria. J. Nanosci. Nanotechnol. 2011, 11, 7242–7245. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Lee, J.; Hong, M.; Roh, Y. Metal reduction and biomineralization by bacteria enriched from intertidal flat sediments, Suncheon Bay, Korea. J. Geol. Soc. Korea 2011, 47, 19–30, (Abstract In English). [Google Scholar]
- Li, W.; Liu, L.-P.; Zhou, P.-P.; Cao, L.; Yu, L.-J.; Jiang, S.-Y. Calcite precipitation induced by bacteria and bacterially produced carbonic anhydrase. Curr. Sci. 2011, 100, 502–508. [Google Scholar]
- Kim, Y.; Oh, J.-M.; Jung, H.-Y.; Lee, S.Y.; Roh, Y. Characterization of microbial diversity of metal-reducing bacteria enriched from groundwater and reduction/biomineralization of iron and manganese. Econ. Environ. Geol. 2014, 47, 431–439. [Google Scholar] [CrossRef]
- Warren, L.A.; Maurice, P.A.; Parmar, N.; Ferris, F.G. Microbially mediated calcium carbonate precipitation: Implications for interpreting calcite precipitation and for solid phase capture of inorganic contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Seo, H.; Roh, Y. Metal Recovery from the Mobile Phone Waste by Chemical and Biological Treatments. Minerals 2018, 8, 8. https://doi.org/10.3390/min8010008
Kim Y, Seo H, Roh Y. Metal Recovery from the Mobile Phone Waste by Chemical and Biological Treatments. Minerals. 2018; 8(1):8. https://doi.org/10.3390/min8010008
Chicago/Turabian StyleKim, Yumi, Hyunhee Seo, and Yul Roh. 2018. "Metal Recovery from the Mobile Phone Waste by Chemical and Biological Treatments" Minerals 8, no. 1: 8. https://doi.org/10.3390/min8010008