Authigenic Mg-Clay Minerals Formation in Lake Margin Deposits (the Cerro de los Batallones, Madrid Basin, Spain)
Abstract
:1. Introduction
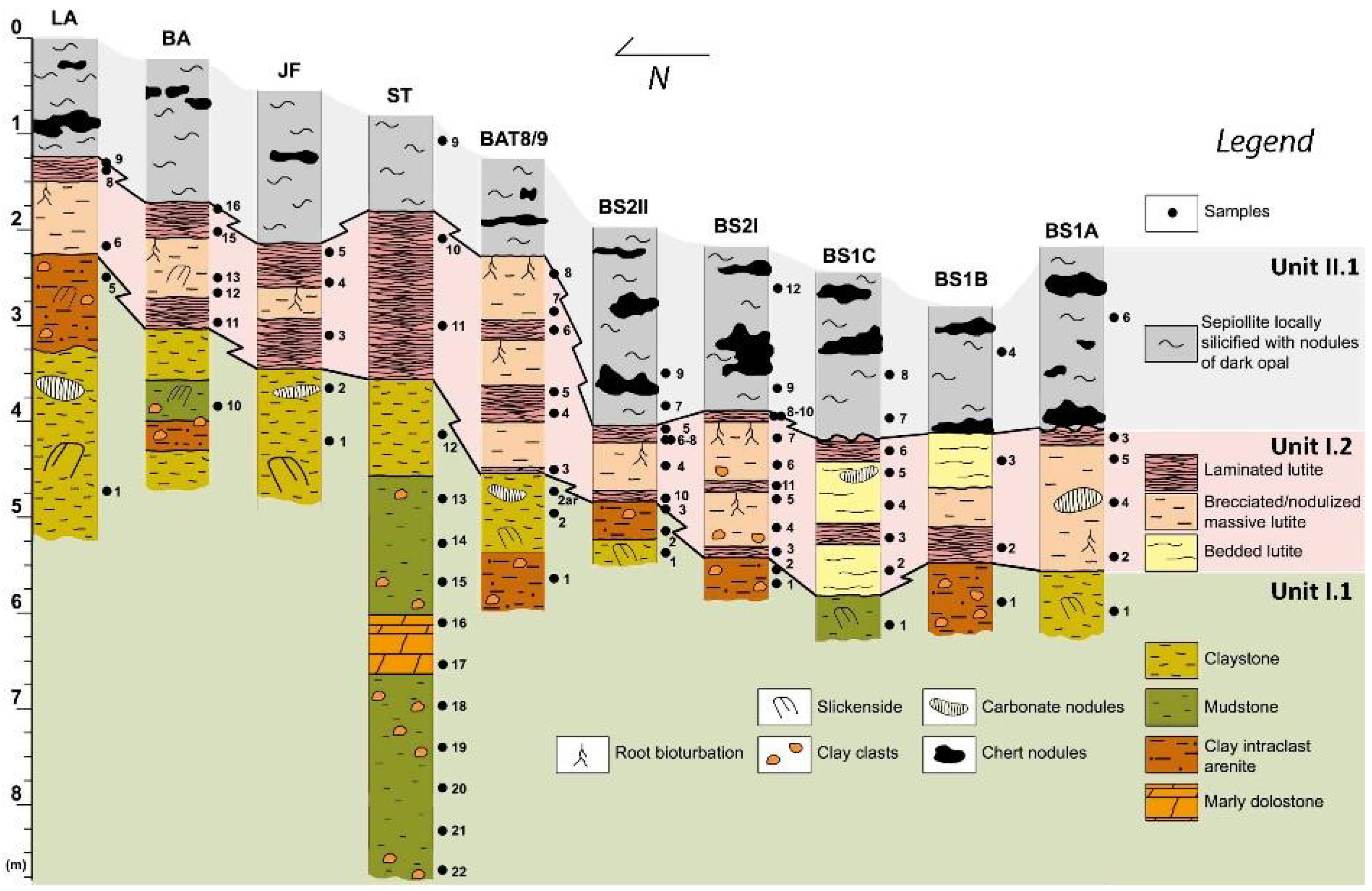
2. Geological Setting and Sampling
3. Analytical Methodology
4. Results and Discussion
4.1. Mineralogical and Chemical Characterization
4.1.1. Unit I.1
Claystone (I.1-A)
Mudstone (I.1-B)
Clay Intraclast Arenite (I.1-C)
Marly Dolostone (I.1-D)
4.1.2. Unit I.2
Laminated Lutite/Mudstone (I.2-A)
Brecciated/Nodulized Massive Lutite (I.2-B)
Bedded Lutite (I.2-C)
4.1.3. Unit II.1
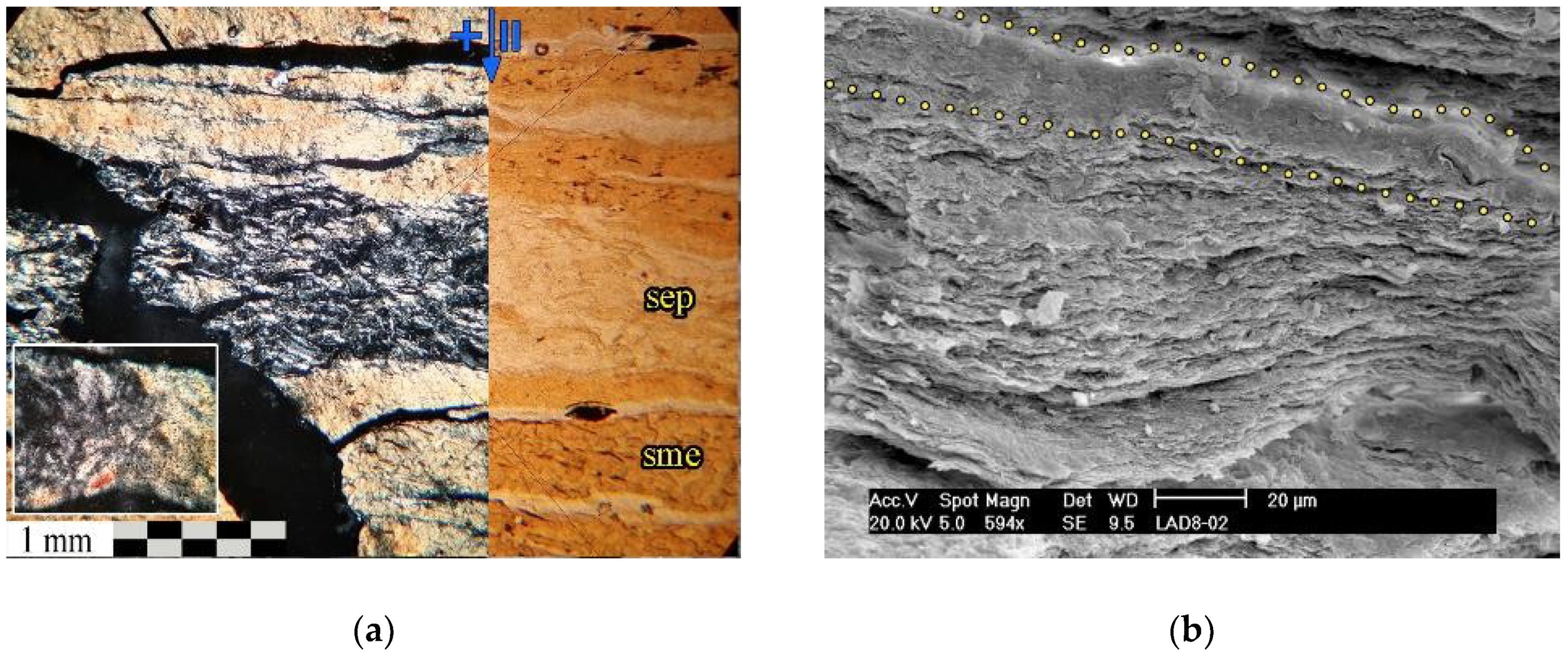
Sepiolite and Opal (II.1-A)
4.2. Mineralogical Considerations
4.2.1. Mg-Smectite Composition
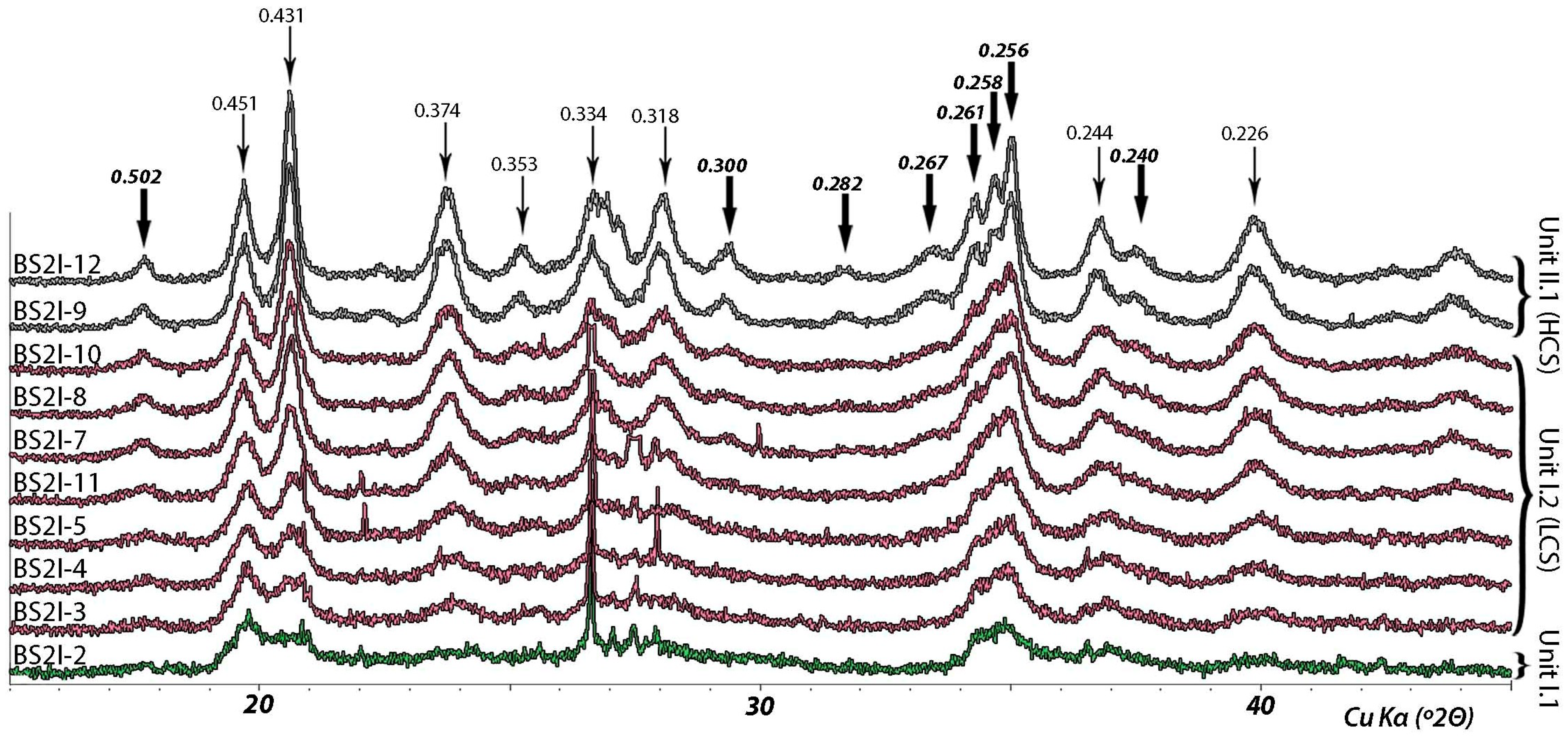
4.2.2. Sepiolite Features
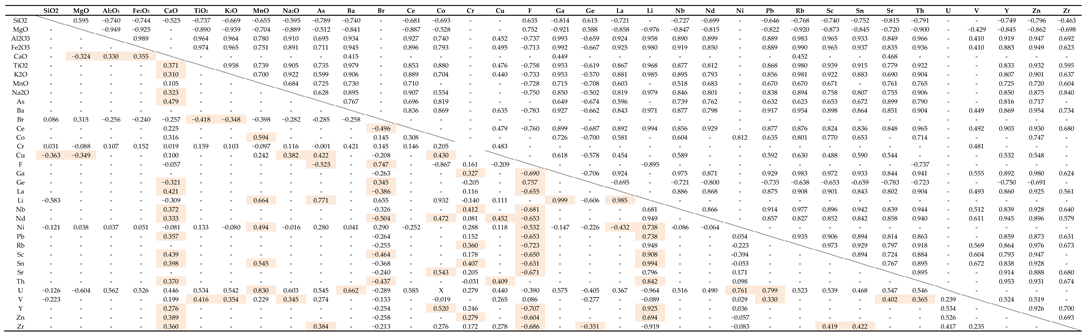
4.3. Geochemistry
Cluster Analysis
4.4. Mg-Clay Minerals Formation Model
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Lf | Sample | SiO2 | MgO | Al2O3 | Fe2O3 | CaO | TiO2 | K2O | MnO | P2O5 | Na2O | F | PPC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II.1-A | ST-9 | 58.12 | 22.18 | 0.6 | 0.17 | 0.08 | 0.05 | 0.12 | 0.02 | - | 0.03 | - | 18.62 |
| II.1-A | BS2-I-12 | 54.64 | 22.14 | 1.53 | 0.54 | 0.37 | <0.1 | <0.4 | <0.05 | <0.045 | 0.09 | - | 19.02 |
| II.1-A | BS2-I-9 | 55.86 | 23.58 | 0.33 | 0.12 | 0.1 | <0.1 | <0.4 | <0.05 | <0.045 | 0.04 | - | 18.76 |
| I.2-A | LA-9 * | 57.25 | 18.909 | 4.45 | 1.654 | 0.552 | 0.191 | 0.767 | 0.021 | - | 0.175 | 0.51 | 15.89 |
| I.2-A | BA-16 * | 54.68 | 21.878 | 0.25 | 0.305 | 1.995 | 0.051 | 0.158 | 0.008 | - | 0.06 | 0.52 | 20.17 |
| I.2-A | BA-15 * | 58.45 | 18.155 | 4.07 | 1.094 | 0.171 | 0.177 | 0.726 | 0.049 | - | 0.192 | 0.35 | 16.56 |
| I.2-A | BA-11 * | 53.46 | 17.284 | 6.49 | 2.194 | 0.413 | 0.267 | 1.149 | 0.04 | - | 0.211 | 0.46 | 17.97 |
| I.2-A | JF-4 * | 56.33 | 22.21 | 1.29 | 0.279 | 0.807 | 0.053 | 0.133 | 0.015 | - | 0.067 | 0.68 | 18.81 |
| I.2-A | JF-2 * | 55.34 | 21.049 | 2.6 | 0.769 | 0.136 | 0.1 | 0.356 | 0.019 | - | 0.094 | 0.49 | 19.51 |
| I.2-A | COSTRA2 * | 55.88 | 20.26 | 2.98 | 1.04 | 0.24 | 0.13 | 0.59 | 0.01 | - | 0.13 | 0.44 | 18.69 |
| I.2-A | ST-10 | 52.52 | 15.06 | 8.01 | 2.61 | 0.47 | 0.32 | 1.54 | 0.03 | - | 0.28 | - | 19.13 |
| I.2-A | ST-11 | 53.15 | 15.66 | 7.59 | 2.35 | 0.47 | 0.29 | 1.33 | 0.03 | - | 0.29 | - | 18.81 |
| I.2-A | B8/9-6 * | 55.04 | 20.315 | 3.24 | 1.01 | 0.295 | 0.155 | 0.592 | 0.025 | - | 0.135 | 0.12 | 19.08 |
| I.2-A | B8/9-4 * | 51.95 | 15.55 | 6.89 | 2.152 | 0.34 | 0.286 | 1.151 | 0.044 | - | 0.255 | 0.21 | 21.18 |
| I.2-A | B8/9-3 * | 54.8 | 20.598 | 2.9 | 0.897 | 0.215 | 0.141 | 0.512 | 0.02 | - | 0.121 | 0.51 | 19.31 |
| I.2-A | BS2-I-8 | 55.19 | 20.61 | 2.77 | 0.86 | 0.12 | 0.11 | 0.51 | <0.05 | <0.045 | 0.15 | - | 18.1 |
| I.2-A | BS2-I-11 | 53.11 | 14.99 | 7.57 | 2.88 | 0.21 | 0.29 | 1.59 | <0.05 | <0.045 | 0.2 | - | 17.42 |
| I.2-A | BS2-I-5 | 52.69 | 15.02 | 8.04 | 2.66 | 0.3 | 0.27 | 1.54 | 0.05 | <0.045 | 0.2 | - | 17.74 |
| I.2-A | BS2-I-3 | 49.53 | 10.4 | 10.89 | 3.52 | 0.43 | 0.38 | 1.95 | <0.05 | <0.045 | 0.32 | - | 21.9 |
| I.2-A | BS-1B-2 | 56.27 | 16.48 | 5.34 | 1.44 | 0.26 | 0.23 | 1.29 | <0.05 | <0.045 | 0.32 | - | 17.82 |
| I.2-B | B8/9-8 * | 55.29 | 22.791 | 1.4 | 0.459 | 0.041 | 0.084 | 0.247 | 0.02 | - | 0.065 | 0.7 | 18.99 |
| I.2-B | BS2-I-7 | 54.99 | 21.2 | 2.69 | 0.85 | 0.11 | 0.1 | 0.49 | <0.05 | <0.045 | 0.11 | - | 17.98 |
| I.2-B | BS2-I-6 | 54.12 | 18.61 | 4.78 | 1.56 | 0.13 | 0.17 | 0.88 | <0.05 | <0.045 | 0.13 | - | 18.36 |
| I.2-B | BS2-I-4 | 52.87 | 11.56 | 10.31 | 3.45 | 0.28 | 0.36 | 2.1 | <0.05 | <0.045 | 0.24 | - | 17.78 |
| I.2-C | BS-1C-4 | 54.78 | 20.18 | 2.22 | 0.73 | 0.95 | <0.1 | 0.4 | <0.05 | 0.051 | 0.08 | - | 19.18 |
| I.2-C | BS-1C-2 | 54.56 | 16.38 | 6.25 | 1.77 | 0.26 | 0.26 | 1.38 | <0.05 | <0.045 | 0.31 | - | 17.38 |
| I.2-C | BS-1B-3 | 62.28 | 17.31 | 1.77 | 0.57 | 0.2 | <0.1 | 0.26 | <0.05 | <0.045 | 0.07 | - | 15.5 |
| I.1-A | LA-1 * | 52.06 | 14.42 | 10.61 | 3.98 | 1.01 | 0.4 | 1.92 | 0.047 | - | 0.31 | 0.23 | 15.1 |
| I.1-A | JF-1 * | 54.83 | 16.633 | 6.67 | 2.219 | 0.319 | 0.284 | 1.349 | 0.04 | - | 0.249 | 0.29 | 17.11 |
| I.1-A | ST-12 | 49.98 | 18.1 | 7.01 | 2.36 | 1.63 | 0.28 | 0.95 | 0.04 | - | 0.21 | - | 19.43 |
| I.1-A | B8/9-2ar * | 48.24 | 13.031 | 10.66 | 3.61 | 0.682 | 0.402 | 1.663 | 0.057 | - | 0.31 | 0.11 | 21.18 |
| I.1-B | BA-10 * | 52.48 | 11.689 | 9.85 | 3.984 | 0.686 | 0.427 | 2.634 | 0.043 | - | 0.512 | 0.13 | 17.53 |
| I.1-B | ST-13 | 50.35 | 16.56 | 8.91 | 3.2 | 0.9 | 0.38 | 1.49 | 0.05 | - | 0.28 | - | 17.81 |
| I.1-B | ST-14 | 50.83 | 13.76 | 10.15 | 3.55 | 1.1 | 0.44 | 1.84 | 0.05 | - | 0.36 | - | 17.84 |
| I.1-B | ST-15 | 51.66 | 11.76 | 12.05 | 4.45 | 0.5 | 0.57 | 2.93 | 0.04 | - | 0.37 | - | 15.64 |
| I.1-B | ST-18 | 45.65 | 10.05 | 12.76 | 4.77 | 4.17 | 0.6 | 2.67 | 0.04 | - | 0.4 | - | 18.86 |
| I.1-B | ST-19 | 50.91 | 9.83 | 14.37 | 5.33 | 0.67 | 0.74 | 3.2 | 0.05 | - | 0.57 | - | 14.28 |
| I.1-B | ST-20 | 50.71 | 11.51 | 13.59 | 5.03 | 0.7 | 0.68 | 2.84 | 0.05 | - | 0.62 | - | 14.2 |
| I.1-B | ST-21 | 50.59 | 17.03 | 8.87 | 3.32 | 0.69 | 0.42 | 1.54 | 0.05 | - | 0.28 | - | 17.18 |
| I.1-B | ST-22 | 49.84 | 16.9 | 9.28 | 3.41 | 1.01 | 0.45 | 1.65 | 0.05 | - | 0.27 | - | 17.1 |
| I.1-B | BS-1C-1 | 54.03 | 13.06 | 7.86 | 2.22 | 2.1 | 0.33 | 1.86 | <0.05 | <0.045 | 0.46 | - | 16.55 |
| I.1-C | LA-5 * | 53.88 | 6.47 | 15.71 | 5.58 | 0.88 | 0.59 | 2.83 | 0.04 | - | 0.55 | 0.22 | 13.39 |
| I.1-C | B8/9-1 * | 51.04 | 12.58 | 10.48 | 3.23 | 0.648 | 0.409 | 1.72 | 0.043 | - | 0.44 | 0.1 | 19.36 |
| I.1-C | BS2-I-1 | 50.44 | 12.13 | 12.45 | 3.94 | 0.69 | 0.46 | 2.28 | 0.05 | 0.07 | 0.48 | - | 14.5 |
| I.1-C | BS-1B-1 | 53.3 | 13.49 | 8.48 | 2.77 | 0.33 | 0.29 | 1.81 | <0.05 | <0.045 | 0.27 | - | 17.87 |
| Lithofacies | As | Ba | Br | Ce | Co | Cr | Cs | Cu | Ga | Ge | Hf | I | La | Li | Nb | Nd | Ni | Pb | Rb | Sc | Sn | Sr | Th | U | V | Y | Zn | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | ||||||||||||||||||||||||||||
| Max. | 17.5 | 322.6 | 44.7 | 83.9 | 9.9 | 33.3 | 7.4 | 17.1 | 23.2 | 4.0 | 4.9 | 10.1 | 37.3 | 207.0 | 19.3 | 35.7 | 19.0 | 19.0 | 178.5 | 12.8 | 11.0 | 161.6 | 18.0 | 2.5 | 91.1 | 31.0 | 89.3 | 120.7 |
| Min. | 0.8 | 20.1 | 1.1 | 5.9 | 1.2 | 3.2 | 7.3 | 0.8 | 1.3 | 0.6 | 3.2 | 5.9 | 0.9 | 45.0 | 1.3 | 4.3 | 1.8 | 1.9 | 3.4 | 1.0 | 2.2 | 5.0 | 0.9 | 0.6 | 21.1 | 1.9 | 2.9 | 5.9 |
| Mean | 4.8 | 126.8 | 5.3 | 42.4 | 4.9 | 13.1 | 7.4 | 8.0 | 11.4 | 2.2 | 4.0 | 7.8 | 19.3 | 99.3 | 9.0 | 20.4 | 8.1 | 9.4 | 74.1 | 7.1 | 5.3 | 63.3 | 9.4 | 1.4 | 46.9 | 14.0 | 41.2 | 50.7 |
| Samples | 27 | 39 | 27 | 35 | 23 | 36 | 2 | 37 | 36 | 32 | 4 | 3 | 36 | 8 | 37 | 30 | 22 | 39 | 37 | 30 | 30 | 37 | 36 | 8 | 41 | 38 | 42 | 37 |
| II1A | ||||||||||||||||||||||||||||
| Max. | 2.8 | 28.5 | 3.6 | 4.9 | 2.0 | 1.9 | 3.8 | 7.3 | 5.9 | 1.3 | 2.9 | 12.7 | 10.3 | 31.9 | 2.1 | 8.9 | 11.3 | |||||||||||
| Min. | 2.8 | 20.1 | 1.6 | 4.9 | 2.0 | 1.3 | 2.1 | 7.3 | 5.9 | 1.3 | 2.9 | 3.4 | 5.0 | 22.9 | 1.9 | 2.9 | 5.9 | |||||||||||
| Mean | 2.8 | 23.0 | 2.4 | 4.9 | 2.0 | 1.6 | 2.8 | 7.3 | 5.9 | 1.3 | 2.9 | 7.8 | 8.4 | 27.4 | 2.0 | 6.0 | 8.6 | |||||||||||
| Samples | 1 | 3 | 3 | 0 | 0 | 1 | 0 | 1 | 2 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 3 | 3 |
| I2A | ||||||||||||||||||||||||||||
| Max. | 5.9 | 160.0 | 8.2 | 48.9 | 5.0 | 33.3 | 7.3 | 17.1 | 18.2 | 3.6 | 3.9 | 10.1 | 28.0 | 118.0 | 12.9 | 22.6 | 17.3 | 14.4 | 117.8 | 8.5 | 7.4 | 86.5 | 14.3 | 2.5 | 91.1 | 21.4 | 61.4 | 94.2 |
| Min. | 0.8 | 21.0 | 1.5 | 7.0 | 1.2 | 3.2 | 7.3 | 3.9 | 4.0 | 1.4 | 3.9 | 10.1 | 0.9 | 45.0 | 3.0 | 6.5 | 1.8 | 1.9 | 24.6 | 1.0 | 2.2 | 15.9 | 0.9 | 0.6 | 25.5 | 3.0 | 14.0 | 24.6 |
| Mean | 2.8 | 93.5 | 5.3 | 32.1 | 3.1 | 13.9 | 7.3 | 8.6 | 9.5 | 2.2 | 3.9 | 10.1 | 14.4 | 78.3 | 7.2 | 16.5 | 7.8 | 7.5 | 62.4 | 5.1 | 4.3 | 47.0 | 6.9 | 1.2 | 44.9 | 11.8 | 33.5 | 48.4 |
| Samples | 13 | 13 | 12 | 13 | 6 | 14 | 1 | 14 | 12 | 12 | 1 | 1 | 12 | 6 | 13 | 11 | 13 | 15 | 12 | 11 | 12 | 12 | 15 | 5 | 16 | 13 | 16 | 12 |
| I2B | ||||||||||||||||||||||||||||
| Max. | 3.8 | 120.9 | 44.7 | 42.2 | 3.3 | 16.5 | 7.4 | 4.9 | 17.2 | 4.0 | 5.9 | 25.3 | 11.6 | 21.1 | 13.9 | 10.9 | 107.6 | 9.5 | 6.9 | 55.5 | 10.8 | 0.9 | 57.6 | 13.3 | 49.9 | 46.3 | ||
| Min. | 1.5 | 32.9 | 1.6 | 5.9 | 3.3 | 4.0 | 7.4 | 0.8 | 2.7 | 2.0 | 5.9 | 1.2 | 1.3 | 4.3 | 2.9 | 2.5 | 12.3 | 2.0 | 2.4 | 9.0 | 1.4 | 0.9 | 21.1 | 3.9 | 7.9 | 22.0 | ||
| Mean | 3.1 | 64.6 | 13.2 | 22.7 | 3.3 | 9.9 | 7.4 | 2.9 | 7.8 | 2.8 | 5.9 | 13.2 | 5.3 | 11.7 | 7.1 | 6.4 | 48.8 | 5.8 | 3.9 | 25.1 | 5.2 | 0.9 | 36.0 | 7.4 | 25.3 | 29.4 | ||
| Samples | 4 | 4 | 4 | 4 | 1 | 4 | 1 | 4 | 4 | 4 | 0 | 1 | 4 | 0 | 4 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 4 | 1 | 4 | 4 | 4 | 4 |
| I2C | ||||||||||||||||||||||||||||
| Max. | 3.9 | 115.6 | 2.0 | 41.3 | 3.0 | 4.7 | 3.7 | 8.5 | 3.6 | 8.7 | 7.0 | 11.7 | 3.1 | 6.3 | 63.1 | 5.1 | 3.1 | 36.1 | 8.4 | 71.0 | 10.6 | 39.8 | 70.9 | |||||
| Min. | 3.9 | 37.3 | 1.5 | 10.4 | 2.8 | 4.7 | 2.9 | 2.7 | 2.4 | 5.7 | 1.8 | 11.7 | 3.1 | 2.1 | 14.5 | 5.1 | 3.1 | 16.3 | 2.0 | 36.5 | 4.3 | 7.9 | 14.7 | |||||
| Mean | 3.9 | 65.9 | 1.7 | 25.8 | 2.9 | 4.7 | 3.3 | 5.0 | 3.2 | 7.1 | 3.7 | 11.7 | 3.1 | 4.2 | 32.7 | 5.1 | 3.1 | 24.3 | 5.2 | 52.6 | 6.9 | 19.9 | 35.2 | |||||
| Samples | 1 | 3 | 3 | 2 | 2 | 1 | 0 | 2 | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 1 | 1 | 3 | 3 | 1 | 1 | 3 | 2 | 0 | 3 | 3 | 3 | 3 |
| I1A | ||||||||||||||||||||||||||||
| Max. | 17.5 | 208.0 | 9.2 | 63.1 | 3.7 | 20.0 | 12.4 | 17.9 | 2.7 | 21.5 | 117.0 | 16.7 | 33.3 | 19.0 | 13.0 | 108.4 | 9.0 | 8.0 | 161.6 | 16.4 | 2.5 | 73.0 | 31.0 | 68.1 | 65.3 | |||
| Min. | 3.5 | 118.3 | 1.6 | 33.5 | 3.7 | 6.5 | 4.0 | 10.0 | 0.6 | 10.7 | 117.0 | 6.8 | 14.9 | 18.0 | 8.1 | 62.7 | 4.7 | 4.2 | 40.4 | 8.1 | 1.6 | 31.3 | 10.4 | 38.8 | 48.1 | |||
| Mean | 10.5 | 153.2 | 5.4 | 46.7 | 3.7 | 13.8 | 8.7 | 13.0 | 1.7 | 15.7 | 117.0 | 10.3 | 21.7 | 18.5 | 10.2 | 80.5 | 6.2 | 5.7 | 102.1 | 11.6 | 2.1 | 52.4 | 17.4 | 50.3 | 55.7 | |||
| Samples | 2 | 3 | 2 | 3 | 1 | 3 | 0 | 3 | 3 | 2 | 0 | 0 | 3 | 1 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 |
| I1B | ||||||||||||||||||||||||||||
| Max. | 11.8 | 322.6 | 1.5 | 76.0 | 9.9 | 22.6 | 15.6 | 23.2 | 2.1 | 4.9 | 37.3 | 207.0 | 19.3 | 31.5 | 4.1 | 19.0 | 178.5 | 12.8 | 11.0 | 123.8 | 18.0 | 63.6 | 26.7 | 89.3 | 111.7 | |||
| Min. | 6.8 | 175.0 | 1.1 | 47.3 | 3.0 | 6.3 | 3.3 | 11.1 | 1.1 | 4.9 | 20.2 | 207.0 | 9.8 | 20.6 | 4.1 | 10.6 | 86.4 | 6.9 | 4.5 | 68.5 | 11.0 | 45.1 | 17.4 | 44.9 | 52.9 | |||
| Mean | 9.7 | 227.2 | 1.3 | 58.3 | 7.1 | 15.1 | 10.3 | 17.6 | 1.4 | 4.9 | 30.4 | 207.0 | 14.1 | 25.5 | 4.1 | 14.3 | 125.7 | 9.7 | 7.1 | 104.6 | 14.1 | 51.4 | 20.6 | 68.2 | 71.6 | |||
| Samples | 4 | 10 | 2 | 10 | 10 | 10 | 0 | 10 | 9 | 7 | 1 | 0 | 10 | 1 | 10 | 9 | 1 | 10 | 9 | 10 | 9 | 9 | 9 | 0 | 10 | 10 | 10 | 9 |
| I1C | ||||||||||||||||||||||||||||
| Max. | 10.6 | 189.2 | 2.4 | 83.9 | 4.5 | 11.9 | 9.6 | 18.2 | 2.4 | 3.9 | 34.6 | 13.2 | 35.7 | 7.1 | 16.5 | 114.2 | 9.8 | 8.1 | 148.6 | 17.9 | 81.7 | 28.7 | 72.0 | 120.7 | ||||
| Min. | 3.5 | 118.1 | 2.4 | 48.9 | 2.9 | 10.4 | 7.3 | 13.6 | 2.4 | 3.2 | 22.4 | 9.3 | 20.1 | 3.6 | 9.0 | 88.3 | 7.3 | 5.4 | 52.9 | 9.9 | 41.3 | 13.0 | 44.1 | 54.3 | ||||
| Mean | 7.1 | 157.5 | 2.4 | 67.6 | 3.8 | 11.1 | 8.8 | 16.1 | 2.4 | 3.5 | 29.9 | 11.5 | 30.4 | 5.3 | 13.0 | 101.4 | 8.6 | 6.7 | 110.2 | 14.7 | 58.9 | 22.3 | 60.9 | 78.8 | ||||
| Samples | 2 | 3 | 1 | 3 | 3 | 3 | 0 | 3 | 3 | 1 | 2 | 0 | 3 | 0 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 0 | 3 | 3 | 3 | 3 |
| Lithofacies | As | Ba | Br | Ce | Co | Cr | Cs | Cu | Ga | Ge | Hf | I | La | Li | Nb | Nd | Ni | Pb | Rb | Sc | Sn | Sr | Th | U | V | Y | Zn | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | ||||||||||||||||||||||||||||
| Max. | 3.65 | 0.51 | 27.94 | 1.33 | 0.57 | 0.36 | 1.51 | 0.61 | 1.33 | 2.86 | 0.92 | 7.22 | 1.20 | 8.63 | 1.61 | 1.32 | 0.40 | 1.12 | 2.13 | 0.91 | 5.24 | 0.51 | 1.71 | 0.93 | 0.94 | 1.48 | 1.33 | 0.63 |
| Min. | 0.17 | 0.03 | 0.68 | 0.09 | 0.07 | 0.03 | 1.49 | 0.03 | 0.07 | 0.43 | 0.60 | 4.22 | 0.03 | 1.88 | 0.11 | 0.16 | 0.04 | 0.11 | 0.04 | 0.07 | 1.05 | 0.02 | 0.09 | 0.22 | 0.22 | 0.09 | 0.04 | 0.03 |
| Mean | 1.00 | 0.20 | 3.34 | 0.67 | 0.29 | 0.14 | 1.50 | 0.29 | 0.65 | 1.61 | 0.75 | 5.54 | 0.62 | 4.14 | 0.75 | 0.76 | 0.17 | 0.55 | 0.88 | 0.51 | 2.54 | 0.20 | 0.90 | 0.51 | 0.48 | 0.67 | 0.62 | 0.26 |
| Samples | 27 | 39 | 27 | 35 | 23 | 36 | 2 | 37 | 36 | 32 | 4 | 3 | 36 | 8 | 37 | 30 | 22 | 39 | 37 | 30 | 30 | 37 | 36 | 8 | 41 | 38 | 42 | 37 |
| II1A | ||||||||||||||||||||||||||||
| Max. | 0.58 | 0.05 | 2.25 | 0.05 | 0.07 | 0.11 | 2.73 | 5.19 | 0.19 | 0.11 | 0.17 | 0.15 | 0.03 | 0.33 | 0.10 | 0.13 | 0.06 | |||||||||||
| Min. | 0.58 | 0.03 | 0.99 | 0.05 | 0.07 | 0.07 | 1.50 | 5.19 | 0.19 | 0.11 | 0.17 | 0.04 | 0.02 | 0.24 | 0.09 | 0.04 | 0.03 | |||||||||||
| Mean | 0.58 | 0.04 | 1.49 | 0.05 | 0.07 | 0.09 | 2.03 | 5.19 | 0.19 | 0.11 | 0.17 | 0.09 | 0.03 | 0.28 | 0.10 | 0.09 | 0.04 | |||||||||||
| Samples | 1 | 3 | 3 | 0 | 0 | 1 | 0 | 1 | 2 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 3 | 3 |
| I2A | ||||||||||||||||||||||||||||
| Max. | 1.23 | 0.25 | 5.13 | 0.78 | 0.29 | 0.36 | 1.49 | 0.61 | 1.04 | 2.60 | 0.73 | 7.22 | 0.90 | 4.92 | 1.08 | 0.84 | 0.37 | 0.84 | 1.40 | 0.61 | 3.54 | 0.27 | 1.37 | 0.93 | 0.94 | 1.02 | 0.92 | 0.49 |
| Min. | 0.17 | 0.03 | 0.94 | 0.11 | 0.07 | 0.03 | 1.49 | 0.14 | 0.23 | 0.96 | 0.73 | 7.22 | 0.03 | 1.88 | 0.25 | 0.24 | 0.04 | 0.11 | 0.29 | 0.07 | 1.05 | 0.05 | 0.09 | 0.22 | 0.26 | 0.14 | 0.21 | 0.13 |
| Mean | 0.58 | 0.15 | 3.31 | 0.51 | 0.18 | 0.15 | 1.49 | 0.31 | 0.54 | 1.60 | 0.73 | 7.22 | 0.46 | 3.26 | 0.60 | 0.61 | 0.17 | 0.44 | 0.74 | 0.36 | 2.03 | 0.15 | 0.65 | 0.45 | 0.46 | 0.56 | 0.50 | 0.25 |
| Samples | 13 | 13 | 12 | 13 | 6 | 14 | 1 | 14 | 12 | 12 | 1 | 1 | 12 | 6 | 13 | 11 | 13 | 15 | 12 | 11 | 12 | 12 | 15 | 5 | 16 | 13 | 16 | 12 |
| I2B | ||||||||||||||||||||||||||||
| Max. | 0.80 | 0.19 | 27.94 | 0.67 | 0.19 | 0.18 | 1.51 | 0.17 | 0.98 | 2.86 | 4.22 | 0.82 | 0.97 | 0.78 | 0.30 | 0.64 | 1.28 | 0.68 | 3.30 | 0.17 | 1.03 | 0.33 | 0.59 | 0.64 | 0.74 | 0.24 | ||
| Min. | 0.31 | 0.05 | 1.02 | 0.09 | 0.19 | 0.04 | 1.51 | 0.03 | 0.15 | 1.46 | 4.22 | 0.04 | 0.11 | 0.16 | 0.06 | 0.15 | 0.15 | 0.14 | 1.12 | 0.03 | 0.13 | 0.33 | 0.22 | 0.19 | 0.12 | 0.11 | ||
| Mean | 0.65 | 0.10 | 8.23 | 0.36 | 0.19 | 0.11 | 1.51 | 0.10 | 0.45 | 2.03 | 4.22 | 0.43 | 0.44 | 0.43 | 0.15 | 0.38 | 0.58 | 0.41 | 1.85 | 0.08 | 0.49 | 0.33 | 0.37 | 0.35 | 0.38 | 0.15 | ||
| Samples | 4 | 4 | 4 | 4 | 1 | 4 | 1 | 4 | 4 | 4 | 0 | 1 | 4 | 0 | 4 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 4 | 1 | 4 | 4 | 4 | 4 |
| I2C | ||||||||||||||||||||||||||||
| Max. | 0.82 | 0.18 | 1.25 | 0.66 | 0.17 | 0.05 | 0.13 | 0.48 | 2.60 | 0.28 | 0.58 | 0.43 | 0.07 | 0.37 | 0.75 | 0.37 | 1.48 | 0.11 | 0.80 | 0.73 | 0.50 | 0.59 | 0.37 | |||||
| Min. | 0.82 | 0.06 | 0.91 | 0.16 | 0.16 | 0.05 | 0.10 | 0.16 | 1.69 | 0.18 | 0.15 | 0.43 | 0.07 | 0.12 | 0.17 | 0.37 | 1.48 | 0.05 | 0.19 | 0.38 | 0.20 | 0.12 | 0.08 | |||||
| Mean | 0.82 | 0.11 | 1.05 | 0.41 | 0.17 | 0.05 | 0.12 | 0.28 | 2.28 | 0.23 | 0.31 | 0.43 | 0.07 | 0.25 | 0.39 | 0.37 | 1.48 | 0.08 | 0.49 | 0.54 | 0.33 | 0.30 | 0.18 | |||||
| Samples | 1 | 3 | 3 | 2 | 2 | 1 | 0 | 2 | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 1 | 1 | 3 | 3 | 1 | 1 | 3 | 2 | 0 | 3 | 3 | 3 | 3 |
| I1A | ||||||||||||||||||||||||||||
| Max. | 3.65 | 0.33 | 5.75 | 1.00 | 0.21 | 0.22 | 0.44 | 1.02 | 1.93 | 0.69 | 4.88 | 1.39 | 1.23 | 0.40 | 0.76 | 1.29 | 0.64 | 3.81 | 0.51 | 1.56 | 0.93 | 0.75 | 1.48 | 1.02 | 0.34 | |||
| Min. | 0.73 | 0.19 | 1.00 | 0.53 | 0.21 | 0.07 | 0.14 | 0.57 | 0.43 | 0.35 | 4.88 | 0.57 | 0.55 | 0.38 | 0.48 | 0.75 | 0.34 | 2.00 | 0.13 | 0.77 | 0.59 | 0.32 | 0.50 | 0.58 | 0.25 | |||
| Mean | 2.19 | 0.24 | 3.38 | 0.74 | 0.21 | 0.15 | 0.31 | 0.74 | 1.18 | 0.51 | 4.88 | 0.86 | 0.80 | 0.39 | 0.60 | 0.96 | 0.45 | 2.70 | 0.32 | 1.10 | 0.76 | 0.54 | 0.83 | 0.75 | 0.29 | |||
| Samples | 2 | 3 | 2 | 3 | 1 | 3 | 0 | 3 | 3 | 2 | 0 | 0 | 3 | 1 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 |
| I1B | ||||||||||||||||||||||||||||
| Max. | 2.46 | 0.51 | 0.94 | 1.21 | 0.57 | 0.25 | 0.56 | 1.33 | 1.49 | 0.92 | 1.20 | 8.63 | 1.61 | 1.17 | 0.09 | 1.12 | 2.13 | 0.91 | 5.24 | 0.39 | 1.71 | 0.66 | 1.27 | 1.33 | 0.58 | |||
| Min. | 1.41 | 0.28 | 0.68 | 0.75 | 0.17 | 0.07 | 0.12 | 0.64 | 0.79 | 0.92 | 0.65 | 8.63 | 0.82 | 0.76 | 0.09 | 0.62 | 1.03 | 0.49 | 2.16 | 0.21 | 1.05 | 0.46 | 0.83 | 0.67 | 0.27 | |||
| Mean | 2.02 | 0.36 | 0.81 | 0.92 | 0.41 | 0.16 | 0.37 | 1.00 | 1.03 | 0.92 | 0.98 | 8.63 | 1.17 | 0.95 | 0.09 | 0.84 | 1.50 | 0.69 | 3.37 | 0.33 | 1.34 | 0.53 | 0.98 | 1.02 | 0.37 | |||
| Samples | 4 | 10 | 2 | 10 | 10 | 10 | 0 | 10 | 9 | 7 | 1 | 0 | 10 | 1 | 10 | 9 | 1 | 10 | 9 | 10 | 9 | 9 | 9 | 0 | 10 | 10 | 10 | 9 |
| I1C | ||||||||||||||||||||||||||||
| Max. | 2.21 | 0.30 | 1.47 | 1.33 | 0.26 | 0.13 | 0.34 | 1.04 | 1.69 | 0.73 | 1.12 | 1.10 | 1.32 | 0.15 | 0.97 | 1.36 | 0.70 | 3.86 | 0.46 | 1.70 | 0.84 | 1.37 | 1.07 | 0.63 | ||||
| Min. | 0.74 | 0.19 | 1.47 | 0.78 | 0.17 | 0.11 | 0.26 | 0.78 | 1.69 | 0.60 | 0.72 | 0.78 | 0.75 | 0.08 | 0.53 | 1.05 | 0.52 | 2.56 | 0.17 | 0.94 | 0.43 | 0.62 | 0.66 | 0.28 | ||||
| Mean | 1.47 | 0.25 | 1.47 | 1.07 | 0.22 | 0.12 | 0.31 | 0.92 | 1.69 | 0.67 | 0.96 | 0.96 | 1.13 | 0.11 | 0.76 | 1.21 | 0.62 | 3.21 | 0.34 | 1.40 | 0.61 | 1.06 | 0.91 | 0.41 | ||||
| Samples | 2 | 3 | 1 | 3 | 3 | 3 | 0 | 3 | 3 | 1 | 2 | 0 | 3 | 0 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 0 | 3 | 3 | 3 | 3 |
References
- Calvo, J.P.; Blanc-Valleron, M.M.; Rodríguez-Aranda, J.P.; Rouchy, J.M.; Sanz, M.E. Authigenic clay minerals in continental evaporitic environments. In Palaeoweathering, Palaeosurfaces and Related Continental Deposits; Thiry, M., Simon-Coincon, R., Eds.; Special Publications of the International Association of Sedimentologists: Oxford, UK, 1999; Volume 27, pp. 129–151. [Google Scholar]
- Cowardin, L.M.; Carter, V.; Golet, F.C.; LaRoe, E.T. Classification of Wetlands and Deepwater Habitats of the United States. In Office of Biological Services, Fish and Wildlife Service, U.S. Dept. of the Interior, Washington, D.C.; Office of Biological Services, Fish and Wildlife Service, U.S. Dept. of the Interior: Washington, DC, USA, 1979. [Google Scholar]
- Jones, B.F. Clay mineral diagenesis in lacustrine sediments. US Geol. Surv. Bull. 1986, 1578, 291–300. [Google Scholar]
- Ordoñez, S.; Calvo, J.P.; García del Cura, M.A.; Alonso Zarza, A.M.; Hoyos, M. Sedimentology of sodium sulphate deposits and special clays from the Tertiary Madrid Basin (Spain). In Lacustrine Facies Analysis; Anadón, P., Cabrera, L., Kelts, K., Eds.; Spec. Publ. Int. Assoc. Sedimentol. Blackwell Sci. Publ. Oxf.: Oxford, UK, 1991; Volume 13, pp. 39–55. [Google Scholar]
- Pozo, M.; Calvo, J.P. Madrid Basin (Spain): A natural lab for the formation and evolution of magnesian clay minerals. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; AIPEA Educational Series, Pub. No. 2; Digilabs: Bari, Italy, 2015; pp. 229–281. [Google Scholar]
- Clauer, N.; Fallick, A.E.; Galán, E.; Pozo, P.; Taylor, C. Varied crystallization conditions for neogene sepiolite and associated Mg-Clays from Madrid Basin (Spain) traced by oxygen and hidrogen isotope geochemistry. Geochim. Cosmochim. Acta 2012, 94, 181–198. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M.; Calvo, J.P. Tajo Basin. In The Geology of Spain; Gibbons, W., Moreno, T., Eds.; The Geological Society: London, UK, 2002; pp. 315–320. [Google Scholar]
- Calvo, J.P.; Jones, B.F.; Bustillo, M.; Fort, R.; Alonso-Zarza, A.M.; Kendall, C. Sedimentology and geochemistry of carbonates from lacustrine sequences in the Madrid Basin, Central Spain. Chem. Geol. 1995, 123, 173–191. [Google Scholar] [CrossRef]
- Cañaveras, J.C.; Calvo, J.P.; Hoyos, M.; Ordóñez, S. Paleomorphologic features of a Intra-Vallesian Paleokarst, Tertiary Madrid Basin. Significance of Paleokarstic Surfaces in Continental Basin Analysis; Friend, P., Dabrio, C.J., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 278–284. [Google Scholar]
- Rodríguez-Aranda, J.P.; Calvo, J.P.; Sanz-Montero, M.E. Lower Miocene gypsum palaeokarst in the Madrid Basin (central Spain): Dissolution diagenesis, morphological relics and karst-end products. Sedimentology 2002, 49, 1385–1400. [Google Scholar]
- Pozo, M.; Casas, J. Distribucion y caracterizacion de litofacies en el yacimiento de arcillas magnesicas de Esquivias (Neogeno de la Cuenca de Madrid). Bol. Geol. Min. 1995, 106, 65–82. [Google Scholar]
- Cuevas, J.; Vigil de la Villa, R.; Ramírez, S.; Petit, S.; Meunier, A.; Leguey, S. Chemistry of Mg smectites in lacustrine sediments from the Vicálvaro sepiolite deposit, Madrid Neogene Basin (Spain). Clays Clay Miner. 2003, 51, 457–472. [Google Scholar] [CrossRef]
- Pozo, M. Origin and evolution of magnesium clays in lacustrine environments: Sedimentology and geochemical pathways. In First Latin-American Clay Conference. Invited Lectures. Proceedings Vol. 1.; Gomes, C.S.F., Ed.; Funchal: Madeira, Portugal, 2000; pp. 117–133. [Google Scholar]
- Galán, E.; Pozo, M. Palygorskite and sepiolite deposits in continental environments. Description, genetic patterns and sedimentary settings. In Developments in Palygorskite–Sepiolite Research. A New Outlook on These Nanomaterials. Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 125–173. [Google Scholar]
- Galán, E.; Pozo, M. The mineralogy, geology, and main occurrences of sepiolite and palygorskite clays. In Natural Mineral Nanotubes; Apple Academic Press: New York, NY, USA, 2015; pp. 117–130. ISBN 978-1-77188-056-5. [Google Scholar]
- Pozo, M.; Galán, E. Magnesian clay deposits: Mineralogy and origin. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; AIPEA Educational Series, Pub. No. 2; Digilabs: Bari Italy, 2015; pp. 175–228. [Google Scholar]
- Pozo, M.; Calvo, J.P.; Pozo, E.; Moreno, A. Genetic constraints on crystallinity, thermal behaviour and surface area of sepiolite from the Cerro de los Batallones deposits (Madrid Basin, Spain). Appl. Clay Sci. 2014, 91–92, 30–45. [Google Scholar] [CrossRef]
- Pozo, M.; Calvo, J.P.; Silva, P.G.; Morales, J.; Peláez-Campomanes, P.; Nieto, M. Geología del sistema de yacimientos de mamíferos miocenos del Cerro de los Batallones, Cuenca de Madrid. Geogaceta 2004, 35, 143–146. [Google Scholar]
- Bustillo, M.A.; Bustillo, M. Miocene silcretes in argillaceous playa deposits, Madrid Basin, Spain: Petrological and geochemical features. Sedimentology 2000, 47, 1023–1037. [Google Scholar] [CrossRef]
- Bustillo, M.A.; Alonso-Zarza, A.M. Overlapping of pedogenesis andmeteoric diagenesis in distal alluvial and shallow lacustrine deposits in the Miocene Madrid Basin, Spain. Sediment. Geol. 2007, 198, 255–271. [Google Scholar] [CrossRef] [Green Version]
- Chung, F.H. Quantitative Interpretation of X-ray Diffraction Patterns of Mixtures. I. Matrix-Flushing Method for Quantitative Multicomponent Analysis. J. Appl. Cryst. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Schultz, L.G. Quantitative Interpretation of Mineralogical Composition from X-ray and Chemical Data for the Pierre Shale. Geol. Surv. Prof. Pap. 1964. Available online: https://pubs.usgs.gov/pp/0391c/report.pdf (accessed on 20 September 2018).
- Van der Marel, H.W. Quantitative analysis of clay minerals and their admixtures. Contrib. Miner. Pet. 1966, 12, 96–138. [Google Scholar] [CrossRef]
- Sánchez del Río, M.; García-Romero, E.; Suárez, M.; Da Silva, I.; Fuentes-Montero, L.; Martínez-Criado, G. Variability in sepiolite: Diffraction studies. Am. Miner. 2011, 96, 1443–1454. [Google Scholar] [CrossRef]
- Christidis, G.E.; Koutsopoulou, E. A simple approach to the identification of trioctahedral smectites by X-ray diffraction. Clay Miner. 2013, 48, 687–696. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3. [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for name of rock-forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Calvo, J.P.; Pozo, M.; Silva, P.G.; Morales, J. Pattern of sedimentary infilling of fossil mammal traps formed in pseudokarst at Cerro de los Batallones, Madrid Basin, Central Spain. Sedimentology 2013, 60, 1681–1708. [Google Scholar] [CrossRef]
- Galán, E.; Aparicio, P. Methodology for the identification and characterization of magnesian clays. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; AIPEA Educational Series, Pub. No. 2; Digilabs: Bari, Italy, 2015; pp. 63–121. [Google Scholar]
- Guggenheim, S. Introduction to Mg-Rich clay minerals: Structure and composition. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; AIPEA Educational Series, Pub. No. 2; Digilabs: Bari, Italy, 2015; pp. 1–62. [Google Scholar]
- Pozo, M.; Casas, J.C. Origin of kerolite and associated Mg clays in palustrine–lacustrine environments. The Esquivias deposit (Neogene Madrid Basin, Spain). Clay Miner. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Yalçin, H.; Bozkaya, O. Sepiolite–palygorskite occurrences in Turkey. In Developments in Palygorskite–Sepiolite Research. A New Outlook on These Nanomaterials. Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 175–200. [Google Scholar]
- Ece, Ö.I.; Çoban, F. Geology, occurrence, and genesis of Eskisehir sepiolites, Turkey. Clays Clay Miner. 1994, 42, 81–92. [Google Scholar] [CrossRef]
- Hay, R.L.; Hughes, R.E.; Kyser, T.K.; Glass, H.D.; Lin, J. Magnesium-rich clays of the Meerschaum mines in the Amboseli Basin, Tanzania and Kenya. Clays Clay Miner. 1995, 43, 455–466. [Google Scholar] [CrossRef]
- Tosca, N. Geochemical pathways to Mg-silicate formation. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; AIPEA Educational Series, Pub. No. 2; Digilabs: Bari, Italy, 2015; pp. 283–330. [Google Scholar]
- Pozo, M.; Leguey, S.; Medina, J.A. Sepiolite and palygorskite genesis in carbonate lacustrine environments. (Duero Basin, Spain). Chem. Geol. 1990, 84, 290–291. [Google Scholar] [CrossRef]
- Torres-Ruíz, J.; López-Galindo, A.; González-López, J.M.; Delgado, A. Geochemistry of Spanish sepiolite-palygorskite deposits: Genetic considerations based on trace elements and isotopes. Chem. Geol. 1994, 112, 221–245. [Google Scholar] [CrossRef]
- Moreno, A.; Pozo, M.; Martín Rubí, J.A. Geoquímica del yacimiento de arcillas magnésicas de Esquivias. (Cuenca de Madrid). Bol. Geol. Min. 1995, 106, 59–70. [Google Scholar]
- Jones, B.F.; Conko, K.M. Environmental influences on the occurrences of sepiolite and palygorskite: A brief review. In Developments in Palygorskite–Sepiolite Research. A New Outlook on These Nanomaterials. Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 69–83. [Google Scholar]
- Birsoy, R. Formation of sepiolite–palygorskite and related minerals from solution. Clays Clay Miner. 2002, 50, 736–745. [Google Scholar] [CrossRef]
- Pozo, M.; Carretero, M.I.; Galán, E. Approach to the trace element geochemistry of non-marine sepiolite deposits: Influence of the sedimentary environment (Madrid Basin, Spain). Appl. Clay Sci. 2015, 131, 27–43. [Google Scholar] [CrossRef]
- Khoury, H.N.; Eberl, D.D.; Jones, B.F. Origin of magnesium clays from the Amargosa Desert, Nevada. Clays Clay Miner. 1982, 30, 327–336. [Google Scholar] [CrossRef]
- Eberl, D.D.; Jones, B.F.; Khoury, H.N. Mixed-layer kerolite/stevensite from the Amargosa Desert, Nevada. Clays Clay Miner. 1982, 30, 321–326. [Google Scholar] [CrossRef]
- Post, J.L.; Janke, N.C. Barallat sepiolite in Inyo County California. In Palygorskite–Sepiolite. Occurrences, Genesis and Uses. Developments in Sedimentology; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 159–167. [Google Scholar]
- Chai, A.; Fritz, B.; Duplay, J.; Weber, F.; Lucas, J. Textural transition and genetic relationship between precursor stevensite and sepiolite in lacustrine sediments (Jbel Rhassoul, Morocco). Clays Clay Miner. 1997, 45, 378–389. [Google Scholar] [CrossRef]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogy Society of America: Chantilly, VA, USA, 1988; Volume 19, pp. 631–674. [Google Scholar]
- Trauth, N. Argiles évaporitiques dans la sédimentation carbonatée continentale et épicontinentale tertiaire: Bassins de Paris, de Mormoiron et de Salinelles (France), Jbel Ghassoul (Maroc). Sci. Géol. Mém. 1977, 49, 1–195. [Google Scholar]










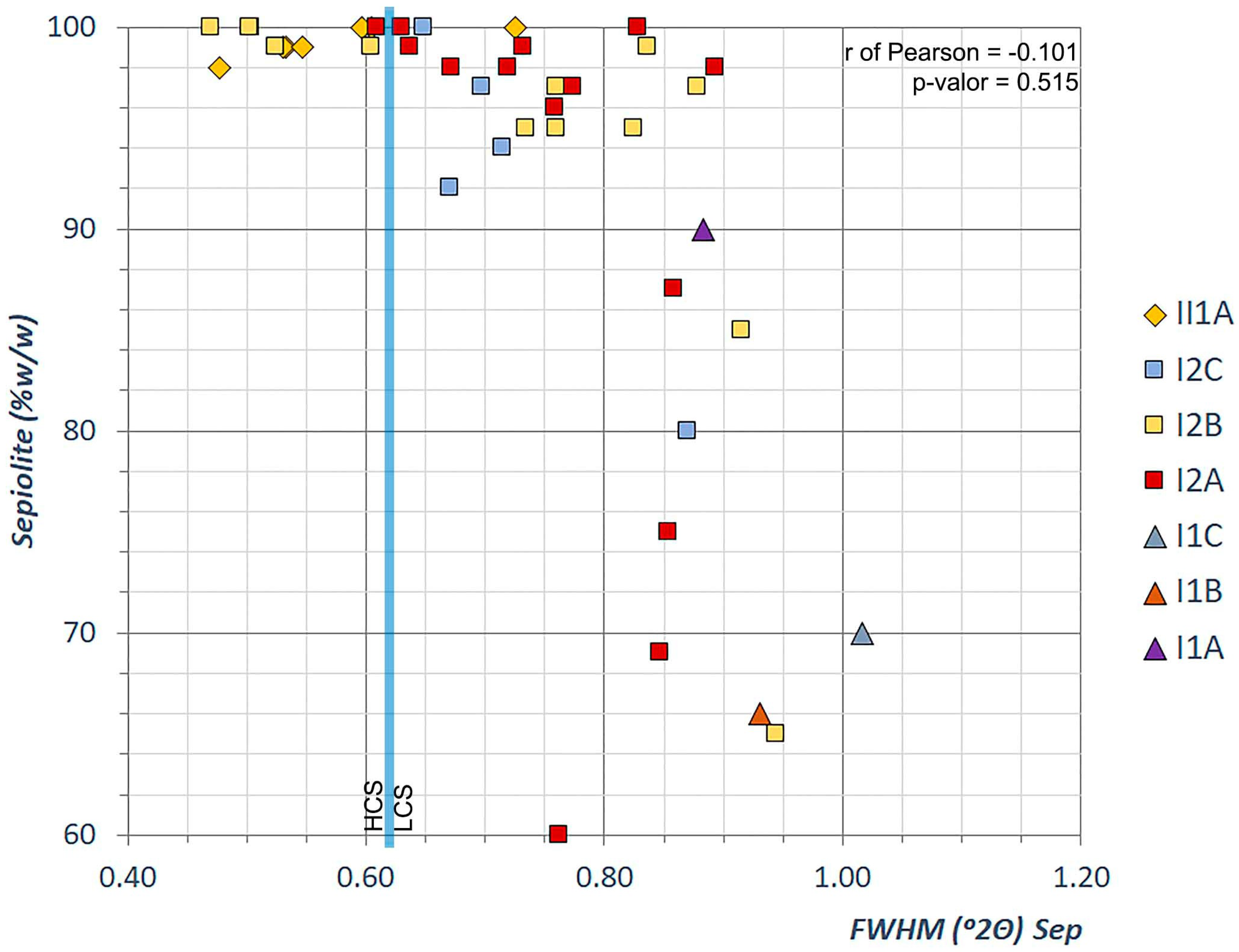
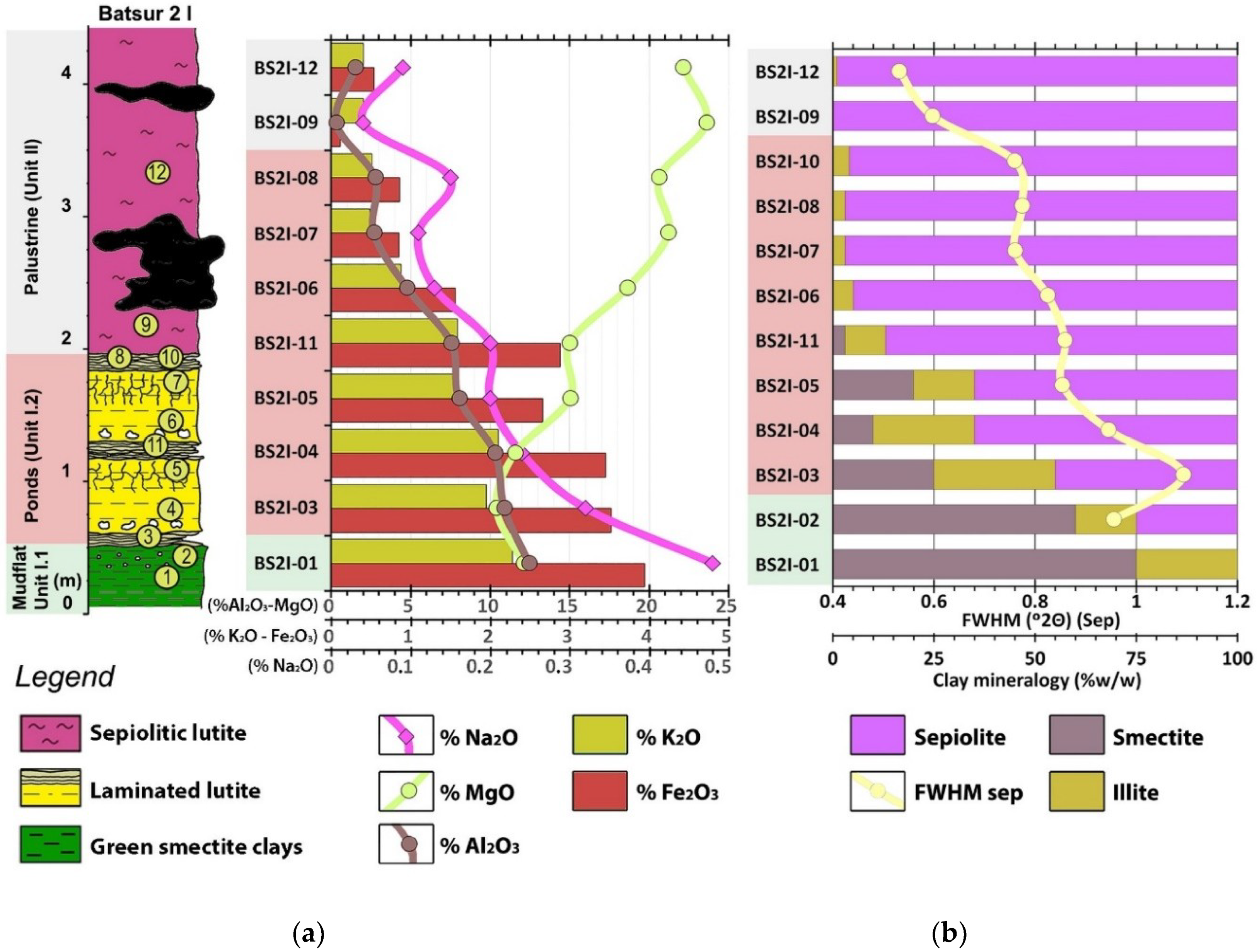
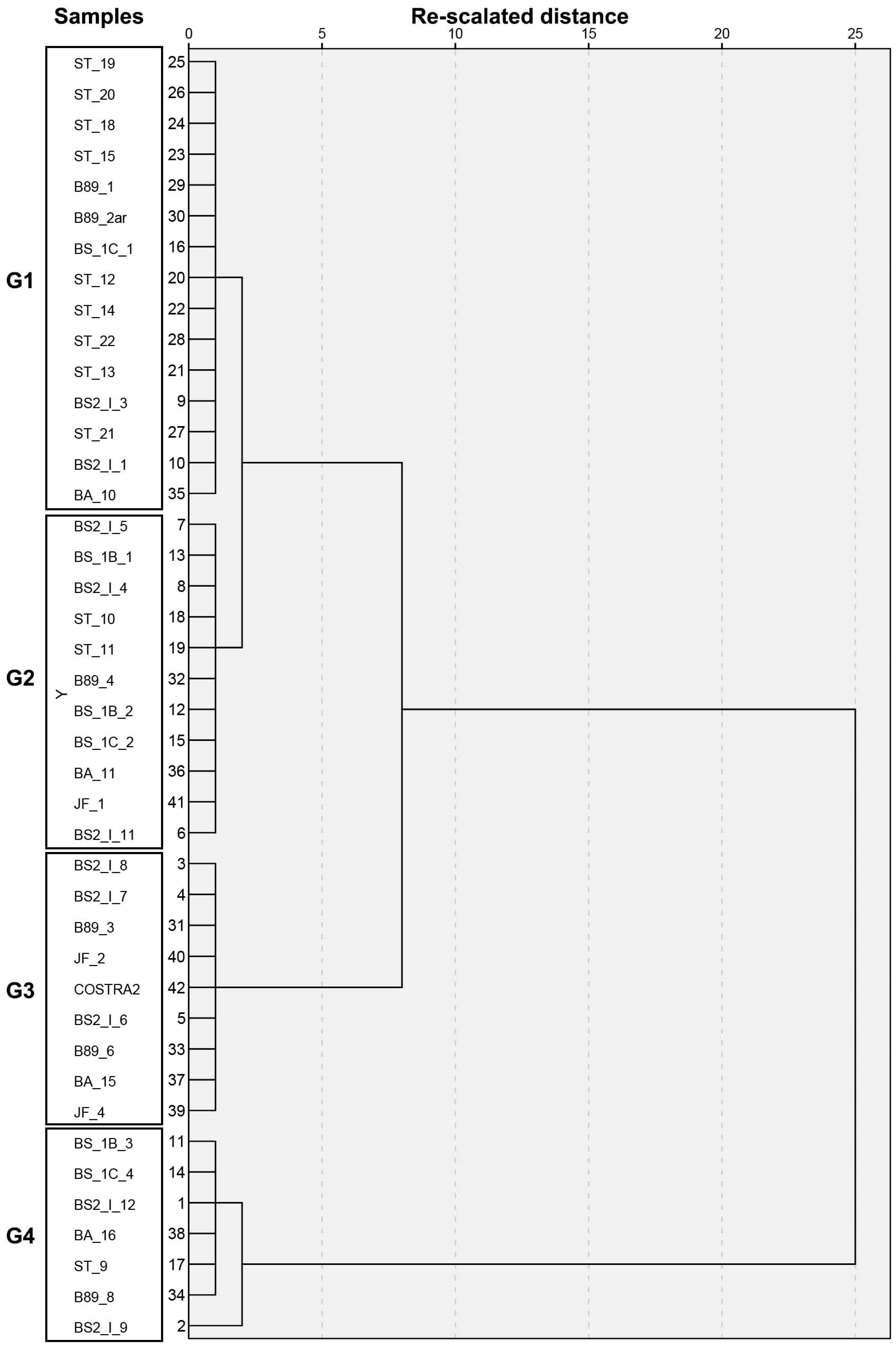

| Unit I.1 | Bulk Mineralogy | Clay Fraction | Sepiolite Features | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lf | Sample | Phy | Qz | Fsp | Cal | Dol | Sme | Sep | Ilt | Kln | FWHM | Cr. | Type |
| A | LA-1 * | 97 | 3 | Tr | - | - | 87 | - | 13 | - | |||
| A | JF-1 * | 100 | Tr | Tr | - | - | 6 | 82 | 12 | - | 0.809 | - | LCS |
| A | ST-12 | 98 | 2 | Tr | - | - | 98 | Tr | 2 | - | |||
| A | B8/9-2ar * | 100 | Tr | Tr | - | - | 91 | - | 9 | - | |||
| A | B8/9-2 * | 98 | 2 | - | Tr | - | 87 | - | 13 | - | |||
| A | BS2II-1 | 97 | 2 | 1 | - | - | 75 | - | 25 | - | |||
| A | BS1A-1 | 98 | 2 | Tr | - | - | - | 90 | 10 | - | 0.883 | 11 | LCS |
| B | BA-10 * | 76 | 17 | 7 | - | - | 78 | Tr | 22 | - | |||
| B | ST-13 | 95 | 5 | Tr | - | - | 95 | Tr | 5 | - | |||
| B | ST-14 | 96 | 4 | Tr | - | - | 84 | Tr | 16 | - | |||
| B | ST-15 | 98 | 2 | Tr | - | - | 85 | Tr | 15 | - | |||
| B | ST-18 | 90 | 2 | Tr | - | 8 | 77 | - | 19 | 4 | |||
| B | ST-19 | 92 | 2 | 6 | - | - | 73 | - | 20 | 7 | |||
| B | ST-20 | 89 | 4 | 7 | - | - | 83 | - | 15 | 2 | |||
| B | ST-21 | 91 | 5 | 4 | - | - | 80 | - | 20 | - | |||
| B | ST-22 | 97 | 2 | 1 | - | - | 83 | - | 17 | - | |||
| B | BS1C-1 | 89 | 3 | 4 | 4 | - | 12 | 66 | 22 | - | 0.931 | - | LCS |
| C | LA-5 * | 74 | 9 | 9 | 8 | - | 64 | - | 31 | 5 | |||
| C | B8/9-1 * | 96 | 2 | 2 | - | - | 100 | - | - | - | |||
| C | BS2II-3 | 96 | 3 | 1 | - | - | 70 | 10 | 20 | - | 0.645 | - | LCS |
| C | BS2II-2 | 98 | 1 | - | - | - | 75 | 5 | 20 | - | 0.800 | - | LCS |
| C | BS2I-2 | 97 | 2 | 1 | - | - | 60 | 25 | 15 | - | 0.956 | - | LCS |
| C | BS2I-1 | 97 | 2 | 1 | - | - | 75 | - | 25 | Tr | |||
| C | BS1B-1 | 98 | 2 | Tr | - | - | 10 | 70 | 20 | - | 1.017 | 6 | LCS |
| D | ST-16 | 67 | - | Tr | 1 | 31 | |||||||
| D | ST-17 | 30 | - | - | - | 70 | |||||||
| Unit I.2 | Bulk Mineralogy | Clay Fraction | Sepiolite Features | BET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lf. | Sample | Phy | Qz | Fsp | Cal | Sme | Sep | Ilt | FWHM | Cr. | Type | |
| A | LA-9 * | 100 | Tr | Tr | - | 13 | 78 | 9 | ||||
| A | LA-8 * | 100 | Tr | Tr | - | 23 | 64 | 13 | ||||
| A | BA-16 * | 95 | Tr | - | 5 | - | 100 | - | 0.609 | 15 | LCS | 328.0 |
| A | BA-15 * | 96 | 3 | 1 | - | |||||||
| A | BA-11 * | 99 | 1 | Tr | - | 11 | 83 | 6 | ||||
| A | JF-4 * | 100 | - | - | - | - | 100 | - | 0.630 | 14 | LCS | |
| A | JF-3 * | 100 | Tr | - | Tr | |||||||
| A | JF-2 * | 100 | Tr | - | - | |||||||
| A | COSTRA2 * | 100 | - | - | - | - | 100 | - | ||||
| A | ST-10 | 100 | Tr | Tr | - | 23 | 73 | 4 | LCS | |||
| A | ST-11 | 100 | Tr | Tr | - | 19 | 76 | 4 | LCS | |||
| A | B8/9-6 * | 95 | 5 | - | - | - | 100 | - | 0.829 | - | LCS | 360.0 |
| A | B8/9-5 * | 100 | Tr | - | - | - | 98 | 2 | 0.893 | - | LCS | 299.0 |
| A | B8/9-4 * | 95 | 3 | 2 | - | 7 | 88 | 5 | ||||
| A | B8/9-3 * | 100 | - | - | - | - | 98 | 2 | 0.719 | 12 | LCS | 355.0 |
| A | BS2II-5 | 98 | 1 | - | - | - | 99 | 1 | 0.638 | 13 | LCS | |
| A | BS2I-10 | 100 | - | - | - | - | 96 | 4 | 0.760 | 12 | LCS | |
| A | BS2I-8 | 98 | 2 | - | - | - | 97 | 3 | 0.774 | 11 | LCS | 352.4 |
| A | BS2I-11 | 98 | 1 | 1 | - | 2 | 87 | 10 | 0.859 | 8 | LCS | 305.7 |
| A | BS2I-11O | 18 | 65 | 17 | ||||||||
| A | BS2I-11C | - | 98 | 2 | ||||||||
| A | BS2I-5 | 95 | 2 | 2 | - | 5 | 75 | 20 | 0.854 | - | LCS | |
| A | BS2I-3 | 97 | 1 | 2 | - | 25 | 45 | 30 | 1.093 | - | LCS | |
| A | BS1C-6 | 90 | - | - | 10 | - | 98 | 2 | 0.672 | 15 | LCS | |
| A | BS1C-3 | 99 | 1 | - | - | 3 | 60 | 37 | 0.762 | 11 | LCS | |
| A | BS1B-2 | 95 | 3 | 2 | - | 1 | 69 | 30 | 0.847 | 9 | LCS | 323.5 |
| A | BS1A-3 | 99 | - | - | 1 | - | 99 | 1 | 0.733 | 13 | LCS | |
| B | LA-6 * | 72 | 8 | 7 | 13 | 8 | 85 | 7 | 0.915 | - | LCS | |
| B | BA-13 * | 100 | Tr | Tr | - | - | 95 | 5 | 0.760 | 12 | LCS | |
| B | BA-12 * | 100 | Tr | Tr | - | - | 97 | 3 | 0.878 | 10 | LCS | |
| B | B8/9-8 * | 100 | - | - | - | - | 99 | 1 | 0.525 | 17 | HCS | |
| B | B8/9-7 * | 100 | - | - | - | - | 100 | - | 0.504 | 18 | HCS | |
| B | BS2II-10 | 1 | 3 | - | 96 | |||||||
| B | BS2II-4 | 99 | Tr | Tr | - | - | 99 | 1 | 0.837 | 10 | LCS | 392.6 |
| B | BS2I-7 | 99 | 1 | - | - | - | 97 | 3 | 0.760 | 12 | LCS | 348.0 |
| B | BS2I-6 | 98 | 1 | 1 | - | Tr | 95 | 5 | 0.825 | 10 | LCS | 358.8 |
| B | BS2I-4 | 97 | 2 | 1 | - | 10 | 65 | 25 | 0.945 | - | LCS | 397.1 |
| B | BS1C-8 | 100 | - | - | - | - | 100 | Tr | 0.470 | 18 | HCS | |
| B | BS1C-7 | 100 | - | - | - | - | 100 | Tr | 0.503 | 17 | HCS | |
| B | BS1C-5 | 2 | - | - | 98 | - | 100 | Tr | 0.649 | 13 | LCS | |
| B | BS1A-5 | 100 | - | - | - | - | 99 | 1 | 0.605 | 14 | HCS | |
| B | BS1A-4 | 30 | - | - | 70 | - | 97 | 3 | 0.698 | 12 | LCS | |
| B | BS1A-2 | 100 | - | - | - | - | 95 | 5 | 0.735 | 13 | LCS | |
| C | BS1C-4 | 95 | - | - | 5 | - | 94 | 6 | 0.715 | 13 | LCS | 345.3 |
| C | BS1C-2 | 93 | 3 | 4 | - | 3 | 80 | 17 | 0.870 | 9 | LCS | |
| C | BS1B-3 | 99 | 1 | - | - | - | 92 | 8 | 0.670 | 13 | LCS | 213.9 |
| Unit II.1 | Bulk min. | Clay Fract. | Sepiolite Features | BET | |||||
|---|---|---|---|---|---|---|---|---|---|
| Lf | Sample | Phy | Cal | Sep | Ilt | FWHM | Cr. | Type | |
| A | ST-9 | 100 | - | 100 | - | 0.726 | 13 | LCS | |
| A | BS2II-9 | 100 | - | 100 | Tr | 0.605 | 15 | HCS | |
| A | BS2II-7 | 100 | - | 99 | 1 | 0.533 | 17 | HCS | |
| A | BS2I-12 | 100 | - | 99 | 1 | 0.531 | 18 | HCS | |
| A | BS2I-9 | 100 | - | 100 | - | 0.597 | 16 | HCS | 371.08 |
| A | BS1B-4 | 100 | - | 98 | 2 | 0.477 | 18 | HCS | |
| A | BS1A-6 | 50 | 50 | 99 | 1 | 0.547 | 16 | HCS | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz, J.E.; Pozo, M. Authigenic Mg-Clay Minerals Formation in Lake Margin Deposits (the Cerro de los Batallones, Madrid Basin, Spain). Minerals 2018, 8, 418. https://doi.org/10.3390/min8100418
Herranz JE, Pozo M. Authigenic Mg-Clay Minerals Formation in Lake Margin Deposits (the Cerro de los Batallones, Madrid Basin, Spain). Minerals. 2018; 8(10):418. https://doi.org/10.3390/min8100418
Chicago/Turabian StyleHerranz, Juan Emilio, and Manuel Pozo. 2018. "Authigenic Mg-Clay Minerals Formation in Lake Margin Deposits (the Cerro de los Batallones, Madrid Basin, Spain)" Minerals 8, no. 10: 418. https://doi.org/10.3390/min8100418





