Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Geomorphoedaphic Division of the Study Area
3.2. Soil Characterization on the Different Geomorphological Units
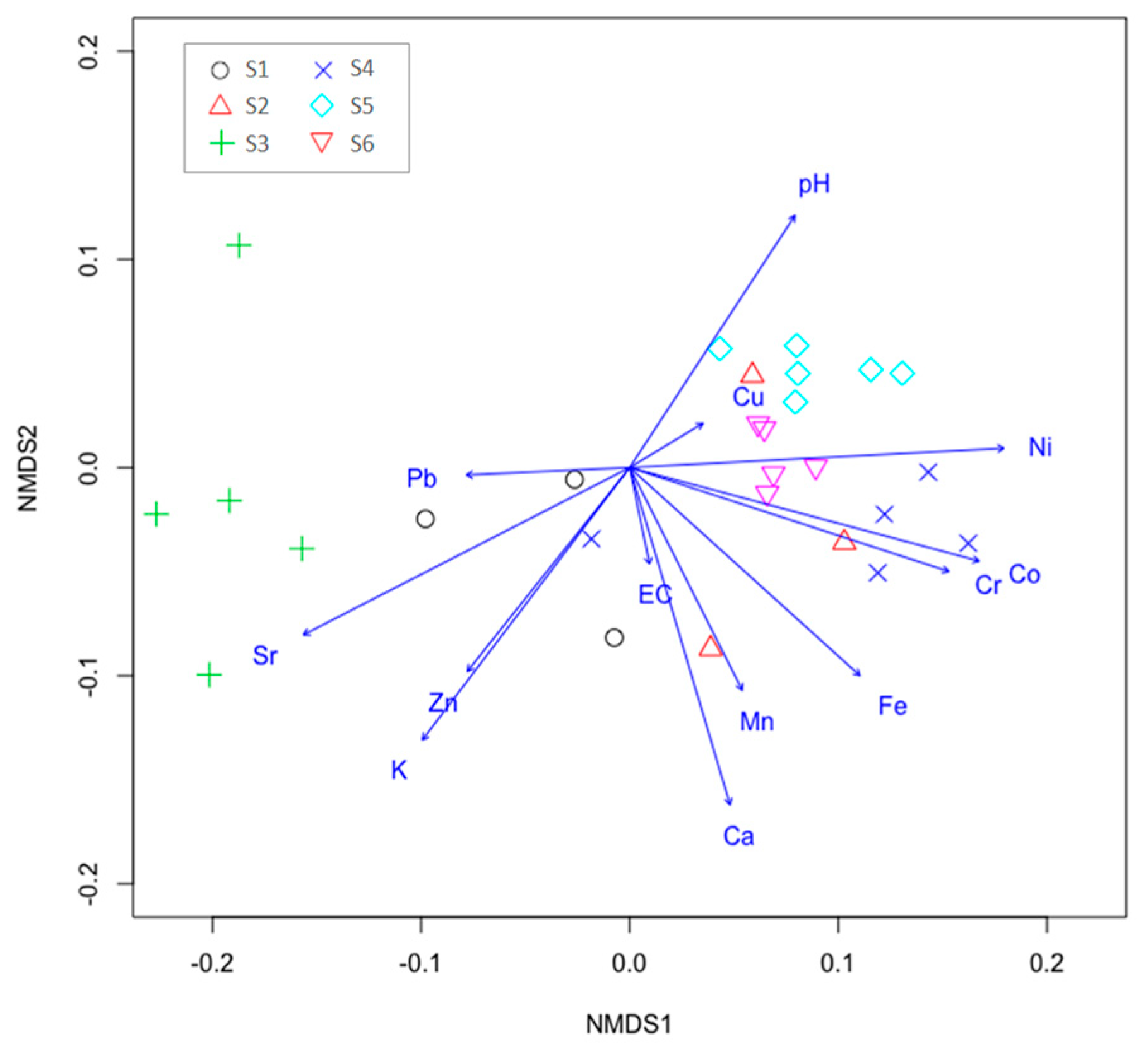
3.3. Potentially Toxic Metals and the Distribution of Microelements in Study Sub-Units
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sequeira, E.M.D.; Pinto da Silva, A.R. The ecology of serpentinized areas of north–east Portugal. In The Ecology of Areas with Serpentinized Rocks; A World Review; Roberts, B.A., Proctor, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 169–197. [Google Scholar]
- Freitas, H.; Prasad, M.N.V.; Pratas, J. Analysis of serpentinophytes from north–east of Portugal for trace metal accumulation–Relevance to the management of mine environment. Chemosphere 2004, 54, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Velde, B.D. Geochemistry at the Earth’s Surface Movement of Chemical Elements; Springer: Berlin, Germany, 2014; ISBN 978-3-642-31359-2. [Google Scholar]
- Díez-Lázaro, J.; Kidd, P.S.; Monterroso-Martínez, C. A phytogeochemical study of the Trás-os-Montes region (NE Portugal): Possible species for plant-based soil remediation technologies. Sci. Total. Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Khurana, M.P.; Jhanji, S. Influence of cadmium on dry matter yield, micronutrient content and its uptake in some crops. J. Environ. Biol. 2014, 35, 865–870. [Google Scholar] [PubMed]
- Zhou, L.L.; Yang, B.; Xue, N.D.; Li, F.S.; Seip, H.M.; Cong, X.; Yann, Y.Z.; Liu, B.; Han, B.L.; Li, H.Y. Ecological risks and potential sources of heavy metals in agricultural soils from Huanghuai Plain, China. Environ. Sci. Pollut. Res. Int. 2014, 21, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Li, X.; Shi, W.; Cheung, S.C.; Thornton, I. Metal contamination in urban, suburban, and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Sci. Total. Environ. 2006, 356, 45–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micó, C.; Recatalá, R.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yuan, X.; Zhao, Y.; Hu, G.; Tu, X. Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J. Environ. Sci. 2008, 20, 664–669. [Google Scholar] [CrossRef]
- Wang, S.L.; Xu, X.R.; Sun, Y.X.; Liu, J.L.; Li, H.B. Heavy metal pollution in coastal areas of South China: A review. Mar. Pollut. Bull. 2013, 76, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.M.; Goldhaber, M.B.; Mills, C.T.; Breit, G.N.; Hooper, R.L.; Holloway, J.M.; Diehl, S.F.; Ranville, J.F. Weathering and transport of chromium and nickel from serpentinite in the Coast Range ophiolite to the Sacramento Valley, California, USA. Appl. Geochem. 2015, 61, 72–86. [Google Scholar] [CrossRef]
- Cheng, C.H.; Jien, S.H.; Tsai, H.; Chang, Y.H.; Chen, Y.C.; Hseu, Z.Y. Geochemical element differentiation in serpentine soils from the ophiolite complexes, Eastern Taiwan. Soil. Sci. 2009, 174, 283–291. [Google Scholar] [CrossRef]
- Duivenvoorden, L.J.; Roberts, D.T.; Tucker, G.M. Serpentine geology links to water quality and heavy metals in sediments of a stream system in central Queensland, Australia. Environ. Earth. Sci. 2017, 76, 320. [Google Scholar] [CrossRef]
- Kumar, A.; Maiti, S.K. Availability of Chromium, Nickel and Other Associated Heavy Metals of Ultramafic and Serpentine Soil /Rock and in Plants. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 256–268. [Google Scholar]
- Pérez-Latorre, A.V.; Hidalgo-Triana, N.; Cabezudo, B. Composition, ecology and conservation of the south-Iberian serpentine flora in the context of the Mediterranean basin. Anales. Jard. Bot. Madrid. 2013, 70, 62–71. [Google Scholar] [Green Version]
- Fernández, S.; Seoane, S.; Merino, A. Plant heavy metal concentrations and soil biological properties in agricultural serpentine soils. Commun. Soil Sci. Plant Anal. 1999, 30, 1867–1884. [Google Scholar]
- Otero, X.L.; Huerta-Diaz, M.A.; Macías, F. Heavy metal geochemistry of saltmarsh soils from the Ría of Ortigueira (mafic and ultramafic areas, NW Iberian Peninsula). Environ. Pollut. 2000, 110, 285–296. [Google Scholar] [CrossRef]
- Shah, M.T.; Ara, J.; Muhammad, S.; Khan, S.; Asad, S.A.; Ali, L. Potential Heavy Metals Accumulation of Indigenous Plant Species along the Mafic and Ultramafic Terrain in the Mohmand Agency, Pakistan. Clean Soil Air Water 2013, 42, 339–346. [Google Scholar] [CrossRef]
- Galey, M.L.; van der Ent, A.; Iqbal, M.C.M.; Rajakaruna, N. Ultramafic geoecology of South and Southeast Asia. Bot. Stud. 2017, 58. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A. Conservation of endemic plants in serpentine landscapes. Biol. Cons. 2001, 100, 35–44. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D.J. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Specht, R.L.; Batianoff, G.N.; Reeves, R.D. Vegetation structure and biodiversity along the eucalypt forest to rainforest continuum on the serpentinite soil catena in a subhumid area of Central Queensland, Australia. Austral. Ecol. 2006, 32, 394–407. [Google Scholar] [CrossRef]
- Gómez-Zotano, J.; Román-Requena, F.; Hidalgo-Triana, N.; Pérez-Latorre, A.V. Biodiversidad y valores de conservación de los ecosistemas serpentínicos en España: Sierra Bermeja (provincia de Málaga). Boletín de la Asociación de Geógrafos Españoles 2014, 65, 187–206. [Google Scholar]
- Rield, H.; Papadopoulou-Vrynioti, K. Comparative investigations on karst generations mainly in the Aegean Archipielago. Mitt. Naturwissenschaftlicher Ver. Für. Steiermark 2001, 131, 23–39. [Google Scholar]
- Graf, W.L.; Clark, S.L.; Kammerer, M.T.; Lehman, T.; Randall, I.C.; Schroeder, T.P. Geomorphlogy of heavy metals in the sediments of Queen Creek, Arizona, USA. Catena 1991, 18, 567–582. [Google Scholar] [CrossRef]
- Gołdyn, B.; Chudzińska, M.; Barałkiewicz, D.; Celewicz-Gołdyn, S. Heavy metal contents in the sediments of astatic ponds: Influence of geomorphology, hydroperiod, water chemistry and vegetation. Ecotoxicol. Environ. Saf. 2015, 118, 103–111. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P. Long-term studies of metal transfer following the application of sewage sludge. In Pollutant Transport and Fate in Ecosystems; Coughtrey, P.J., Martin, M.H., Unsworth, M.H., Eds.; Blackwell Science Publications: Oxford, UK, 1987; pp. 301–317. ISBN 978-0632016273. [Google Scholar]
- Dickey, J.S. Partial fusion products in Alpine-Type peridotites: Serranía de Ronda and other examples. Mineral. Soc. Am. 1970, 3, 33–49. [Google Scholar]
- Gómez-Zotano, J.; Román-Requena, F.; Thorne, J.H. Attributes and roadblocks: A conservation assessment and policy review of the Sierra Bermeja. A Mediterranean serpentine landscape. Nat. Areas J. 2015, 35, 328–343. [Google Scholar] [CrossRef]
- Gómez-Zotano, J.; Alcántara-Manzanares, J.; Martínez-Ibarra, E.; Olmedo-Cobo, J.A. Applying the Technique of Image Classification to Climate Science: The Case of Andalusia (Spain). Geograph. Res. 2016, 54, 461–470. [Google Scholar] [CrossRef]
- Rivas-Martínez, S. Memoria del Mapa de Vegetación Potencial de España. Itiner. Geobot. 2011, 18, 5–800. [Google Scholar]
- Gómez-Zotano, J. The broadleaved tree-conifer controversy at Sierra Bermeja, an ultramafic mountain in southern Spain. In Ultramafic Rocks: Their Soils, Vegetation and Fauna, Proceedings of the Fourth International Conference on Serpentine Ecology; Boyd, R.S., Baker, A.J.M., Proctor, J., Eds.; Science Reviews: St Albans-Herts, UK, 2004; pp. 151–156. ISBN 1900814412. [Google Scholar]
- Olmedo-Cobo, J.A.; Cunill-Artigas, R.; Martínez-Ibarra, E.; Gómez-Zotano, J. Paleoecología de Abies sp. en Sierra Bermeja (sur de la Península Ibérica) durante el Holoceno medio a partir del análisis pedoantracológico. Bosque 2017, 259–270. [Google Scholar] [CrossRef]
- Instituto Geográfico Nacional–IGN. Available online: http://www.ign.es/web/ign/portal/cbg-area-cartografia (accessed on 14 March 2018).
- Instituto Geológico y Minero de España–IGME Hoja 1065 MARBELLA. Available online: http://info.igme.es/cartografiadigital/geologica/Magna50.aspx?language=es (accessed on 17 March 2018).
- Ministerio de Agricultura Pesca y Alimentación–MAPA. Métodos Oficiales de Análisis. Tomo III; Secretaría General Técnica del Ministerio de Agricultura, Pesca y Alimentación: Madrid, España, 1994; ISBN 978-84-491-0003-1. [Google Scholar]
- Martín, F.; Morales, S.; Bagur, M.G.; Estepa, C. A rapid field procedure for screening trace elements in polluted soil using portable X-ray fluorescence (PXRF). Geoderma 2010, 159, 76–82. [Google Scholar]
- Billets, S. Innovative Technology Verification Report Xrf Technologies for Measuring Trace Elements in Soil and Sediment Niton xlt700 Series XRF Analyzer; U.S. Environmental Protection Agency: Washington, DC, USA, 2006; EPA/540/R-06/004 (NTIS PB2006-1090036).
- Priem, H.; Hebeda, E.; Boelrijk, N.; Verdurmen, T.; Oen, I. Isotopic dating of the emplacement of the ultramafic masses in the Serrania de Ronda, Southern Spain. Contrib. Mineral. Petrol. 1979, 70, 103–109. [Google Scholar] [CrossRef]
- Alexander, E.B.; DuShey, J. Topographic and soil differences from peridotite to serpentinite. Geomorphology 2011, 135, 271–276. [Google Scholar] [CrossRef]
- Acosta-Vigil, A. Estudio de los Fenómenos de Fusión Cortical y Generación de Granitoides Asociados a las Peridotitas de Ronda. Ph.D. Thesis, University of Granada, Granada, Spain, 1998. [Google Scholar]
- Gómez-Ortiz, A.; Díaz del Olmo, F.; Simón-Torres, M. Periglaciarismo en las Cordilleras Béticas. In Periglaciarismo en la Península Ibérica, Canarias y Baleares. Estudios Significativos; Gómez-Ortiz, A., Simón-Torres, M., Salvador-Franch, F., Eds.; SEG-Universidad de Granada: Granada, Spain, 1994; pp. 165–188. ISBN 84-338-1982-8. [Google Scholar]
- Sener, E.; Davraz, A.; Ozcelik, M. An integration of GIS and remote sensing in groundwater investigations: A case study in Burdur, Turkey. Hydrogeol. J. 2005, 13, 826–834. [Google Scholar] [CrossRef]
- FAO-Organización de las Naciones Unidas para la Alimentación y la Agricultura. Base Referencial Mundial del Recurso Suelo 2014. Available online: http://www.fao.org/3/i3794es/I3794es.pdf (accessed on 17 January 2018).
- Bini, C.; Maleci, L.; Wahsha, M. Potentially toxic elements in serpentine soils and plants from Tuscany (Central Italy). A proxy for soil remediation. Catena 2017, 148, 60–66. [Google Scholar] [CrossRef]
- Hseu, Z.-Y.; Zehetner, F.; Fujii, K.; Watanabe, T.; Nakao, A. Geochemical fractionation of chromium and nickel in serpentine soil profiles along a temperate to tropical climate gradient. Geoderma 2018, 327, 97–106. [Google Scholar] [CrossRef]
- Aguilar, J.; Gómez, J.L.; Galán, E. Criterios y Estándares Para Declarar un Suelo Contaminado en Andalucía y la Metodología y Técnicas de Toma de Muestra y Análisis Para su Investigación; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 1999. [Google Scholar]
- Suen, C.J. Geochemistry of Peridotites and Associated Mafic Rocks, Ronda Ultramafic Complex, Spain. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005; p. 283. [Google Scholar]
- Visoli, G.; Menta, C.; Gardi, C.; Conti, F.D. Metal toxicity and biodiversity in serpentine soils: Application of bioassay tests and microarthropod index. Chemosphere 2013, 90, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Ecological Soil Screening Level for Iron. Interim Final OSWER Directive 9285.7-69. 2003. Available online: https://semspub.epa.gov/work/10/500006307.pdf (accessed on 11 February 2018).
- Aguilar, J.; Calvo, R.; Fernández, E.; Macías, F. Geoquímica de la alteración y edafogénesis de rocas serpentinizadas de la Sierra Bermeja (Málaga). Edafología 1998, 5, 135–151. [Google Scholar]
- Salminen, R. Geochemical Atlas of Europe. Available online: http://weppi.gtk.fi/publ/foregsatlas/ (accessed on 6 March 2018).
- McKeague, J.A.; Wolynetz, M.S. Background levels of minor elements in some Canadian soils. Geoderma 1980, 24, 299–307. [Google Scholar] [CrossRef]
- Mota, J.F.; Medina-Cazorla, J.M.; Navarro, F.B.; Pérez-García, F.J.; Pérez-Latorre, A.V.; Sánchez-Gómez, P. Dolomite flora of the Baetic Ranges glades (South Spain). Flora 2008, 203, 359–375. [Google Scholar] [CrossRef]
- Rufo, L.; Rodríguez, N.; Fuente, V. Análisis comparado de metales en suelos y plantas de la Sierra Bermeja. In Proceedings II Simposio Nacional de Control de la Degradación de Suelos; Jiménez, R., Álvarez, A.M., Eds.; Universidad Autónoma de Madrid: Madrid, Spain, 2005; pp. 197–201. ISBN 84-689-2620-5. [Google Scholar]
- Yusta, A.; Berahona, E.; Huertas, F.; Reyes, E.; Yáñez, J.; Linares, J. Geochemistry of soils from peridotite in Los Reales, Málaga. Acta Mineral. Petrog. 1985, 29, 439–498. [Google Scholar]
- Olmedo-Cobo, J.A.; Cunill-Artigas, R.; Martínez-Ibarra, E.; Gómez-Zotano, J. Nuevos datos paleoecológicos de Abies ssp. en el Sur de España a partir del análisis pedoantracológico en Sierra Bermeja. In Avances en Biogeografía. Áreas de Distribución: Entre Puentes y Barreras; Gómez-Zotano, J., Arias-García, J., Olmedo-Cobo, J.A., Serrano-Montes, J.L., Eds.; Universidad de Granada-Tundra Ediciones: Granada, Spain, 2016; pp. 582–591. ISBN 978-84-338-5932-7. [Google Scholar]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy Metals in Soils, 2nd ed.; Blackie Academic & Professional: London, UK, 1995; ISBN 978-94-010-4586-5. [Google Scholar]
- Boletín Oficial de la Junta de Andalucía–BOJA. Available online: http://normativa.infocentre.es/sites/normativa.infocentre.es/files/noticies/20206012.pdf (accessed on 22 February 2018).
- Rufo, L.; de la Fuente, V.; Sánchez-Mata, D.; Rodríguez-Rojo, M.P. Studies on Iberian peninsula ultramafic flora: A selected nickel accumulation screening. Lazaroa 2004, 25, 161–167. [Google Scholar]
- Alados-Arboledas, L.; Olmo, F.J.; Alados, I.; Pérez, M. Parametric models to estimate photosynthetically active radiation in Spain. Agric. Forest Meteorol. 2000, 101, 187–201. [Google Scholar] [CrossRef]



| Geomorphoedaphic Sub-Unit | Number of Samples (n) | Parent Material | Extension (m2) | Elevation (masl) | Orientation | Soil Taxonomy |
|---|---|---|---|---|---|---|
| S1—Endorheic basin bottom | 3 | Clays | 1110 | 1368–1364 | Southeast | Regosol Dystric |
| S2—Chaos of blocks | 3 | Peridotites (Hazburgites) | 2138 | 1393–1367 | Southeast | Leptosol Lithic |
| S3—Granitoid dyke | 5 | Leucogranites | 9980 | 1414–1367 | East | Leptosol Mollic |
| S4—Rocky and eroded slopes | 5 | Peridotites (Lherzolites) | 13,261 | 1428–1377 | Southeast | Leptosol Eutric |
| S5—Col | 6 | Serpentinites | 538 | 1430–1421 | East | Leptosol Eutric |
| S6—Notched ridges | 5 | Peridotites (Hazburgites) | 2989 | 1432–1424 | East | Leptosol Eutric |
| Geomorphoedaphic | pH | EC | Ca | K | Fe | Sr:Ca |
|---|---|---|---|---|---|---|
| Sub-Unit | dS m−1 | mg kg−1 | ||||
| S1 | 6.05 ± 0.12 | 0.08 ± 0.01 | 13,000 ± 2400 bc | 8360 ± 1217 bc | 73817 ± 32704 bc | 0.0036 ± 0.0011 |
| (n = 3) | (5.96–6.18) | (0.07–0.12) | (10,600–15,400) | (7144–9577) | (42,612–107,838) | |
| S2 | 6.31 ± 0.32 | 0.13 ± 0.04 | 14,733 ± 4362 bc | 4501 ± 904 ab | 65,544 ± 13,036 abc | 0.0010 ± 0.0002 |
| (n = 3) | (5.98–6.62) | (0.08–0.15) | (10,200–18,900) | (3794–5519) | (54316–79,841) | |
| S3 | 6.01 ± 0.12 | 0.08 ± 0.01 | 5434 ± 4899 a | 11,012 ± 3945 c | 36,213 ± 10,299 a | 0.0503 ± 0.0568 |
| (n = 7) | (5.8–6.1) | (0.07–0.09) | (525–12,200) | (6882–18,700) | (25,300–54,807) | |
| S4 | 6.39 ± 0.13 | 0.08 ± 0.01 | 16,920 ± 3144 b | 6053 ± 2380 ab | 95,726 ± 22,956 c | 0.0009 ± 0.00042 |
| (n = 5) | (6.21–6.51) | (0.06–0.09) | (12,900–21,700) | (3154–9745) | (65,932–126,300) | |
| S5 | 7.12 ± 0.23 | 0.08 ± 0.01 | 8376 ± 844 ab | 2614 ± 617 a | 58408 ± 6042 ab | 0.0009 ± 0.000317 |
| (n = 6) | (6.84–7.39) | (0.06–0.10) | (6848–9312) | (1929–3742) | (50,190–66,382) | |
| S6 | 6.79 ± 0.22 | 0.08 ± 0.01 | 9337 ± 1632 ab | 3966 ± 591 ab | 81,651 ± 14,508 bc | 0.0013 ± 0.00033 |
| (n = 5) | (6.53–7.09) | (0.06–0.09) | (7896–12,000) | (3297–4904) | (62,197–96,051) | |
| pH | Major Ions | PTM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ca | K | Sr | Cr | Ni | Co | Mn | Zn | Cu | ||
| pH | 1 | ||||||||||
| Fe | 1 | ||||||||||
| Ca | 0.525 ** | 1 | |||||||||
| K | −0.742 ** | 1 | |||||||||
| Sr | −0.698 ** | −0.585 ** | 0.645 ** | 1 | |||||||
| Cr | 0.431 * | 0.637 ** | 0.617 ** | −0.593 ** | −0.658 ** | 1 | |||||
| Ni | 0.600 ** | 0.863 ** | 0.471 ** | −0.715 ** | −0.805 ** | 0.734 ** | 1 | ||||
| Co | 0.418 * | 0.831 ** | 0.508 ** | −0.580 ** | −0.769 ** | 0.802 ** | 0.901 ** | 1 | |||
| Mn | 0.889 ** | 0.578 ** | 0.484 ** | 0.650 ** | 0.601 ** | 1 | |||||
| Zn | −0.561 ** | 0.610 ** | 0.651 ** | −0.458 * | −0.556 ** | −0.409 * | 1 | ||||
| Cu | 0.412 * | 0.526 ** | 1 | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Freire, A.; Olmedo-Cobo, J.A.; Gómez-Zotano, J. Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain). Minerals 2018, 8, 447. https://doi.org/10.3390/min8100447
Romero-Freire A, Olmedo-Cobo JA, Gómez-Zotano J. Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain). Minerals. 2018; 8(10):447. https://doi.org/10.3390/min8100447
Chicago/Turabian StyleRomero-Freire, Ana, José Antonio Olmedo-Cobo, and José Gómez-Zotano. 2018. "Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain)" Minerals 8, no. 10: 447. https://doi.org/10.3390/min8100447





