Carbothermic Reduction of Ore-Coal Composite Pellets in a Tall Pellets Bed
Abstract
:1. Introduction
2. Experimental
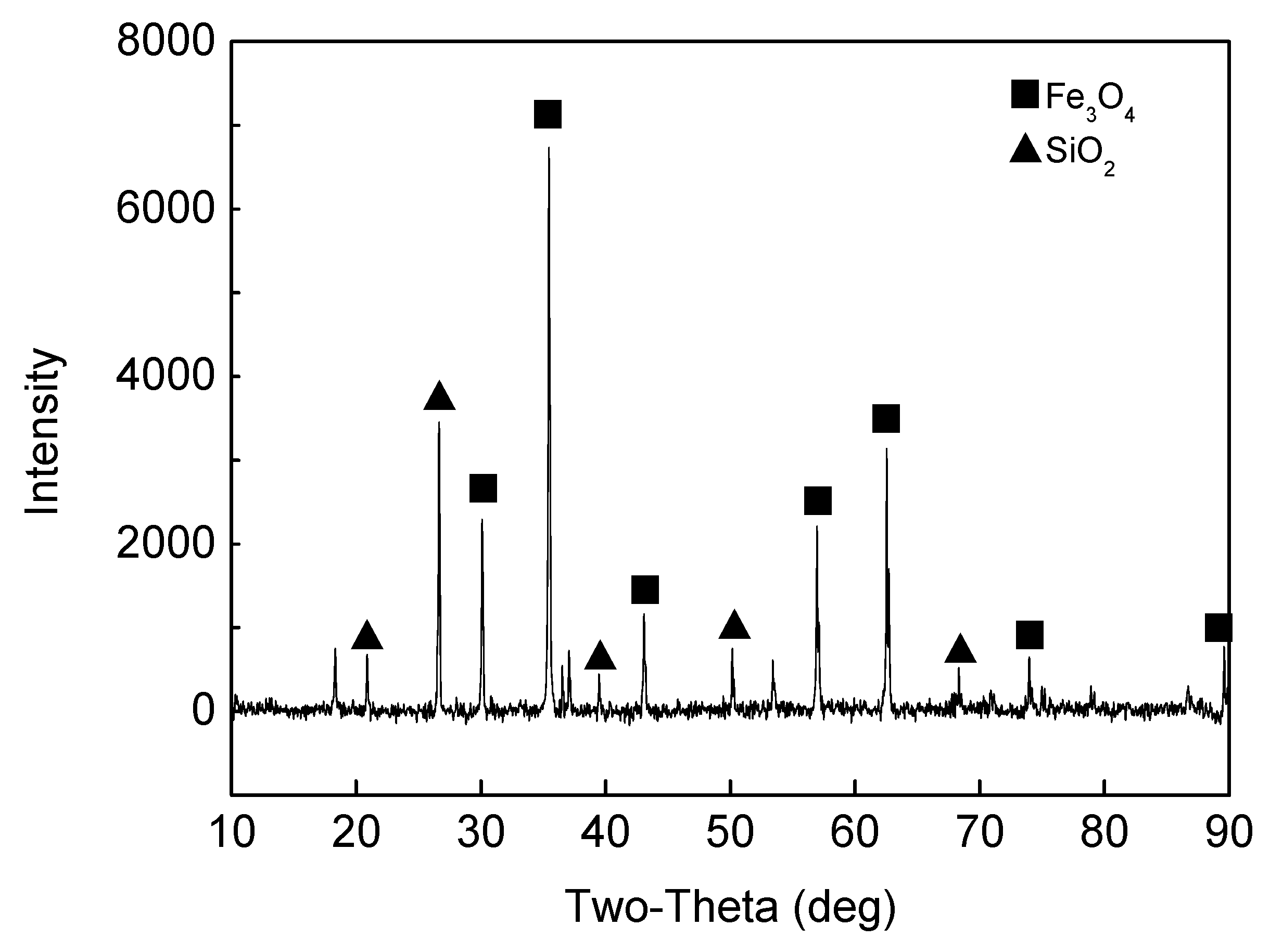
2.1. Raw Materials
2.2. Experimental Set-Up
- Mixing of raw materials. Effective mixing of ore and coal is an essential step to ensure proper homogeneous reaction throughout the pellets. Therefore, first the ore and coal were mixed in an intensive mixer to ensure the uniform mixing of ore and coal, and a homogeneous reaction in the ore-coal composite pellets.
- Pelletizing. The diameter of the ore-coal composite pellet is about 16–18 mm. The total H2O content in the wet green ball is about 8.5–9.0%. In order to avoid the crack of pellets in the high temperature furnace due to evaporation, the wet green balls should be dried before charging into the furnace.
- A special crucible was made for holding composite pellets in muffle furnace (Figure 5). The crucible consists of two parts: (a) the mullite ring (the inner diameter is 80 mm) to retain the upward gas in a vertical direction in the pellets bed; and (b) the insulating materials surrounding the mullite ring to obstruct the heat transfer from the horizontal direction of the pellets bed.
- The special crucible was put into the furnace. Then, the temperature of the furnace was heated to 1200 °C in air atmosphere, and the crucible was pre-heated to 1200 °C in the furnace.
- The furnace door was opened, and 500 g of the dried composite pellets was charged into the crucible. The height of pellets bed is about 80 mm (5 layers, and 12–13 pellets each layer), and the void ratio of packing bed is about 14–18%.
- The furnace temperature was kept at 1200 °C for 5 min. Then, it was heated to 1500 °C in about 20 min (15 °C/min), and kept at 1500 °C until the target time.
- When the target reduction time was reached, the entire crucible was taken out of the furnace, and the surrounding insulating materials were immediately taken away from the crucible to stop the reduction reaction. Then, the hot DRI bed is quenched in liquid N2 to prevent the DRI from re-oxidation.
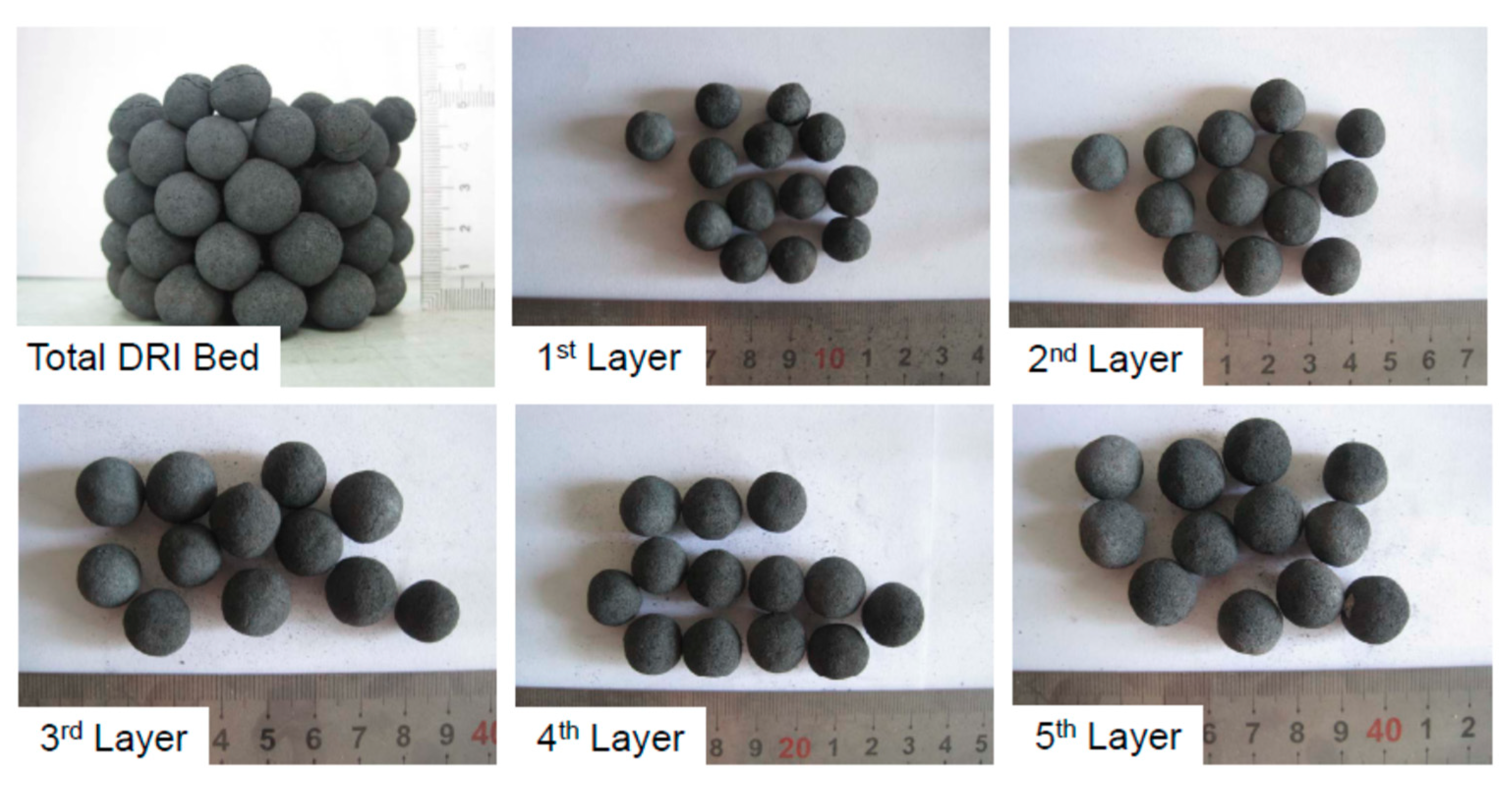
- Divide the DRI bed. The cooled DRI bed is shown in Figure 6. From top to bottom, the first 12–13 pellets are defined as the 1st layer, then the second 12–13 pellets are defined as the 2nd layer, then the 3rd layer, 4th layer, and 12–13 pellets on the bottom are defined as the 5th layer. Finally, the total DRI bed is divided into 5 layers from top to bottom.
- Eight pellets in each layer were randomly selected for chemical analysis. Total Fe (TFe) and Metallic Fe (MFe) were obtained. Then, for each layer, the ratio of metallic Fe to total Fe was defined as metallization degree (MD = MFe/TFe), which was an actual value (not average value). The metallization degrees (MD) of the total DRI bed, which is an actual value too, can be calculated by the following equation:MD (total bed) = [MFe (1st layer) × Weight (1st layer) + MFe (2nd layer) × Weight (2nd layer) + … + MFe (5th layer) × Weight (5th layer)]/[TFe (1st layer) × Weight (1st layer) + TFe (2nd layer) × Weight (2nd layer) + … + TFe (5th layer) × Weight (5th layer)]
3. Experimental Results and Analysis
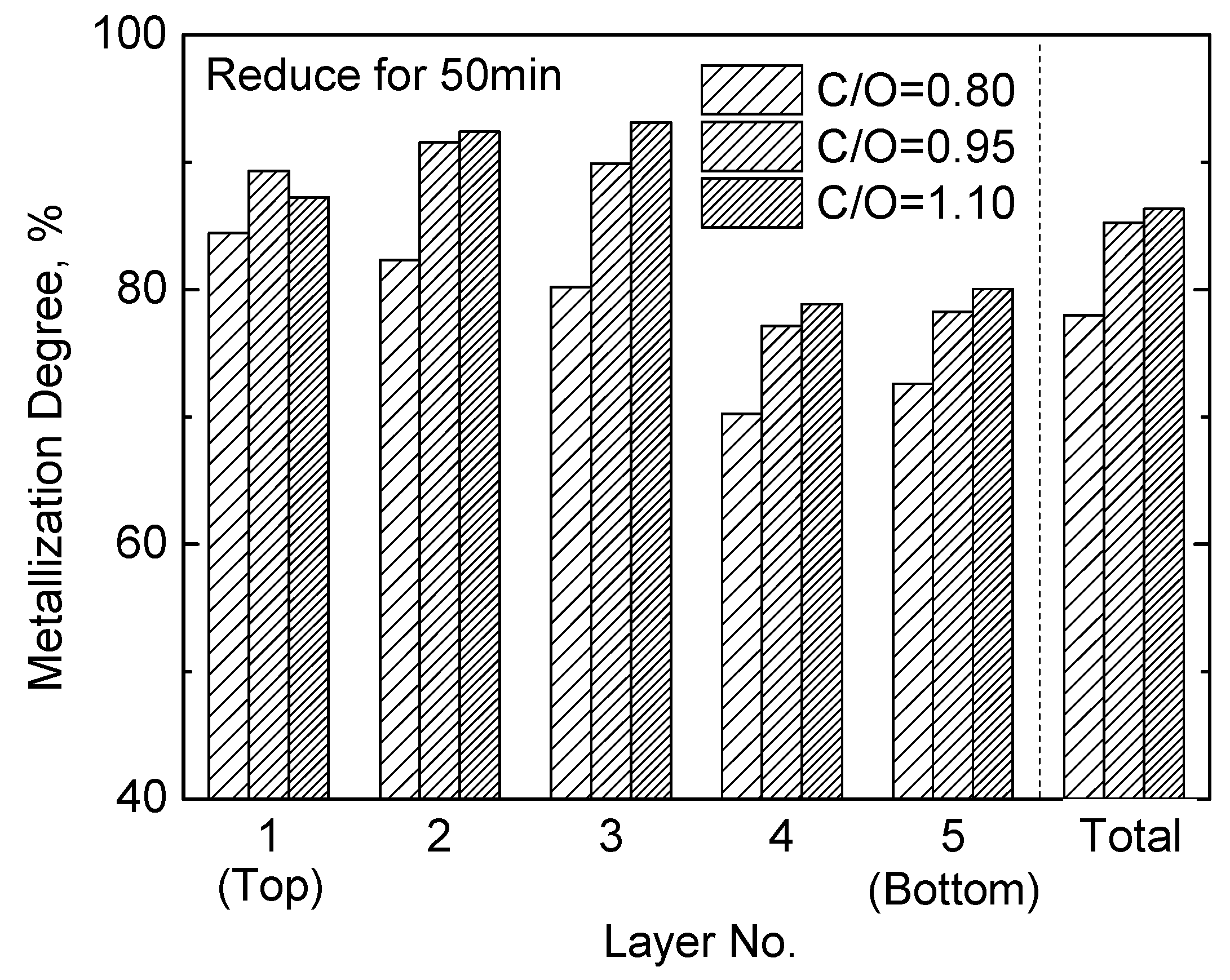
3.1. Effects of Amount of Carbon Addition (C/O)
3.1.1. Metallization Degree
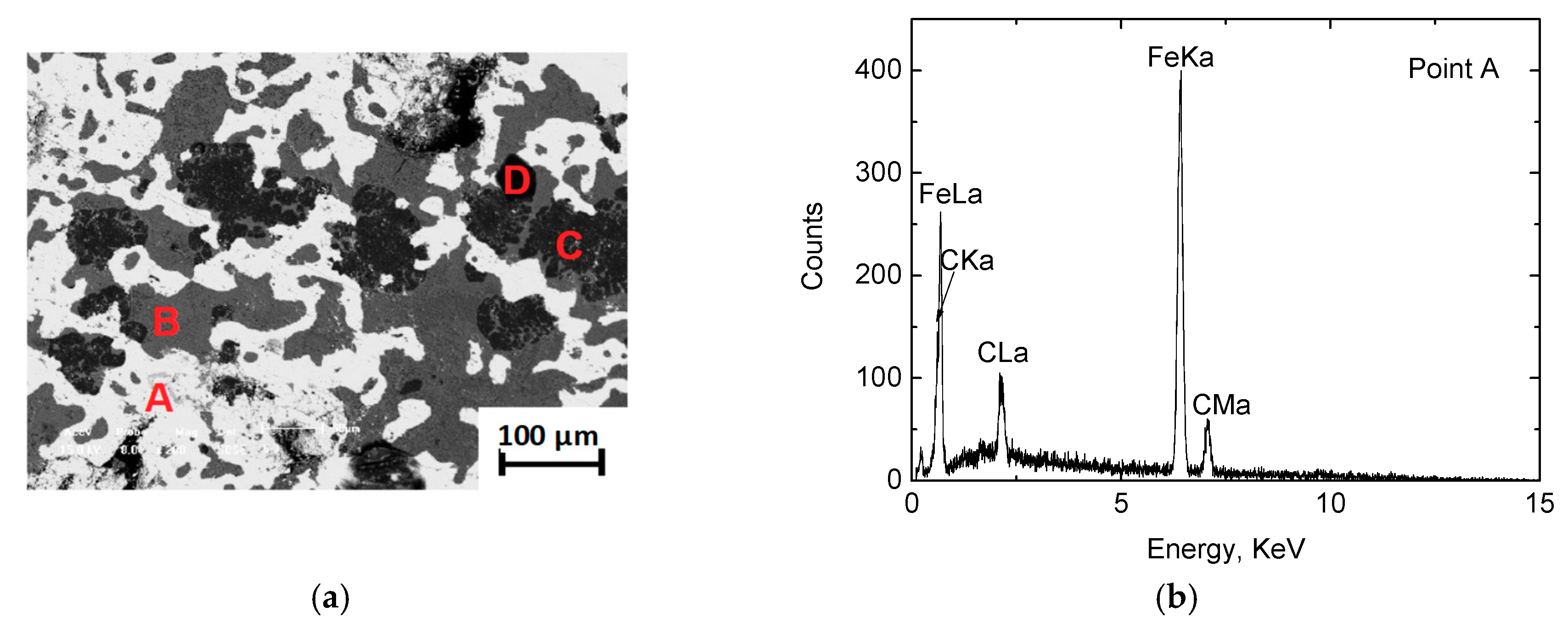
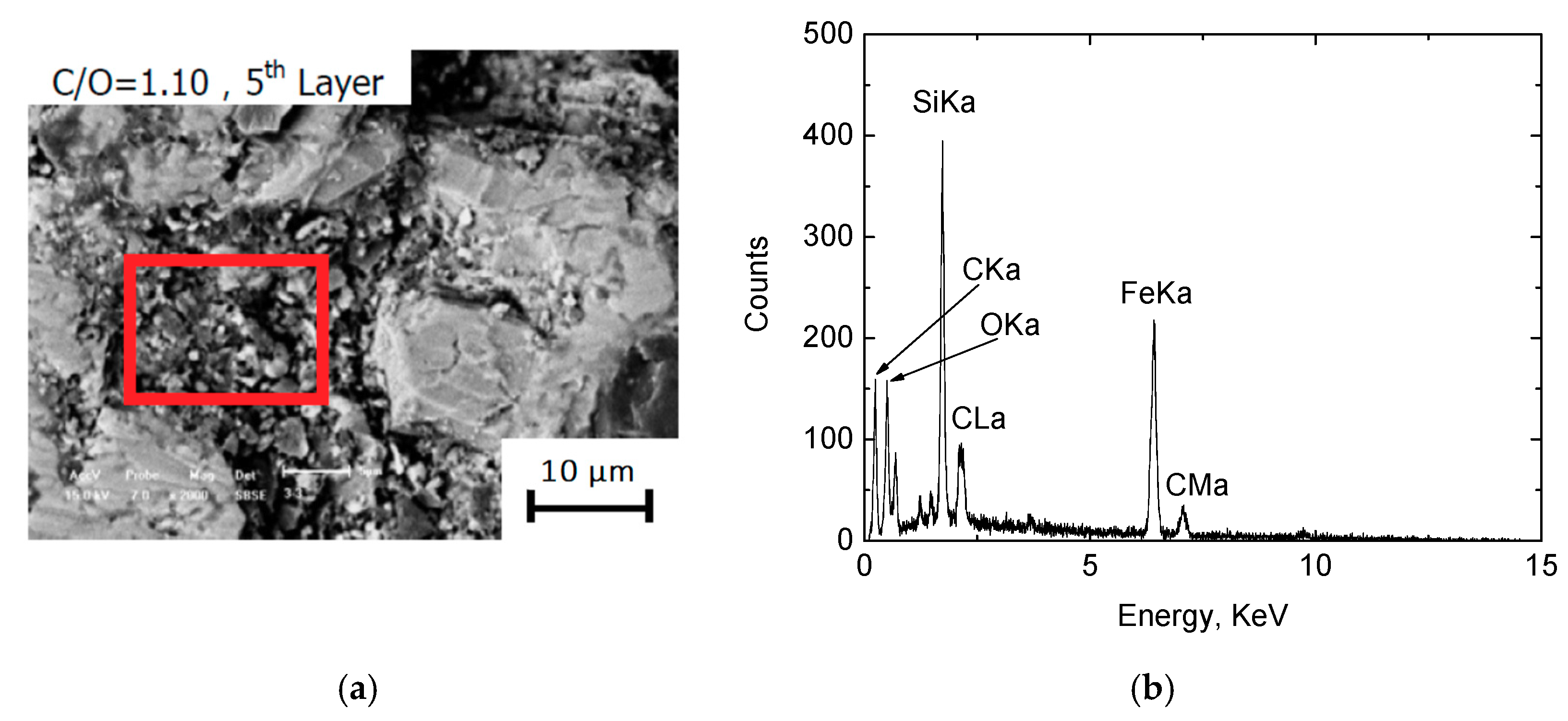
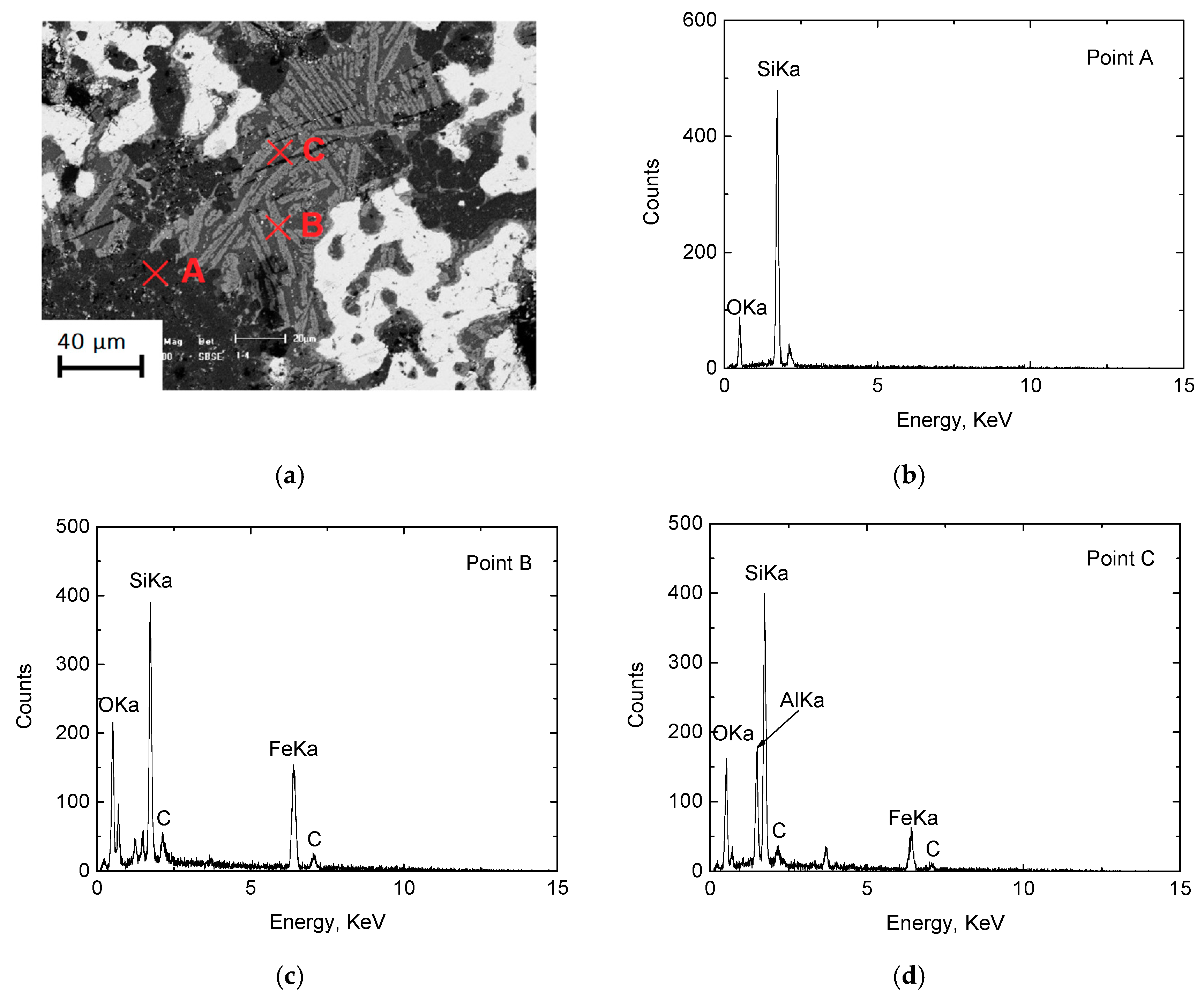
3.1.2. Metallographic Analysis
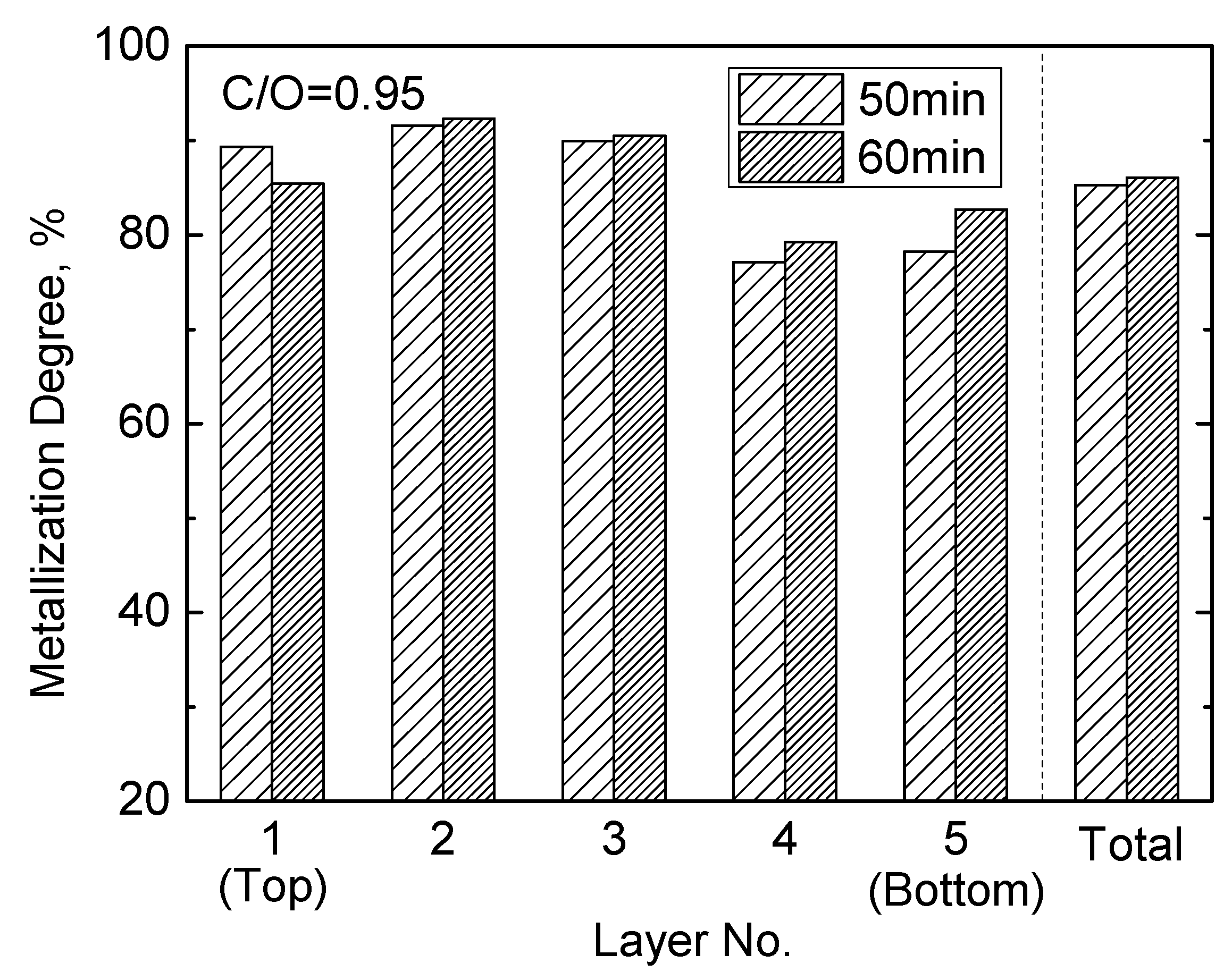
3.2. Carbothermic Reduction for 50 min and 60 min
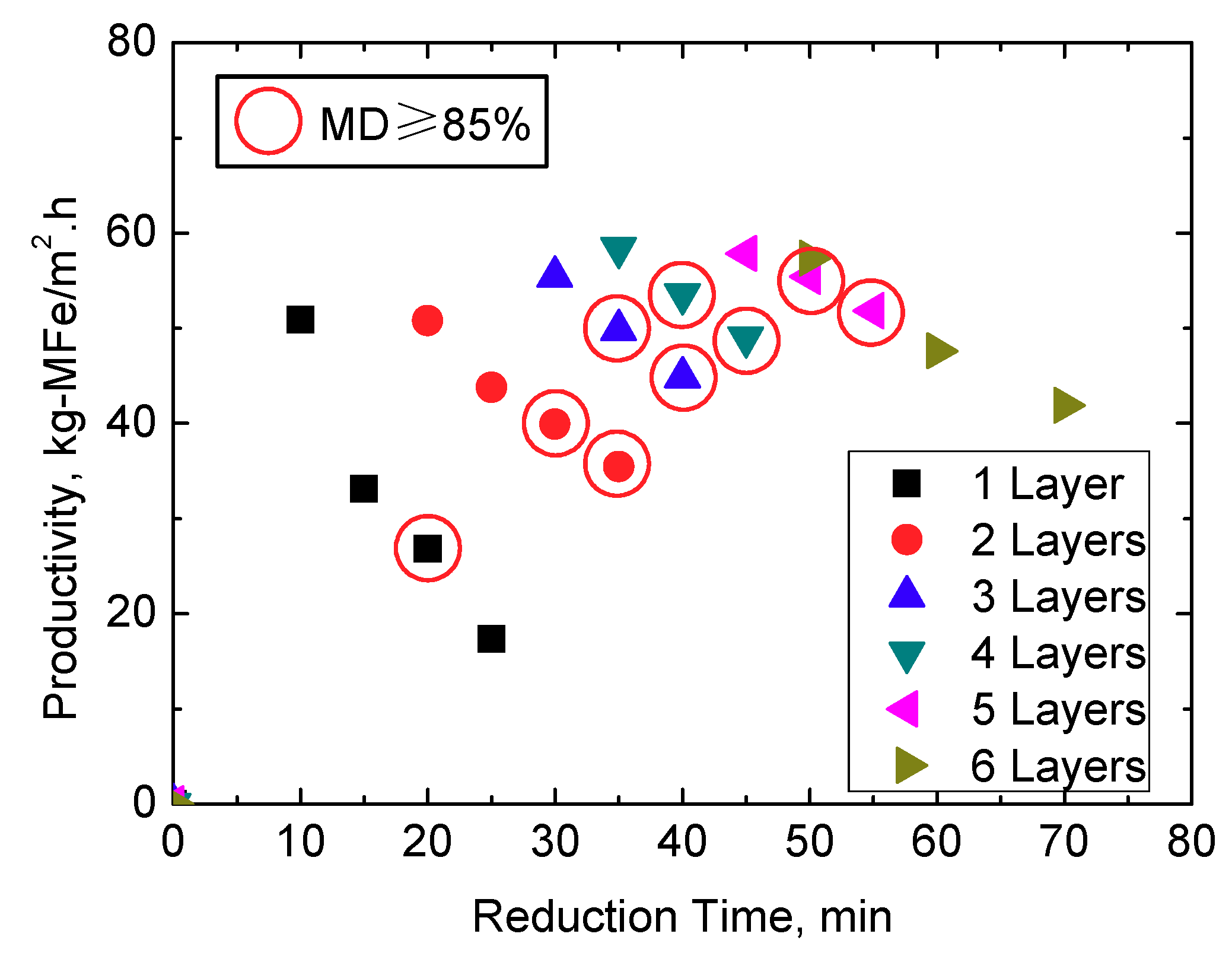
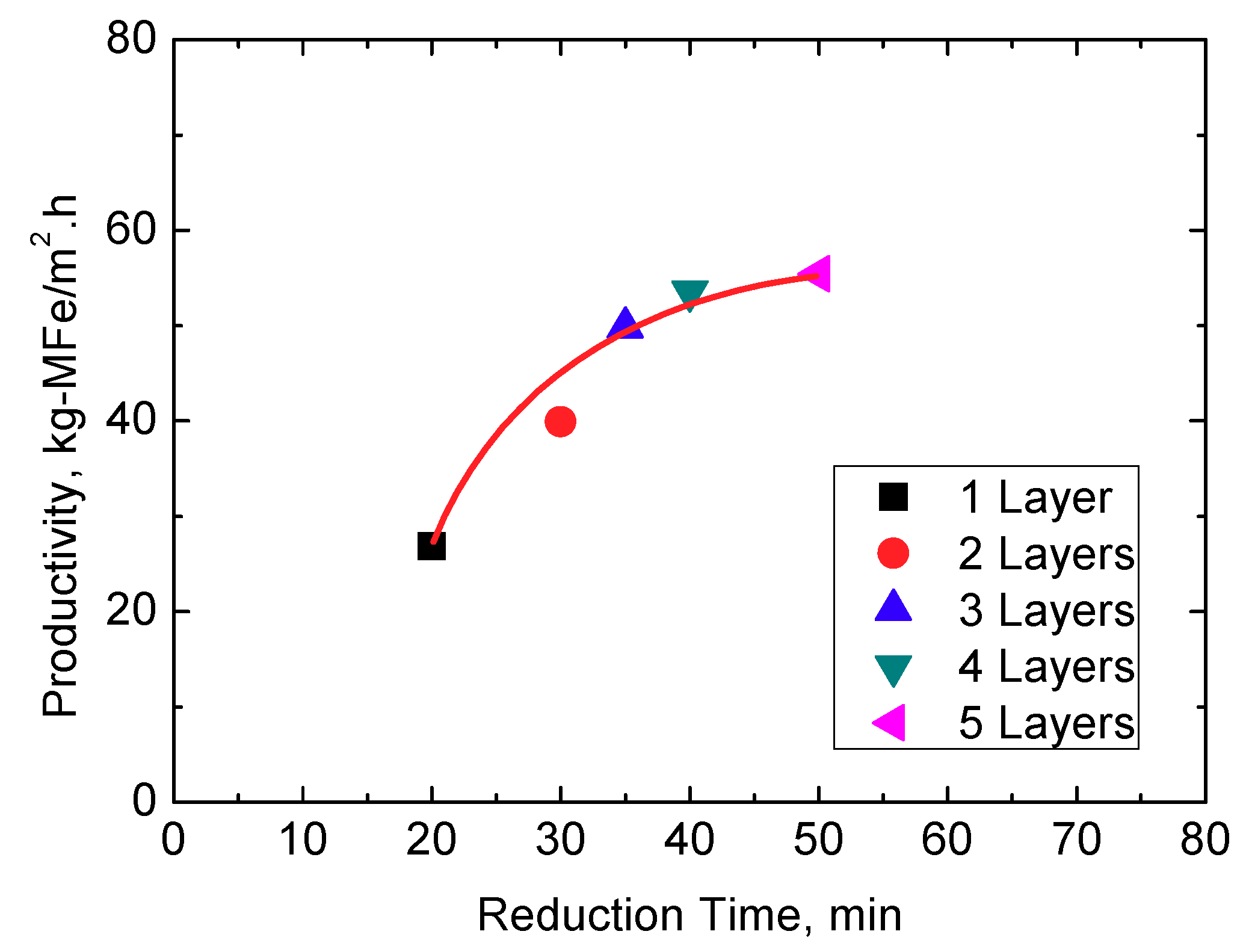
3.3. Productivity of the Tall Pellets Bed
4. Discussion
4.1. Optimal Parameters
4.1.1. Carbon Addition
4.1.2. Reduction Time
4.2. Heat Transfer
4.3. Simultaneous Tall Pellets Bed and High Temperature
4.4. Economics and Challenges
5. Conclusions
- (1)
- When the height of the pellets bed is 80 mm (16–18 mm each layer, and 5 layers), C/O = 0.80 may result in a lower metallization degree due to an insufficient reductant, and C/O = 1.10 may result in more residual carbon in the DRI of the bottom layer. Therefore, under the present experimental conditions, the optimal amount of carbon to add is C/O = 0.95. Addition of either more or less carbon does not benefit the production of high quality DRI.
- (2)
- The longer reduction time (60 min) may result in more melting and corrosive slag in the top layer of DRI, which does not benefit the actual operation of the PSH process. With a reduction time of 50 min, the metallization degree may be up to 85.24%. Therefore, 50min is considered to be the optimal reduction time for an 80 mm bed (5 layers).
- (3)
- In the present work, kg-MFe/m2·h is used as the unit to evaluate the productivity of metallic iron. In lab-scale experiments, under the condition of 5 layers of pellets (about 80 mm in height) and 50 min, the productivity of metallic iron can reach 55.41 kg-MFe/m2·h (or 75.26 kg-DRI/m2·h). Therefore, compared with the traditional shallow bed (one or two layers), the productivity of the DRI can be effectively increased in a tall pellets bed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasai, E.; Kitajima, T.; Kawaguchi, T. Carbothermic reduction in the combustion bed packed with composite pellets of iron oxide and coal. ISIJ Int. 2000, 40, 842–849. [Google Scholar] [CrossRef]
- Qi, Z.; Murakami, T.; Kasai, E. Gasification and reduction behavior of iron ore-carbon composite under high pressure. ISIJ Int. 2012, 52, 1778–1784. [Google Scholar] [CrossRef]
- Murakami, T.; Kasai, E. Utilization of ores with high combined water content for ore-carbon composite and iron coke. ISIJ Int. 2011, 51, 1220–1226. [Google Scholar] [CrossRef]
- Murakami, T.; Kasai, E. Reduction mechanism of iron oxide–carbon composite with polyethylene at lower temperature. ISIJ Int. 2011, 51, 9–13. [Google Scholar] [CrossRef]
- Yunus, N.A.; Ani, M.H.; Salleh, H.M.; Aborashid, R.Z.; Akiyama, T.; Purwanto, H. Reduction of iron ore/empty fruit bunch char briquette composite. ISIJ Int. 2013, 53, 1749–1755. [Google Scholar] [CrossRef]
- Takyu, Y.; Murakami, S.H.; Son, S.H.; Kasai, E. Reduction mechanism of composite consisted of coal and hematite ore by volatile matter at 700–1100 K. ISIJ Int. 2015, 55, 1188–1196. [Google Scholar] [CrossRef]
- Yi, L.Y.; Huang, Z.C.; Jiang, T.; Zhao, P.; Zhong, R.H.; Liang, Z.K. Carbothermic reduction of ferruginous manganese ore for Mn/Fe beneficiation: Morphology evolution and separation characteristic. Minerals 2017, 7, 167. [Google Scholar] [CrossRef]
- Yu, D.W.; Paktunc, D. Calcium chloride-assisted segregation reduction of chromite: Influence of reductant type and the mechanism. Minerals 2018, 8, 45. [Google Scholar] [CrossRef]
- Suzuki, H.; Mizoguchi, H.; Hayashi, S. Influence of ore reducibility on reaction behavior of ore bed mixed with coal composite iron ore hot briquettes. ISIJ Int. 2011, 51, 1255–1261. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ueno, T.; Okumura, K.; Hayashi, S. Reaction behavior of coal rich composite iron ore hot briquettes under load at high temperatures until 1400 °C. ISIJ Int. 2011, 51, 1240–1246. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Shen, F.M.; Lu, W.K. Adiabatic carbon rate of alternative ironmaking processes to produce hot metal. Steel Res. Int. 2014, 85, 35–43. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.C.; Cui, Q.; Xu, Y.; Kou, J. Effect of coal type on the reduction and magnetic separation of a high-phosphorus oolitic hematite ore. ISIJ Int. 2015, 55, 536–543. [Google Scholar] [CrossRef]
- Xu, C.B.; Cang, D.Q. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Iguchi, Y.; Yokomoto, S. Kinetics of the reactions in carbon composite iron ore pellets under various pressures from vacuum to 0.1 MPa. ISIJ Int. 2004, 44, 2008–2017. [Google Scholar] [CrossRef]
- Yu, W.; Wen, X.J.; Chen, J.G.; Huang, W.Q.; Qiu, T.S. Preparation of direct reduced iron and titanium nitride from Panzhihua titanomagnetite concentrate through carbothermic reduction-magnetic separation. Minerals 2017, 7, 220. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.H.; Huang, T.Y.; Shen, F.M. Effects of reducing time on metallization degree of carbothermic reduction of tall pellets bed. ISIJ Int. 2016, 56, 88–93. [Google Scholar] [CrossRef]
- Sun, S.; Lu, W.K. A theoretical investigation of kinetics and mechanisms of iron ore reduction in an ore/coal composite. ISIJ Int. 1999, 39, 123–129. [Google Scholar] [CrossRef]







| TFe | FeO | SiO2 | CaO | MgO | Al2O3 | LOI |
|---|---|---|---|---|---|---|
| 63.26 | 26.99 | 6.68 | 0.15 | 0.12 | 0.20 | −1.45 |
| Proximate Analysis | Ash Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Fixed C | Total C | Ash | VM | SiO2 | Al2O3 | Fe2O3 | MgO | CaO |
| 61.31 | 77.5 | 9.38 | 26.00 | 49.14 | 29.98 | 10.22 | 0.70 | 5.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Ding, G.; Guo, H.; Gao, Q.; Shen, F. Carbothermic Reduction of Ore-Coal Composite Pellets in a Tall Pellets Bed. Minerals 2018, 8, 550. https://doi.org/10.3390/min8120550
Jiang X, Ding G, Guo H, Gao Q, Shen F. Carbothermic Reduction of Ore-Coal Composite Pellets in a Tall Pellets Bed. Minerals. 2018; 8(12):550. https://doi.org/10.3390/min8120550
Chicago/Turabian StyleJiang, Xin, Guangen Ding, He Guo, Qiangjian Gao, and Fengman Shen. 2018. "Carbothermic Reduction of Ore-Coal Composite Pellets in a Tall Pellets Bed" Minerals 8, no. 12: 550. https://doi.org/10.3390/min8120550




